Abstract
In chronic granulomatous disease (CGD), defective phagocytic nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity causes reduced superoxide anion (O2·̄) radical production leading to frequent infections as well as granulomas and impaired wound healing indicative of excessive inflammation. Based on recent mouse studies, the lack of O2·̄-dependent interferon γ (IFNγ)–induced synthesis of kynurenine (kyn), an anti-inflammatory tryptophan metabolite produced by indolamine 2,3 deoxygenase (IDO), was proposed as a cause of hyperinflammation in CGD and this pathway has been considered for clinical intervention. Here, we show that IFNγ induces normal levels of kynurenine in cultures of O2·̄-deficient monocytes, dendritic cells, and polymorphonuclear leukocytes from gp91PHOX- or p47PHOX-deficient human CGD donors. Kynurenine accumulation was dose- and time-dependent as was that of a downstream metabolite, anthranilic acid. Furthermore, urinary and serum levels of kynurenine and a variety of other tryptophan metabolites were elevated rather than suppressed in CGD donors. Although we did not specifically evaluate kyn metabolism in local tissue or inflamed sites in humans, our data demonstrates that O2·̄ anion is dispensable for the rate-limiting step in tryptophan degradation, and CGD patients do not appear to have either hematopoietic cell or systemic deficits in the production of the anti-inflammatory kynurenine molecule.
Introduction
Excessive inflammation causes significant morbidity in chronic granulomatous disease (CGD) patients. In addition to causing granulomas, strictures and impaired wound healing, dysregulated inflammation may complicate recovery from infections.1 CGD results from defective O2·̄ production by the phagocytic nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, an enzyme complex of gp91PHOX, p22PHOX and cytosolic regulators p47PHOX and p67PHOX. Superoxide, either directly or through downstream oxidative transformations, plays an antimicrobial role but is increasingly seen as an important regulator of inflammatory responses including transcription,2 apoptosis,3 neutrophil extracellular trap (NET) formation,4 and leukotriene metabolism.5 Knockout mice lacking either gp91PHOX or p47PHOX recapitulate many of these aspects of human CGD and are an accepted model of CGD.6,7
Using p47PHOX-deficient mice, a recent study proposed that hyperinflammation of CGD was due to defective production of kynurenine,8 an anti-inflammatory tryptophan metabolite produced during inflammation by indolamine 2,3-dioxygenase (IDO) using O2·̄ as a required cofactor.9 In these CGD mouse studies, the defective conversion of tryptophan into N-formylkynurenine by superoxide-dependent mouse IDO could be circumvented by providing exogenous kynurenine and interferon γ (IFNγ) thereby dramatically reversing inflammation.8 Consequently, the IDO-kynurenine pathway was rapidly proposed as a therapeutic target to control inflammation in human CGD.10 Tryptophan and kynurenine are important immunoregulatory modulators of tolerance during pregnancy, infection, and autoimmunity.11 While the bulk of systemic tryptophan conversion is performed by the constitutively expressed hepatic tryptophan 2,3-dioxygenase enzyme, inflammatory stimuli such as IFNγ strongly up-regulate IDO activity in cells such as macrophages.12 Furthermore, several tryptophan metabolites are antibacterial13 and may contribute to controlling some protozoan parasites,14 raising the possibility that absence of such metabolites in CGD could contribute to infections. Given the pressing clinical need to control hyperinflammation in CGD and better understand regulatory mechanisms of human inflammation in general, we examined whether O2·̄ played a similar regulatory role in the tryptophan/kynurenine pathway in leukocytes from human CGD subjects.
Methods
Patients
Human subjects included X-linked (gp91PHOX-deficient, X-CGD) and autosomal (p47PHOX-deficient, A-CGD) patients and normal volunteers (Institutional Review Board–approved protocol NIH#05-I-0213).
Sample preparation and stimulation
Polymorphonuclear leukocytes (PMNs) were isolated as previously described15 and purity exceeded 90%. For the Western blot experiments, PMN were further purified with magnetic affinity cell sorting (MACS) to a purity of 99%. Monocytes were prepared using the Dynal Monocyte Negative Isolation bead kit (Invitrogen) mean 87% purity by FACS using CD45:FITC, CD14:PE; Becton Dickinson). PMN and monocytes were suspended in 0.5 mL (5 × 106/mL and 1 × 106/mL, respectively) RPMI 1640/10% fetal calf serum/50μM Trp and incubated with buffer or indicated doses of IFNγ (Actimmune) at 37°C and 5% CO2 for the indicated periods of time.
Dendritic cells generated from monocytes in RPMI 1640, fetal calf serum, penicillin/streptomycin l-glutamine, 25mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 1% nonessential amino acids, sodium pyruvate, and 50nM 2-mercaptoethanol, with 50 ng/mL interleukin-4 (IL-4) and 100 U/mL granulocyte/macrophage-colony-stimulating factor added on day 3 for 7 days, were stimulated with IFNγ as described above.
Sera were collected in serum separator tubes and stored frozen until analysis. Urine (24-hour collections) from 2 A-CGD, 4 X-CGD, and 3 normal donors were stored frozen until analysis.
Liquid chromatography–electrospray ionization–tandem mass spectrometry for tryptophan and metabolites
Tryptophan, kynurenine, 3-hydroxy-kynurenine, α-picolinic acid, anthranilic acid, quinolinic acid, trichloroacetic acid (TCA) and heptafluorobutyric acid (HFBA) solutions were obtained from Sigma-Aldrich. Deuterated standards D5-tryptophan and D6-kynurenine were obtained from Cambridge Isotope Laboratories and D4-picolinic acid was obtained from CDN Isotopes. Samples and/or standards were mixed for 10 minutes with a TCA solution containing deuterated tracers and centrifuged at 14 000g for 5 minutes.
Liquid chromatography–electrospray ionization–tandem mass spectrometry (LC-ESI-MS) was performed with an Agilent 1200 system coupled to a Finnigan Quantum-AM triple quadrupole mass spectrometer (Thermo Finnigan) operating at selected reaction monitoring (SRM) mode with positive ionization voltage. Separation was performed with a Waters 1 × 50 mm, 3 μm T3 column. Mobile phase A consisted of 0.1% HFBA in water and mobile phase B consisted of 0.1% HFBA in methanol. Samples (5 μL) were resolved at 50 μL/min with a multisegment gradient of 0-2 minutes/2%B, 12 minutes/90%B, 12.5-16 minutes/2%B), and detected using the following m/z precursor/product ions: picolinic acid (124/78); quinolinic acid (168/78); 3-hydroxy-kynurenine (225/110); kynurenine (209/146); anthranilic acid (138/120); tryptophan (205/146), D4-picolinic acid (128/82); D6-kynurenine (215/152); D5-tryptophan (210/150). The detection sensitivity (S/N = 100) of the standards were as follows: tryptophan (2nM), anthranilic acid (4nM), kynurenine (9nM), 3-hydroxy-kynurenine (9nM), picolinic acid (10nM), quinolinic acid (40nM). The general MS conditions were as follows: spray voltage, 4800 V; sheath and auxiliary gas, nitrogen; sheath gas pressure, 49 arbitrary units; auxiliary gas pressure, 25 arbitrary units; ion transfer capillary temperature, 350°C; collision gas pressure, argon; collision gas pressure, 1.5 mTorr; scan width, 0.70 u; scan time, 0.03 s; Q1 peak width, 0.7 u full-width half-maximum (fwhm); Q3 peak width, 0.70 u fwhm. The collision energy voltages for PA, D4-PA, QA, 3-HKYN, KYN, D6-KYN, AA, TRP, and D5-TRP were 19, 19, 28, 18, 26, 26, 10, 21, and 21, respectively.
The metabolite concentrations in the samples were calculated using a second-order polynomial fit constructed by plotting the concentrations of standard metabolites versus peak area ratios. The concentrations used were 0.14, 0.41, 1.2, 3.7, 11, 33, and 100μM. The peak area ratios were calculated by dividing the peak areas of metabolites by the deuterated internal standards (ie, picolinic acid/D4-picolinic acid, quinolinic acid/D4-picolinic acid, 3-hydroxykynurenine/D6-kynurenine, kynurenine/D6-kynurenine, anthranilic acid/D6-kynurenine, tryptophan/D5-tryptophan).
IDO protein detection
After incubation of cells with buffer or IFNγ as indicated, adherent cells were washed twice with PBS and solubilized in 40 μL of sample buffer (Invitrogen) containing protease inhibitors (Complete Protease Inhibitor Cocktail, Roche) and 5mM diisopropylfluorophosphate (Sigma-Aldrich) by shaking for 5 minutes. Samples were then transferred to polypropylene tubes and sonicated to shear DNA, then boiled for 10 minutes in presence of reducing agent (per manufacturer's instructions). Cell lysates (30 μL) were resolved on 10% Bis-Tris polyacrylamide gels and transferred overnight to Immobilon-P membrane (Millipore) using a Trans-blot unit. After blocking with 5% nonfat dry milk in Tris-buffered saline for 2 hours, the membranes were incubated overnight with a 1:3000 dilution (in 5% bovine serum albumin) of anti-IDO antibody (clone EPR1230Y, Epitomics), washed 3 times, and then incubated for 1 hour with anti–rabbit IgG-HRP secondary antibody (GE Healthcare; 1:1000 in blocking buffer). Blots were developed using enhanced chemiluminescence (Bio-Rad) and visualized using the ChemiDoc XRS System (Bio-Rad). The membranes were then stripped (GM Biosciences) and reprobed with anti-actin antibody (Millipore, clone C4 1:10 000 in blocking buffer) and developed as described above.
Statistics
Statistical testing was by paired t test using a 2-tailed P value or by nonparametric analysis of variance (ANOVA; Kruskal-Wallis) performed using the GraphPad Prism 5.0b for Mac.
Results
Defects in gp91PHOX or p47PHOX were confirmed in these patients by gene sequencing (gp91PHOX) and Western blot protein expression (gp91PHOX and p47PHOX). To confirm the extent of functional impairment of the NADPH oxidase, phorbol 12-myristate 13-acetate–induced O2·̄ production by PMN was measured as previously described15 by cytochrome C reduction resulting in values (in units of nmoles/106 PMNs/60 minutes) of 1.77 (± 0.58) for A-CGD (mean ± SD, n = 3) and 2.02 (± 1.79) for X-CGD (n = 5) versus a reference value of 232.55 (± 57.14) for normal PMNs (n = 170).
IFNγ induction of IDO protein in normal and CGD leukocytes
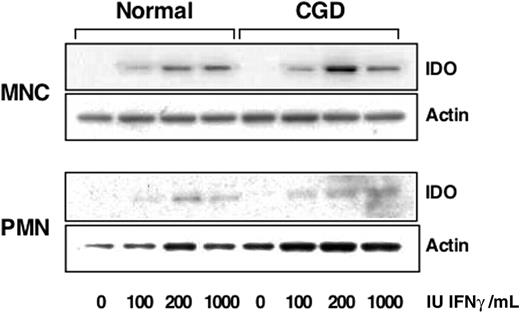
To confirm that human monocytes and PMN from CGD patients were capable of producing IDO, the initial enzyme in the kynurenine synthesis pathway,11 we treated cells with different doses of IFNγ and detected IDO with specific antibodies by Western blotting. As shown in Figure 1, the level of IDO increased both in normal and CGD monocytes. Highly purified PMN were also found to up-regulate expression of IDO protein after IFNγ treatment and no difference was noted between normal and CGD cells however the amount of IDO expressed by PMN appeared to be at least 5 times less than that expressed by monocytes.
CGD monocytes and PMN express IDO after IFNγ treatment. Immunoblots of lysates from monocytes (MNC) or PMN treated with indicated doses of IFNγ were probed for indoleamine 2,3-dioxygenase (IDO) expression and β-actin as a loading control. Blots shown are representative of 2 experiments.
CGD monocytes and PMN express IDO after IFNγ treatment. Immunoblots of lysates from monocytes (MNC) or PMN treated with indicated doses of IFNγ were probed for indoleamine 2,3-dioxygenase (IDO) expression and β-actin as a loading control. Blots shown are representative of 2 experiments.
IDO enzymatic activity in normal and CGD leukocytes
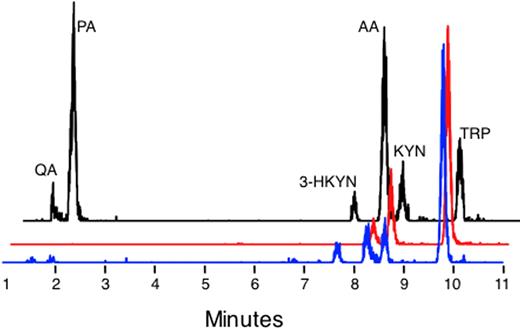
To detect tryptophan metabolites, we used a LC-ESI-MS to measure their concentrations in cell-culture supernatants, serum, and urine. As shown in Figure 2, simultaneous detection of several metabolites was possible with this technique.
LC-ESI-MS detection of metabolites in cell culture supernatants and urine. Representative traces are provided of standards (black line), a normal monocyte culture treated with IFNγ and supplemented with 500μM tryptophan (red trace), and a 24-hour urine sample from a CGD patient (blue trace). The traces are staggered to facilitate comparison.
LC-ESI-MS detection of metabolites in cell culture supernatants and urine. Representative traces are provided of standards (black line), a normal monocyte culture treated with IFNγ and supplemented with 500μM tryptophan (red trace), and a 24-hour urine sample from a CGD patient (blue trace). The traces are staggered to facilitate comparison.
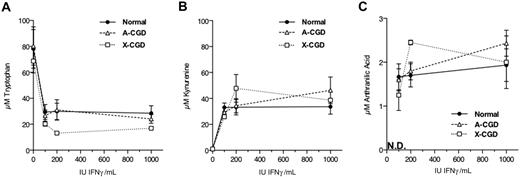
We next examined the IFNγ dose dependence of tryptophan metabolism in normal and CGD monocytes. As shown in Figure 3, all donor groups appeared to respond near maximally to IFNγ doses of as low as 100 IU/mL in terms of depletion of tryptophan (Figure 3A) and accumulation of kynurenine (Figure 3B) and anthranilic acid (Figure 3C) in culture supernatants.
Dose response to IFNγ of tryptophan metabolism in monocytes. Cells were incubated in media alone or with the indicated amounts of IFNγ for 48 hours before collection and analysis of cell culture supernatants. N.D. indicates that anthranilic acid was not detected in the absence of IFNγ treatment.
Dose response to IFNγ of tryptophan metabolism in monocytes. Cells were incubated in media alone or with the indicated amounts of IFNγ for 48 hours before collection and analysis of cell culture supernatants. N.D. indicates that anthranilic acid was not detected in the absence of IFNγ treatment.
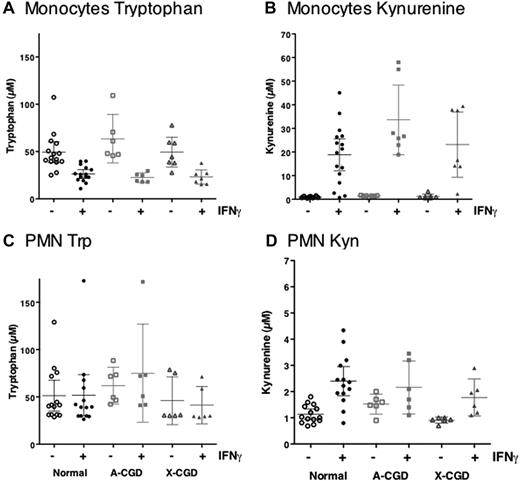
To maximize the likelihood of detecting metabolites, we chose to use an IFNγ dose of 1000 IU/mL for the remaining studies. As shown in Figure 4, treatment of normal human monocytes with IFNγ dramatically decreased residual tryptophan concentrations (Figure 4A) and increased those of kynurenine (Figure 4B). Despite 5-fold larger numbers of PMN (albeit incubated for 24 vs 48 hours for monocytes), residual tryptophan levels in PMN cultures treated with IFNγ remained constant (Figure 4C) although a small but significant increase in kynurenine concentration was measured (Figure 4D). IFNγ-induced IDO activity in either A-CGD or X-CGD cells, as measured by reduction in tryptophan or an increase in kynurenine concentration in monocyte (Figure 4A-B) or PMN culture supernatants (Figure 4C-D), was essentially comparable with normal cells. Nonparametric ANOVA (Kruskal-Wallis) indicated that while groups treated with IFNγ were significantly different from controls, none of the variance could be accounted for by patient type (ie, all subject groups were statistically indistinguishable). Cell viabilities of PMNs and monocytes (MNCs) in all doses were greater than 80% at 24 and 48 hours.
Kynurenine production by Normal and CGD monocytes and PMN. Tryptophan and kynurenine concentrations were measured in culture supernatants after 24 (PMNs) or 48 hours (monocytes) of treatment with 1000 IU IFNγ/mL. Data shown represent mean kynurenine or tryptophan concentration (micromolar) ± SE from normal (circles) monocytes (n = 16) or PMNs (n = 14), A-CGD (squares) monocytes (n = 7) or A-CGD PMNs (n = 6), or X-CGD (triangles) monocytes (n = 7) or PMNs (n = 6).
Kynurenine production by Normal and CGD monocytes and PMN. Tryptophan and kynurenine concentrations were measured in culture supernatants after 24 (PMNs) or 48 hours (monocytes) of treatment with 1000 IU IFNγ/mL. Data shown represent mean kynurenine or tryptophan concentration (micromolar) ± SE from normal (circles) monocytes (n = 16) or PMNs (n = 14), A-CGD (squares) monocytes (n = 7) or A-CGD PMNs (n = 6), or X-CGD (triangles) monocytes (n = 7) or PMNs (n = 6).
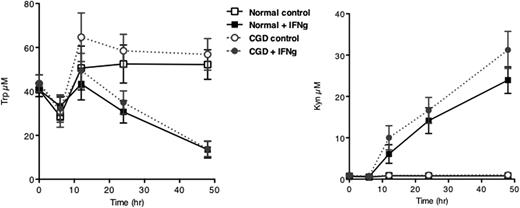
Another cell type implicated in regulation of inflammation are dendritic cells. We therefore tested whether monocyte-derived dendritic cells were capable of tryptophan catabolism. As shown in Figure 5, no difference was detected between normal and CGD monocyte-derived dendritic cells (MDDCs) in terms of kynurenine accumulation which was inversely proportional to the amount of detected tryptophan.
Kinetics of IFNγ-induced tryptophan metabolism of MDDC from normal and CGD donors. MDDC were treated as described for monocytes and tryptophan and kynurenine concentrations measured at the indicated time points. Plotted are means ± SEM of 4 to 8 normal donors and 2 to 6 CGD donors (both genotypes).
Kinetics of IFNγ-induced tryptophan metabolism of MDDC from normal and CGD donors. MDDC were treated as described for monocytes and tryptophan and kynurenine concentrations measured at the indicated time points. Plotted are means ± SEM of 4 to 8 normal donors and 2 to 6 CGD donors (both genotypes).
Systemic tryptophan metabolism in CGD
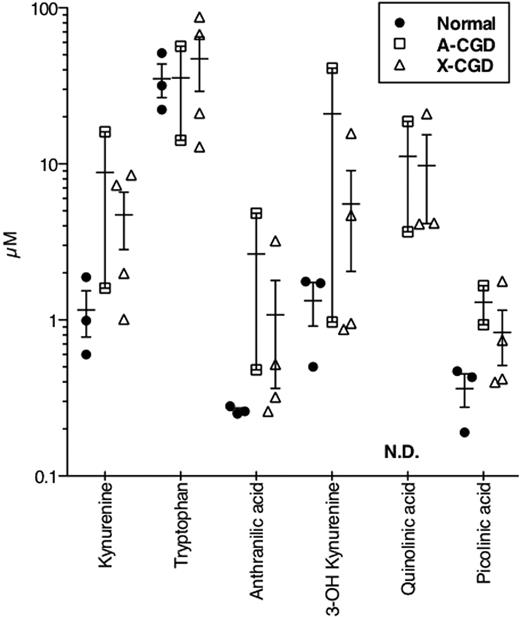
We demonstrated normal tryptophan metabolism in leukocytes from human CGD donors however we were unable to detect other known downstream metabolites (with the exception of anthranilic acid) in culture supernatants from either normal or CGD cells unless we supplemented media with supraphysiologic concentrations of tryptophan. Although systemic tryptophan metabolism relies predominantly on hepatic enzymes such as tryptophan 2,3 dioxygenase, we tested sera from our patients for these metabolites. Kynurenine concentrations in sera from CGD patients were significantly higher than those occurring in normal donors (3.093 ± 0.321μM vs 2.030 ± 0.191μM, respectively; P ≤ .05), while tryptophan levels did not differ significantly between groups (72.59 ± 6.43μM vs 60.93 ± 11.40μM for normal and CGD, respectively). Heeley et al16 had shown in 1970 that human CGD patients excreted hydroxykynurenine, kynurenine, and xanthurenic acid in urine at higher concentrations than normal donors. We therefore tested 24-hour urine samples from normal donors and 2 A-CGD and 4 X-CGD donors. For most urinary tryptophan metabolites tested, levels in CGD patients were the same or elevated compared with normal controls (Figure 6). Quinolinic acid was undetectable in normal urine but was detected in urine from CGD donors.
Urinary trptophan levels in normal and CGD donors. Twenty-four-hour collections of urine were analyzed as described and the resulting concentrations (micromolar) plotted for each replicate (n = 3 normal, 2 A-CGD, 4 X-CGD). A line is drawn at the mean ± SEM for each set. ND indicates not detected.
Urinary trptophan levels in normal and CGD donors. Twenty-four-hour collections of urine were analyzed as described and the resulting concentrations (micromolar) plotted for each replicate (n = 3 normal, 2 A-CGD, 4 X-CGD). A line is drawn at the mean ± SEM for each set. ND indicates not detected.
Discussion
CGD is an inherited disease caused by defects in components of the NADPH oxidase that lead to defective superoxide production. In addition to recurrent infections, a major component of CGD is the exuberant hyperinflammation leading to the characteristic chronic granulomas, delayed wound healing, and autoimmunity. The mechanism(s) underlying the dysregulated inflammation in CGD remains unclear. Recent studies with p47PHOX-knockout mice attributed this hyperinflammatory state to the absence of superoxide-dependent IDO activity8 and offered hopes of phenotypic reversal with supplementary kynurenine and IFNγ.
We found, as had previously been reported,8,17,18 that IFNγ dramatically increased the concentration of kynurenine and decreased that of tryptophan in culture supernatants from normal human monocytes (Figures 3–4). Kynurenine production could also be demonstrated in normal and CGD PMN (Figure 4) as was induction of IDO protein (Figure 1), although both were low compared with monocytes. While PMN and adherent lung cells from CGD mice are apparently unable to form kynurenine,8 this metabolite is formed normally in PMN, monocytes, and monocyte-derived dendritic cells from human CGD donors. We detected no differences between normal and CGD leukocytes in terms of the kinetics of kynurenine accumulation or the IFNγ dose response. One possible explanation for the difference between our results and those reported in mouse CGD is that in the latter case, lung homogenates and isolated phagocytes from infected mice were studied.8 Our studies with human subjects however demonstrated that both the magnitude and rate of kynurenine accumulation in culture supernatants of human peripheral blood leukocytes is independent of O2·̄.
Because the bulk of systemic tryptophan conversion is performed by the constitutively expressed hepatic tryptophan 2,3-dioxygenase enzyme (TDO), isolated human CGD cells may not reveal a systemic defect in tryptophan metabolism. We therefore measured levels of tryptophan and some of its major metabolites in serum and urine from normal and CGD donors. In no case did CGD appear to decrease the levels of these metabolites, on the contrary the concentrations of these molecules were higher in urine from CGD donors than from normal donors. While it is possible that superoxide may be required to further catabolize these metabolites and that levels are increased due to substrate accumulation, a more likely explanation is that the increased levels of infection and inflammation in these patients has caused an up-regulation of this pathway. A study published in 1970 similarly reported an increase in the urine concentrations of kynurenine and 3-OH kynurenine,16 although this early work did not report levels for other metabolites we report here. Taken together, these results reveal normal or elevated tryptophan metabolite levels in CGD patients. Because both IDO and TDO metabolize tryptophan, the serum kynurenine levels may not reflect IDO activity at a systemic level. However, in the in vitro PMN and MNC system, the elevated kynurenine levels correlated with increased IDO expression with IFNγ induction.
The results shown here suggest a difference in the regulation of human and mouse IDO enzymatic activity in leukocytes. In CGD mice, the absence of O2·̄ prevents the activation of murine IDO, thus preventing kynurenine accumulation resulting in an excess of IL17 production from unrestrained γδ-T-cell activity giving rise to a hyperinflammatory phenotype.8 Furthermore, the absence of kynurenine production in mouse also decreases the levels of a variety of downstream kynurenine metabolites that likely play important roles in regulating inflammation. Recent biochemical studies showed that human IDO does not use O2·̄ as a cofactor and coexpression of O2·̄ dismutase (SOD) does not lower kynurenine production in human cells.19 Our data show that human CGD peripheral blood cells produce normal levels of kynurenine in response to IFNγ treatment. While we cannot exclude a role of O2·̄ in regulation of downstream tryptophan metabolite production within tissues, our data do not reveal any defects in human CGD in the regulation of the production of kynurenine from tryptophan when tested in vitro. IFNγ is used in the clinical management of CGD,20 and it remains possible that IDO-dependent formation of other tryptophan metabolites is an important component of IFNγ's therapeutic action. Nevertheless, the underlying cause or causes of dysregulated hyperinflammation seen in human CGD patients remain to be established. Numerous other inflammatory processes are altered in CGD, for example regulation of leukotriene B45,21 IL-8 production,2 adenosine and cAMP metabolism,22 activity of the IL-1β inflammasome,23 as well as global transcriptional regulation.24 Most likely, the complex clinical inflammatory phenotype in CGD depends on multiple pathways and will differ depending on the specific process that initiates inflammation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Steven Holland for patient samples and Susan Strobl for contributions with dendritic cell cultures.
This work was supported by the National Institute of Allergy and Infectious Diseases Division of Intramural Research and the Clinical Center of the National Institutes of Health (NIH). This project has been funded in part with federal funds from the National Cancer Institute, NIH, under contract no. HHSN261200800001E.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
National Institutes of Health
Authorship
Contribution: S.S.D.R., K.A.Z., D.B.K., H.L.M. were responsible for experimental design; K.A.Z., S.S.D.R., D.L.P., D.B.K., J.I.G., and H.L.M. performed data analysis; K.A.Z. and S.S.D.R. wrote the manuscript; and S.D.F. and K.C.C. performed HPLC.
Conflict-of-interest disclosure: S.S.D.R., K.A.Z., J.I.G., and H.L.M. are US Government employees and declare no competing financial interests. D.B.K., D.L.P., K.C.C., and S.D.F. are employees of SAIC-Frederick.
Correspondence: Suk See De Ravin, Laboratory of Host Defenses, National Institute of Allergy and Infectious Diseases, National Institutes of Health, 10CRC/5W-3816, 10 Center Dr, Bethesda, MD 20892; e-mail: sderavin@niaid.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal