Abstract
Apoptosis and necrosis represent distinct cell death processes that regulate mammalian development, physiology and disease. Apoptosis characteristically leads to the silent destruction and removal of cells in the absence of an inflammatory response. In contrast, necrotic cell death can induce physiologic inflammatory responses linked to tissue defense and repair. Although anucleate, platelets undergo programmed cell death, with apoptosis playing an important role in clearing effete platelets from the circulation. While it has long been recognized that procoagulant platelets exhibit characteristic features of dying cells, recent studies have demonstrated that platelet procoagulant function can occur independent of apoptosis. A growing body of evidence suggest that the biochemical, morphologic and functional changes underlying agonist-induced platelet procoagulant function are broadly consistent with cell necrosis, raising the possibility that distinct death pathways regulate platelet function and survival. In this article, we will discuss the mechanisms underlying apoptotic and necrotic cell death pathways and examine the evidence linking these pathways to the platelet procoagulant response. We will also discuss the potential contribution of these pathways to the platelet storage lesion and propose a simplified nomenclature to describe procoagulant platelets.
Introduction
Until the early 1970s it was thought that all cells die as a result of necrosis (Table 1); a form of cell death that is prominent when tissues undergo extensive damage from trauma or disease. This concept changed dramatically in 1972 after the landmark observations of Kerr and colleagues,9 who described a new form of cell death, termed apoptosis (Table 1). Since then, the genetic and biochemical processes responsible for apoptotic cell death have been extensively investigated. It is now well defined that apoptosis is a key process underlying human development, normal physiology and a range of common human diseases.10 In contrast, the concepts underlying necrosis have become less clear-cut. While it has long been assumed that necrosis is predominantly a pathologic process resulting from tissue injury, there is growing evidence that cells can orchestrate their own demise through programmed cell necrosis in a manner that initiates physiologic inflammatory and repair responses.2-4,6-8
Glossary defining cell death and cell death pathways in relation to procoagulant platelets
| . | Glossary: cell death pathways and procoagulant platelets . |
|---|---|
| Cell death | Defines a process that leads to a point of no return—the termination of the biological function of a cell. |
| Programmed cell death | Cell death under regulated genetic control. |
| Apoptosis | Describes a process of programmed cell death used to dispose of damaged, redundant, or superfluous cells, wherein cells die silently and are removed by macrophages in the absence of inflammatory/reparative responses. |
| Apoptotic cell death pathway | Extrinsic (eg, death receptor initiated) or intrinsic (eg, Bcl-2 family-mediated) biochemical signals result in caspase activation, leading to a series of well-characterized morphological changes (including blebbing, loss of cell membrane asymmetry [PS exposure] and attachment, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation), and eventual cell death and clearance. Note: In this article, we have limited our discussion of apoptotic cell death pathways to the intrinsic Bcl-2–mediated pathway implicated in the regulation of platelet lifespan.1 |
| Necrosis | Describes an alternative form of programmed cell death,2-4 often initiated following a chemical (hypoxia, glucose depletion, acidosis), heat-related or mechanical insult. Necrosis results in cell lysis, provoking inflammatory and reparative responses in surrounding cells and tissue. Note: In this article, we refer to cell necrosis, as opposed to tissue necrosis, being the drastic macroscopic tissue changes, visible to the naked eye occurring after cell death.5 |
| Necrotic cell death pathway | Characterized by bioenergetic failure of the cell, followed by loss of plasma membrane integrity, cell and organelle swelling, and cytolysis. These features are a consequence of a defined set of molecular events, including mitochondrial dysfunction (rapid loss of membrane potential, metabolic failure, increased ROS production), sustained toxic intracellular calcium levels and the activation of nonapoptotic proteases such as calpain and lysosomal cathepsins. A key modulator of the necrotic death pathway is the inner mitochondrial membrane protein cyclophilin D (CypD), which controls mitochondrial permeability transition (mPT). |
| Functional necrosis | Certain forms of necrotic cell death are now being reconsidered as normal physiologic programmed cell events. By initiating adaptive responses, necrosis may be considered as an important response to maintain tissue and organism integrity through the initiation of inflammatory and reparative responses.2-4,6-8 |
| Procoagulant platelets | Phosphatidylserine (PS)–positive platelets capable of facilitating thrombin generation. |
| Procoagulant apoptotic platelets | Platelets induced to become procoagulant through a Bak/Bax-mediated apoptotic signaling pathway, in a manner dependent upon caspases but independent of platelet activation and granule release. |
| Procoagulant necrotic platelets | Platelets induced to become procoagulant through potent activation, leading to high sustained levels of cytosolic calcium, rapid mitochondrial dysfunction, and loss of membrane integrity. These platelets are also capable of initiating an inflammatory response. |
| . | Glossary: cell death pathways and procoagulant platelets . |
|---|---|
| Cell death | Defines a process that leads to a point of no return—the termination of the biological function of a cell. |
| Programmed cell death | Cell death under regulated genetic control. |
| Apoptosis | Describes a process of programmed cell death used to dispose of damaged, redundant, or superfluous cells, wherein cells die silently and are removed by macrophages in the absence of inflammatory/reparative responses. |
| Apoptotic cell death pathway | Extrinsic (eg, death receptor initiated) or intrinsic (eg, Bcl-2 family-mediated) biochemical signals result in caspase activation, leading to a series of well-characterized morphological changes (including blebbing, loss of cell membrane asymmetry [PS exposure] and attachment, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation), and eventual cell death and clearance. Note: In this article, we have limited our discussion of apoptotic cell death pathways to the intrinsic Bcl-2–mediated pathway implicated in the regulation of platelet lifespan.1 |
| Necrosis | Describes an alternative form of programmed cell death,2-4 often initiated following a chemical (hypoxia, glucose depletion, acidosis), heat-related or mechanical insult. Necrosis results in cell lysis, provoking inflammatory and reparative responses in surrounding cells and tissue. Note: In this article, we refer to cell necrosis, as opposed to tissue necrosis, being the drastic macroscopic tissue changes, visible to the naked eye occurring after cell death.5 |
| Necrotic cell death pathway | Characterized by bioenergetic failure of the cell, followed by loss of plasma membrane integrity, cell and organelle swelling, and cytolysis. These features are a consequence of a defined set of molecular events, including mitochondrial dysfunction (rapid loss of membrane potential, metabolic failure, increased ROS production), sustained toxic intracellular calcium levels and the activation of nonapoptotic proteases such as calpain and lysosomal cathepsins. A key modulator of the necrotic death pathway is the inner mitochondrial membrane protein cyclophilin D (CypD), which controls mitochondrial permeability transition (mPT). |
| Functional necrosis | Certain forms of necrotic cell death are now being reconsidered as normal physiologic programmed cell events. By initiating adaptive responses, necrosis may be considered as an important response to maintain tissue and organism integrity through the initiation of inflammatory and reparative responses.2-4,6-8 |
| Procoagulant platelets | Phosphatidylserine (PS)–positive platelets capable of facilitating thrombin generation. |
| Procoagulant apoptotic platelets | Platelets induced to become procoagulant through a Bak/Bax-mediated apoptotic signaling pathway, in a manner dependent upon caspases but independent of platelet activation and granule release. |
| Procoagulant necrotic platelets | Platelets induced to become procoagulant through potent activation, leading to high sustained levels of cytosolic calcium, rapid mitochondrial dysfunction, and loss of membrane integrity. These platelets are also capable of initiating an inflammatory response. |
Although anucleate, platelets have the capacity to undergo programmed cell death (Table 1). This has been most clearly demonstrated with platelet apoptosis; a process that is important for clearance of effete platelets from the circulation.1,11 Many of the features of apoptosis (membrane fragmentation, cytoskeletal disruption, microvesiculation, caspase activation and phosphatidylserine [PS] exposure) are observed during prolonged platelet storage ex vivo and during the conversion of activated platelets to a procoagulant state, raising the possibility that apoptosis may also regulate platelet function. However, recent studies have demonstrated that the molecular events underlying agonist-induced platelet procoagulant function can occur independently of apoptotic cell death,12 raising the possibility that alternative cell death pathways contribute to platelet procoagulant function (Table 1).
Distinct biochemical processes regulate apoptosis and necrosis
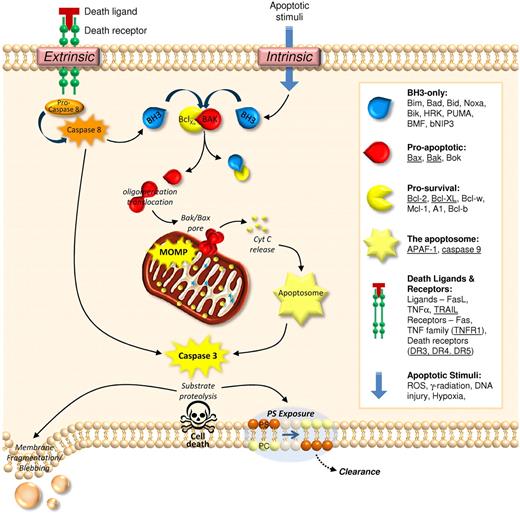
Based on studies in nucleated cells, distinct molecular processes regulate the fate of apoptotic and necrotic cells. Morphologically, apoptosis is characterized by cell shrinkage, nuclear condensation and fragmentation, and the formation of condensed cell bodies. Central to apoptosis are the caspase family of cysteine proteases. Caspases dismantle the cell from within, executing their demise through restricted proteolysis of key cellular structures, resulting in cytoskeletal disassembly, plasma membrane instability (leading to blebbing), proteolytic processing of cell-surface receptors (undermining cell adhesion), and through the surface expression of cell clearance signals (including PS). Caspase activation can be triggered via 2 major pathways,13 an extrinsic death-receptor pathway, and an intrinsic pathway controlled by the Bcl-2 family of proteins (Figure 1). Although platelets possess elements of both pathways, Bcl-2-mediated apoptotic events have the most clear-cut functional role in regulating platelet clearance from the circulation.1 Central to this process is the balance between prosurvival proteins (such as Bcl-XL) and proapoptotic molecules (ie, Bak/Bax). Functional loss of Bcl-XL liberates Bak/Bax to form oligomers in the outer mitochondrial membrane, leading to mitochondrial outer membrane permeabilization (MOMP)14 and release of cytochrome C (CytC). Once released from the mitochondria, CytC initiates apoptosome assembly and caspase activation (Figure 1). Notably, the process of apoptosis is energy-dependent with apoptotic cells maintaining the integrity of their plasma membrane, minimising disruption to neighbouring cells and avoiding unwanted immune responses.
Mechanisms of apoptotic cell death. Cell death via apoptosis can be triggered in defective or unwanted cells via extrinsic or intrinsic means. Ligation of death receptors (extrinsic pathway) leads to the activation of caspase 8, which in turn activates the executioner caspase, caspase 3. Activation of caspase 3 is either direct, or mediated via ‘activation’ of BH3-only proteins. These latter proteins bind to prosurvival Bcl-2 family proteins, releasing their hold on proapoptotic Bcl-2 proteins (Bak/Bax) and allowing them to form pores within the outer mitochondrial membrane, thus causing mitochondrial outer membrane permeabilization (MOMP). The release of cytochrome c (CytC) from the mitochondrial intermembrane space leads to assembly of the apoptosome, leading to caspase 3 activation and substrate proteolysis. Subsequent exposure of phosphatidylserine (PS) provides a major clearance signal for phagocytes. Activation of the intrinsic apoptotic pathway through exposure to radiation, hypoxia, DNA damage or reactive oxygen species (ROS) results in direct activation of BH3-only proteins, with eventual caspase 3 activation. Note: Underlined are the elements of the apoptotic machinery identified in platelets thus far.
Mechanisms of apoptotic cell death. Cell death via apoptosis can be triggered in defective or unwanted cells via extrinsic or intrinsic means. Ligation of death receptors (extrinsic pathway) leads to the activation of caspase 8, which in turn activates the executioner caspase, caspase 3. Activation of caspase 3 is either direct, or mediated via ‘activation’ of BH3-only proteins. These latter proteins bind to prosurvival Bcl-2 family proteins, releasing their hold on proapoptotic Bcl-2 proteins (Bak/Bax) and allowing them to form pores within the outer mitochondrial membrane, thus causing mitochondrial outer membrane permeabilization (MOMP). The release of cytochrome c (CytC) from the mitochondrial intermembrane space leads to assembly of the apoptosome, leading to caspase 3 activation and substrate proteolysis. Subsequent exposure of phosphatidylserine (PS) provides a major clearance signal for phagocytes. Activation of the intrinsic apoptotic pathway through exposure to radiation, hypoxia, DNA damage or reactive oxygen species (ROS) results in direct activation of BH3-only proteins, with eventual caspase 3 activation. Note: Underlined are the elements of the apoptotic machinery identified in platelets thus far.
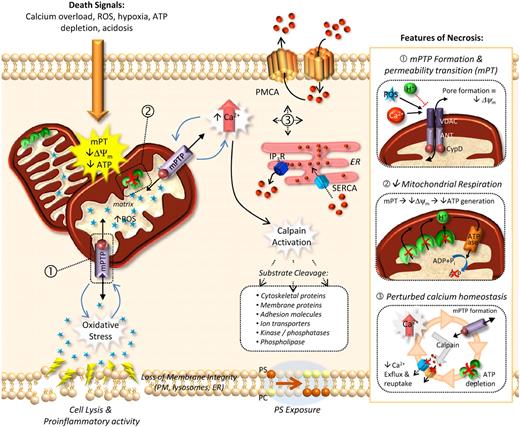
In contrast to apoptosis, necrosis is typified by bioenergetic failure of the cell (ATP depletion), leading to rapid loss of plasma membrane integrity and the release of cellular contents into the extracellular environment. Also referred to as oncosis (based on the Greek term ónkos meaning swelling) necrotic cell death is characterized by swelling of cytoplasmic organelles and the plasma membrane, ultimately leading to cell lysis. Several defined molecular events underlie the necrotic process, with calcium (Ca2+) cytotoxicity and reactive oxygen species (ROS) representing 2 major initiating insults. Both Ca2+ overload and ROS facilitate the formation of the mitochondrial permeability transition pore (MPTP), leading to collapse of mitochondrial membrane potential (Δψm) and ATP depletion. The inner mitochondrial matrix protein, cyclophilin D (CypD), along with the voltage-dependent anion channel (VDAC) and the adenine nucelotide translocator (ANT), form the mitochondrial permeability transition pore (MPTP) and modulate mitochondrial membrane potential (Figure 2). Deficiency of CypD prevents formation of MPTP and abolishes Ca2+ and ROS-dependent necrotic cell death without impacting on apoptosis.15
Mechanisms of necrotic cell death. Necrotic cell death is initiated by numerous cell insults that include calcium overload, excessive ROS production, hypoxia, ATP depletion, or acidosis. A central feature of necrotic cell death is the rapid loss of mitochondrial membrane potential (Δψm), otherwise known as the permeability transition (mPT). mPT is facilitated by the formation of a mitochondrial permeability transition pore (mPTP) traversing the inner and outer mitochondrial membranes (inset 1). Opening of the mPTP allows small ions and metabolites to permeate freely across the inner mitochondrial membrane, essentially shutting down the proton gradient necessary for ATP generation (inset 2). mPT results in the inability of the mitochondria to maintain electrochemical potential, resulting in an increased mitochondrial matrix volume and organelle swelling (and eventually rupture). mPT is catalysed by the inner mitochondrial protein cyclophilin D (CypD; inset 1), with deficiency of this protein leading to resistance to necrosis (but not apoptosis). Another consequence of mitochondrial dysfunction is an increased production of mitochondrial reactive oxygen species (ROS), which when released facilitates mPTP formation as well as damage to DNA and proteins, as well as oxidative modification of lipid components of organelle and plasma membranes. A major instigator of mPTP formation is perturbed calcium homeostasis (inset 3). Many of the channels linked to the control of intracellular Ca2+ levels, including the Plasma Membrane ATPase (PMCA) and the Sarcoplasmic Endoreticulum Ca2+ ATPase (SERCA), are ATP-dependent, such that after an initial Ca2+ insult the subsequent mitochondrial damage and ATP depletion exacerbate elevated intracellular Ca2+ levels. Moreover, elevated intracellular calcium activates proteases such as calpain, leading to the degradation of PMCA, further perturbing calcium homeostasis.
Mechanisms of necrotic cell death. Necrotic cell death is initiated by numerous cell insults that include calcium overload, excessive ROS production, hypoxia, ATP depletion, or acidosis. A central feature of necrotic cell death is the rapid loss of mitochondrial membrane potential (Δψm), otherwise known as the permeability transition (mPT). mPT is facilitated by the formation of a mitochondrial permeability transition pore (mPTP) traversing the inner and outer mitochondrial membranes (inset 1). Opening of the mPTP allows small ions and metabolites to permeate freely across the inner mitochondrial membrane, essentially shutting down the proton gradient necessary for ATP generation (inset 2). mPT results in the inability of the mitochondria to maintain electrochemical potential, resulting in an increased mitochondrial matrix volume and organelle swelling (and eventually rupture). mPT is catalysed by the inner mitochondrial protein cyclophilin D (CypD; inset 1), with deficiency of this protein leading to resistance to necrosis (but not apoptosis). Another consequence of mitochondrial dysfunction is an increased production of mitochondrial reactive oxygen species (ROS), which when released facilitates mPTP formation as well as damage to DNA and proteins, as well as oxidative modification of lipid components of organelle and plasma membranes. A major instigator of mPTP formation is perturbed calcium homeostasis (inset 3). Many of the channels linked to the control of intracellular Ca2+ levels, including the Plasma Membrane ATPase (PMCA) and the Sarcoplasmic Endoreticulum Ca2+ ATPase (SERCA), are ATP-dependent, such that after an initial Ca2+ insult the subsequent mitochondrial damage and ATP depletion exacerbate elevated intracellular Ca2+ levels. Moreover, elevated intracellular calcium activates proteases such as calpain, leading to the degradation of PMCA, further perturbing calcium homeostasis.
Calcium cytotoxicity and ATP depletion contribute to cell damage by promoting excess mitochondrial ROS production. In addition to oxidative modification of DNA and proteins, ROS are highly reactive to lipids, leading to loss of integrity of both the plasma membrane and intracellular membranes, including the lysosomes and ER. Subsequent leakage of lysosomal enzymes such as the cathepsins, in combination with other Ca2+-activated proteases, ie, calpain, results in cytoskeletal protein breakdown and loss of the cell's structural integrity.
Dysregulated calcium flux and cell death
One of the cardinal features of necrotic cell death is dysregulated intracellular calcium flux.2 High, sustained cytosolic calcium levels are toxic to the cell, leading to activation of intracellular proteases, damage to the mitochondria and ultimately disruption of organelles and the plasma membrane.2,16 Therefore to fulfil its intracellular second messenger function, calcium signals must typically be transient or oscillatory in nature.17,18 To prevent calcium toxicity, mammalian cells have evolved a complex network of calcium channels that rapidly remove calcium from the cytosol into specialized storage compartments or to the extracellular space.17-19 Dysregulated calcium homeostasis is also a feature of apoptosis, however the levels of calcium required to trigger apoptosis are typically lower than those that induce necrosis.2
In the context of necrosis, bioenergetic failure and dysregulated Ca2+ homeostasis act in a cooperative manner to enhance organelle damage and cell death. Many of the channels linked to the control of intracellular Ca2+ levels, including the Plasma Membrane ATPase (PMCA) and the Sarcoplasmic Endoreticulum Ca2+ ATPase (SERCA), are ATP-dependent, such that following an initial Ca2+ insult, the subsequent mitochondrial damage and ATP depletion exacerbate elevated intracellular Ca2+ levels.
Agonist-induced procoagulant platelets: apoptotic or necrotic?
A fundamental, but incompletely understood aspect of platelet function is the relationship between a regulated, naturally occurring platelet activation response, ie, platelet procoagulant function, and the cell death pathways regulating platelet survival. In this context, procoagulant platelets are not just highly activated cells, they are undergoing cell death. They have all the biochemical, morphologic and functional features of a dying cell, including caspase and calpain activation, proteolytic processing of cytoskeletal elements, surface exposure of PS, membrane contraction, blebbing and microvesiculation. Morphologically, procoagulant platelets have lost their internal organelles, their cytoskeleton is completely disrupted and functionally these platelets have lost their ability to adhere and aggregate with other platelets. Nonetheless, these cells retain physiologic functions as they support assembly of coagulation complexes leading to thrombin formation and fibrin generation and also have an increased reactivity toward neutrophils, raising the possibility that they may promote inflammatory reactions.20
It has generally been assumed that the biochemical and morphologic changes associated with platelet procoagulant function are linked to apoptosis.21 For example, Bcl-2 family proteins can be posttranscriptionally regulated and caspases activated in agonist-stimulated platelets.22,23 However, recent analysis of Bak/Bax-deficient mouse platelets has revealed that agonist-induced platelet procoagulant function occurs independent of apoptosis.12 Consistent with this, several studies have demonstrated that caspase inhibitors do not block the procoagulant function of activated platelets.12,24,25
There is a growing body of evidence demonstrating that many of the biochemical processes regulating agonist-induced platelet procoagulant function are more typical of necrosis (Figure 3). For example, procoagulant platelet function is initiated by high, sustained levels of cytosolic Ca2+23, leading to opening of the MPTP and mitochondrial dysfunction.2,26 In nucleated cells, such Ca2+-dependent processes initiate a necrotic cell death pathway that is CypD-dependent (but caspase-independent). Consistent with this, platelet CypD deficiency markedly reduces agonist-induced loss of mitochondrial membrane potential (Δψm) and platelet procoagulant function.27 Moreover, ROS production is elevated in platelets undergoing high, sustained cytosolic Ca2+ flux,28,29 and H2O2 has been demonstrated to promote platelet procoagulant function through a CypD-dependent mechanism.27 Whether ROS is responsible for undermining the integrity of the lysosomal and platelet plasma membranes remains to be established,2,30 nonetheless there is a correlation between platelet lysosomal enzyme activity and procoagulant activity.30 Consistent with a necrotic process, calpains (not caspases) play the major role in disassembling the cytoskeleton and promoting the release of procoagulant microvesicles from the surface of platelets.24
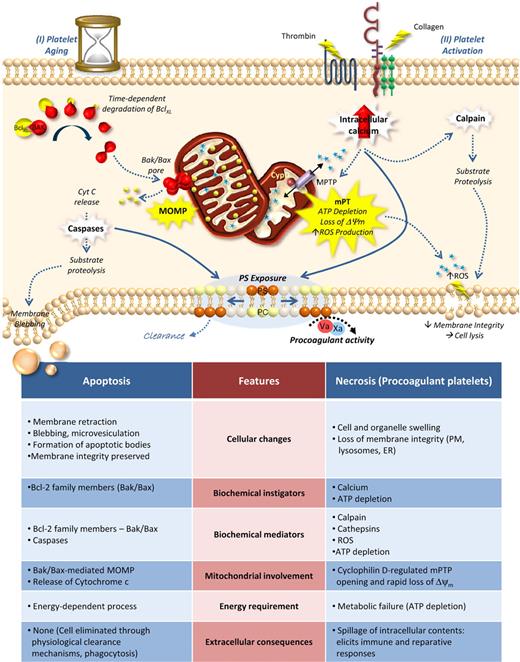
Cell death pathways in platelet biology. Many of the features of apoptotic and necrotic cell death (see bottom table) are common to platelets. (I) Platelet aging: Recent studies have demonstrated an important role for Bak/Bax-mediated apoptosis in control of platelet lifespan. In this model, anucleate circulating platelets possess finite amounts of BclXL, Bak, and Bax. With no significant means for further protein translation, the cellular levels of BclXL decline over time, at a rate faster then their proapoptotic counterparts. After 7 to 10 days, this results in the release of Bak/Bax, the formation of Bak/Bax pores in the outer membrane of the mitochondria, and eventual MOMP. This triggers the typical intrinsic apoptotic cascade including cytochrome c (Cyt C) release, caspase activation and substrate proteolysis, resulting in proteolytic destruction of the cell, membrane blebbing/vesiculation and phosphatidylserine (PS) exposure (II) In contrast, potent activation of platelets by thrombin and collagen leads to the formation of a platelet phenotype consistent with necrosis. Potent activation leads to sustained elevated cytosolic calcium, mitochondrial calcium overload, and formation of the mitochondrial permeability transition pore (MPTP). This facilitates the mitochondrial permeability transition (mitochondrial membrane depolarization, Δψm), ATP depletion and excess production of reactive oxygen species (ROS), facilitated by the mitochondrial inner membrane protein cyclophilin D (CypD). Additional consequences of mitochondrial injury include sustained elevated calcium, calpain activation, PS exposure and loss of plasma membrane integrity. Functionally, platelets undergoing necrosis possess both procoagulant and proinflammatory characteristics.
Cell death pathways in platelet biology. Many of the features of apoptotic and necrotic cell death (see bottom table) are common to platelets. (I) Platelet aging: Recent studies have demonstrated an important role for Bak/Bax-mediated apoptosis in control of platelet lifespan. In this model, anucleate circulating platelets possess finite amounts of BclXL, Bak, and Bax. With no significant means for further protein translation, the cellular levels of BclXL decline over time, at a rate faster then their proapoptotic counterparts. After 7 to 10 days, this results in the release of Bak/Bax, the formation of Bak/Bax pores in the outer membrane of the mitochondria, and eventual MOMP. This triggers the typical intrinsic apoptotic cascade including cytochrome c (Cyt C) release, caspase activation and substrate proteolysis, resulting in proteolytic destruction of the cell, membrane blebbing/vesiculation and phosphatidylserine (PS) exposure (II) In contrast, potent activation of platelets by thrombin and collagen leads to the formation of a platelet phenotype consistent with necrosis. Potent activation leads to sustained elevated cytosolic calcium, mitochondrial calcium overload, and formation of the mitochondrial permeability transition pore (MPTP). This facilitates the mitochondrial permeability transition (mitochondrial membrane depolarization, Δψm), ATP depletion and excess production of reactive oxygen species (ROS), facilitated by the mitochondrial inner membrane protein cyclophilin D (CypD). Additional consequences of mitochondrial injury include sustained elevated calcium, calpain activation, PS exposure and loss of plasma membrane integrity. Functionally, platelets undergoing necrosis possess both procoagulant and proinflammatory characteristics.
Platelet death pathways: a choice between going quietly or raising the alarm
A hallmark feature of apoptotic cell death is the removal of unwanted, aged or damaged cells without an accompanying inflammatory response. Apoptosis is able to proceed virtually unnoticed by neighbouring cells, through a process of internal cellular degradation, followed by the surface exposure of specific “eat-me” signals that trigger phagocytic clearance by scavenger cells. Clearance of aged platelets from the circulation is one such physiologic example, although the precise nature of the “eat-me” signals and their mode of clearance from the circulation remain to be established. In contrast to apoptosis, necrotic cell death is associated which loss of plasma membrane integrity and the consequent release of cellular contents into the extracellular environment. While traditionally viewed as an unregulated by-product of severe pathologic injury, increasing evidence suggests that necrotic cells can elicit deliberate and controlled biologic responses that are important for host defense and repair.3,4,7,8 Thus, necrotic cell death may have evolved as an early warning system to stimulate immune responses and repair mechanisms in organ systems that have sustained damage or invasion. This has become increasingly apparent in the context of viral and microbial infections, in which pathogen-induced necrotic death of cells of the host's immune system helps signal an inflammatory response.6,7 Similarly, in various malignancies, ischemia-reperfusion injury and neurodegenerative diseases, necrotic cell death pathways contribute to inflammatory and immune responses.2
In the context of platelets, the initiation of necrotic cell death at sites of vascular injury may play an important role in inducing inflammatory and repair processes. Agonist-induced procoagulant platelets play a central role in promoting thrombin generation and the development of the 3-dimensional fibrin matrix, 2 processes that are critical for hemostasis and vascular repair.31 Furthermore, procoagulant platelets produce high levels of the proinflammatory lipid Platelet Activating Factor (PAF) and have enhanced reactivity toward neutrophils,20 raising the possibility that they play an important physiologic role in promoting leukocyte recruitment/activation at sites of vascular injury. These functional properties of procoagulant platelets are broadly consistent with the role of cell necrosis in signaling physiologic inflammatory and repair responses.
Procoagulant platelets: searching for their real identity
Because both apoptotic and necrotic death pathways can lead to surface PS exposure and a procoagulant platelet surface, an accurate description of agonist-induced procoagulant platelets is required to differentiate these platelets from other potential procoagulant forms. Several descriptions have previously been used including balloon-shaped procoagulant platelets (reflecting their morphologic appearance),33,33 COAT (COllagen And Thrombin-activated platelets)34 or COATED platelets34 due to their surface coating by adhesive proteins and coagulation factors, and SCIPs (Sustained Calcium-Induced Platelet morphology)24 reflecting the central role of high levels of sustained cytosolic Ca2+ in stimulating platelet procoagulant function.31
Balloon-shaped procoagulant platelets.
A hallmark feature of procoagulant platelets that readily distinguishes them from other forms of activated platelets is their adoption of a balloon-like appearance (Figure 4). The dynamic changes in platelet morphology during transformation from an activated to a procoagulant state were first observed by Heemskerk and colleagues using high-resolution phase- contrast imaging.36,37 Phenotypic conversion of spread platelets to a procoagulant state is associated with contraction of the cell membrane, membrane bleb formation and ballooning, as well as microvesiculation from the cell surface. This dramatic morphologic conversion is associated with loss of membrane phospholipid asymmetry and the surface exposure of anionic phospholipids, particularly PS. As a consequence, all balloon-shaped platelets bind high levels of annexin Va. The combination of their distinct balloon-like appearance, combined with their ability to support coagulation reactions, led to their initial description as balloon-shaped procoagulant platelets.32,33,38,39
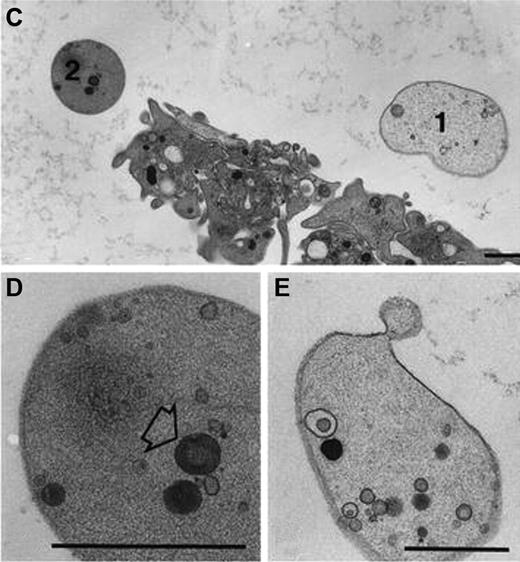
Procoagulant balloon-shaped platelets. Ultrastructure of procoagulant platelets adhering to collagen-related peptide in the presence of calcium (2mM). Balloon platelets appear completely smooth, apart from an occasional membrane bleb. (Top panel) Comparison between normal platelets and balloon platelets (types 1 and 2). The cytoplasm of balloon platelets contain only few (if any) randomly distributed organelles, or remnants thereof. 2 such examples are presented in the bottom panels. (Left panel, type 1): Highly diluted cytoplasm and few inclusions. (Right panel, type 2): Moderately dense cytoplasm and higher levels of organelles. These images have been reproduced from Hess and Siljander33 Figure 1C through E with permission from Springer Science+Business Media.
Procoagulant balloon-shaped platelets. Ultrastructure of procoagulant platelets adhering to collagen-related peptide in the presence of calcium (2mM). Balloon platelets appear completely smooth, apart from an occasional membrane bleb. (Top panel) Comparison between normal platelets and balloon platelets (types 1 and 2). The cytoplasm of balloon platelets contain only few (if any) randomly distributed organelles, or remnants thereof. 2 such examples are presented in the bottom panels. (Left panel, type 1): Highly diluted cytoplasm and few inclusions. (Right panel, type 2): Moderately dense cytoplasm and higher levels of organelles. These images have been reproduced from Hess and Siljander33 Figure 1C through E with permission from Springer Science+Business Media.
COAT/COATED platelets.
In unrelated studies, Dale and colleagues described a subset of platelets that express high levels of factor V on their surface after potent platelet stimulation with thrombin and collagen.34,35 Initially termed COAT platelets, these cells represent a subset of the platelet population that retain high levels of α-granule proteins on their surface, including factor V (FV), fibrinogen, fibronectin and von Willebrand factor (VWF). Several of these proteins are derivatized with serotonin in a transglutaminase-dependent fashion,34 thereby creating a stabilized multivalent coating over the platelet surface. With the subsequent demonstration that these platelet subtypes could be generated by stimuli other than collagen and thrombin, the acronym COAT has been extended to COATED, reflecting the surface coating of these platelets by adhesive proteins and coagulation factors. Whether COATED platelets and balloon-shaped procoagulant platelets are one and the same remains unclear, however the ability of COATED platelets to expose PS and retain high levels of procoagulant proteins on their surface suggests a primary role for these platelet subsets in supporting coagulation reactions. Notably, COATED platelets have been demonstrated to undergo loss of Δψm25 (associated with the CypD-dependent formation of the MPTP formation) and increased plasma membrane permeability. In line with this, COATED platelet formation is potentiated by exposure to ROS.27
SCIP.
More recently, we have demonstrated that spread platelets exposed to experimental conditions leading to high, sustained levels of cytosolic Ca2+, have a selective defect in their ability to form stable adhesion contacts with other platelets, leading to decreased platelet aggregation and thrombus growth.24 These platelets, which almost certainly are the same as the balloon-shaped procoagulant platelets described by Heemskerk and colleagues have been termed SCIP (Sustained Ca2+-Induced Platelet [SCIP] morphology), reflecting the central role of Ca2+ flux in their formation.24 Functionally these platelets exhibit a marked down-regulation in the adhesive function of integrin αIIbβ3,23 and paradoxically, have increased reactivity toward neutrophils, suggesting that high levels of cytosolic Ca2+ converts platelets from a proaggregatory to a proinflammatory phenotype.20 Several features of the SCIP phenotype are consistent with COATED platelets, including increased PS exposure and heightened procoagulant function,12,24,26 loss of Δψm,12,26 and the involvement of transglutaminases. However, while high levels of Ca2+ appear to be a prerequisite for SCIP formation, this is not always the case for COATED platelets, as treatment of platelets with Ca2+ ionophore does not lead to fibrinogen retention.27
Given the acronyms of COAT, COATED platelets,35 and SCIP reflect only specific aspects of platelet procoagulant function, it may be more appropriate to classify agonist-induced procoagulant platelets (those that have undergone a high, sustained cytosolic calcium response) as procoagulant necrotic platelets (Table 1), a term that may more accurately reflect the underlying molecular processes governing the cell's fate.
Apoptotic and necrotic death pathways: implications for the platelet storage lesion
If platelets do have the capacity of undergoing more than one form of programmed cell death, what are the potential implications for the platelet storage lesion (PSL)? It has long been speculated that the shelf-life of stored platelets is primarily regulated by platelet apoptosis.40 Platelets isolated from donor blood undergo time-dependent structural and functional changes that are indicative of apoptosis. The PSL is characterized by mitochondrial damage (Δψm), increased PS, loss of discoid morphology, fragmentation of the plasma membrane, microvesiculation, and ectodomain shedding of GPIb.40,41 Reductions in the levels of Bcl-xl, cytochrome-c release, caspase activation, and gelsolin proteolysis have also been reported during platelet storage.40 Functionally, these platelets have reduced adhesive function, increased procoagulant activity, and reduced survival after transfusion to a recipient.
It is also possible that a number of the changes associated with PSL may be caused by biochemical processes linked to platelet necrosis, in particular those responsible for significant mitochondrial damage, swelling of plasma and organelle membranes, metabolic failure, changes in intracellular pH, membrane lysis, and release of LDH. Although apoptosis and necrosis are often viewed as occurring in a mutually exclusive manner, studies on nucleated cells have demonstrated that these processes may in fact occur in continuum, depending on the nature and strength of the death signal. One example where apoptosis and necrosis may occur in the same cell is during the death of cells in vitro under cell-culture conditions.2 Although a cultured cell may respond to death signals and respond initially by undergoing apoptosis, in the absence of any phagocytic clearance mechanism, eventual ATP depletion will lead to features of necrotic death such as compromise of the plasma membrane, which has been designated “apoptotic” or “secondary” necrosis.2 Apoptosis with ensuing secondary necrosis may therefore be relevant to the PSL. Consistent with this, metabolic disturbance has been reported to play a role in the loss of platelet viability during PSL.42-47 The possibility of stored platelets undergoing secondary necrosis may partly explain the inability of apoptosis inhibitors to maintain the viability of stored platelet concentrates.48,49
Would agents that preserve mitochondrial function improve the viability of stored platelets?
Much of the current effort into improving the viability of stored platelet concentrates has focused on reducing platelet activation, maintaining pH, buffering lactate production and enhancing gas exchange. This has been achieved with the use of platelet activation inhibitors (NO, phosphodiesterase inhibitors, apyrase and prostacyclin, protease inhibitors),50-57 through inclusion of additives such as acetate, phosphate, gluconate, potassium, and magnesium41,43-47,58-60 and through the implementation of modified storage bags with agitation.61 However, these measures have provided only modest improvements in platelet viability. This may not be surprising given that these approaches only partially mitigate the consequences of mitochondria damage, and do not target the primary underlying cause of the PSL, namely irreversible mitochondrial injury.62-65 Whether inhibitors of platelet activation and apoptosis, in combination with agents that preserve mitochondrial function (such as inhibitors of cyclophilin D), will further improve platelet viability, remains to be determined.
Perspectives and future directions
Significant advances in understanding the molecular events regulating procoagulant platelet function have occurred over the past decade, with a growing body of evidence demonstrating a central role for mitochondrial dysfunction in the induction of a procoagulant platelet response.26,27 In this context, the procoagulant platelet response is distinct from all other physiologic platelet activation responses, in that it is primarily initiated by cell death pathways that produce fundamental alterations in platelet morphology, ultrastructure and function.
The description of procoagulant necrotic platelets throughout this article refers specifically to annexin V+ platelets that have undergone biochemical, morphologic and functional changes that are consistent with cell necrosis. As such, necrotic platelets specifically refer to platelets that have undergone a high, sustained cytosolic calcium response after agonist stimulation, and should be distinguished from procoagulant apoptotic platelets that have primarily been stimulated by proapoptotic agents. Whether other programmed cell death pathways, ie, autophagy, can contribute to platelet procoagulant function remains to be established. It is noteworthy that platelets with a morphologic appearance consistent with necrotic cell death (balloon-shaped platelets with loss of internal organelles) have previously been identified at sites of vascular injury in vivo.32,38,39 While the importance of these platelets in supporting blood coagulation and thrombin generation in vivo remains to be established there is considerable clinical66-68 and experimental evidence69-71 supporting an important role for PS-expressing cells in promoting blood coagulation reactions and thrombin generation in vivo.31,64-68
Are there multiple pathways regulating platelet procoagulant function in vivo?
It is notable that CypD-deficient mice do not exhibit a marked bleeding diathesis despite defective agonist-induced PS exposure in platelets.27 This may partially reflect redundancy in the pathways controlling platelet procoagulant function in vivo. Notably, CypD-deficient platelets retain their ability to express PS after potent platelet stimulation with calcium-ionophore.27 Whether this is via an apoptotic mechanism remains unclear.12 It is possible that CypD-independent pathways leading to PS expression on the surface of leukocyte, endothelial or smooth muscle cells may also compensate for defects in platelet procoagulant function. Future studies on genetically modified mice with defects in cell death pathways in specific cell types will be required to address these issues.
A major challenge for the future will be the development of techniques to accurately analyze the contribution of distinct cell death pathways to blood coagulation reactions in vivo. In recent years there has been considerable progress in the development of intravital imaging techniques that enable monitoring of different platelet activation states (platelet Ca2+ flux, morphologic change, P-selectin expression, and PS exposure), which in concert with real-time measurements of thrombin generation and fibrin formation, should provide new insight into the specific platelet activation pathways supporting blood coagulation. Progress in this field will require the development of new methodologies to accurately identify (and differentiate) necrotic from apoptotic platelets in vivo and to elucidate their relative contributions to various coagulation reactions.
Acknowledgment
The authors thank Dr Benjamin Kile for his constructive comments.
Authorship
Contribution: S.P.J. contributed ideas and wrote the manuscript; and S.M.S. contributed ideas, wrote the manuscript, and prepared figures.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shaun P. Jackson, Australian Centre for Blood Diseases, 6th Level Burnet Bldg, AMREP, 89 Commercial Rd, Melbourne, Victoria, Australia 3004; e-mail: Shaun.Jackson@med.monash.edu.au.