Abstract
Targeted therapy with imatinib in chronic myeloid leukemia (CML) prompted a new treatment paradigm. Unlike CML, chronic lymphocytic leukemia (CLL) lacks an aberrant fusion protein kinase but instead displays increased phosphatidylinositol 3-kinase (PI3K) activity. To date, PI3K inhibitor development has been limited because of the requirement of this pathway for many essential cellular functions. Identification of the hematopoietic-selective isoform PI3K-δ unlocks a new therapeutic potential for B-cell malignancies. Herein, we demonstrate that PI3K has increased enzymatic activity and that PI3K-δ is expressed in CLL cells. A PI3K-δ selective inhibitor CAL-101 promoted apoptosis in primary CLL cells ex vivo in a dose- and time-dependent fashion that was independent of common prognostic markers. CAL-101–mediated cytotoxicity was caspase dependent and was not diminished by coculture on stromal cells. In addition, CAL-101 abrogated protection from spontaneous apoptosis induced by B cell–activating factors CD40L, TNF-α, and fibronectin. In contrast to malignant cells, CAL-101 does not promote apoptosis in normal T cells or natural killer cells, nor does it diminish antibody-dependent cellular cytotoxicity. However, CAL-101 did decrease activated T-cell production of various inflammatory and antiapoptotic cytokines. Collectively, these studies provide rationale for the clinical development of CAL-101 as a first-in-class targeted therapy for CLL and related B-cell lymphoproliferative disorders.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common type of adult leukemia in the United States, with approximately 15 000 new cases and approximately 4500 deaths per year.1 CLL is characterized by a B1 monoclonal lymphocyte immunophenotype with expression of the surface antigens CD19, CD5, CD20, CD23, and dim surface immunoglobulin G. The cell of origin of CLL is uncertain, but a gene expression pattern most similar to a mature memory B cell has been hypothesized.2 In addition, CLL cells display disrupted apoptosis that is caused by both primary tumor features and codependent stromal elements.3 Although many patients are asymptomatic at diagnosis, CLL is a progressive disease that in most patients eventually will require treatment. Once they become symptomatic, patients have a relatively short overall survival, ranging from 18 months to 6 years, with a 22.5% 10-year survival expectation.4 Common treatments for CLL include alkylating chemotherapeutic drugs (such as chlorambucil and cyclophosphamide), purine analogs (such as fludarabine), and rituximab (used in combination with fludarabine, fludarabine and cyclophosphamide, or pentostatin and cyclophosphamide). Newer studies with either single-agent bendamustine or alemtuzumab have been shown to have improved response and progression-free survival over alkylator-based therapy. However, no current treatment option results in curative therapy, and all patients eventually relapse. This provides strong justification for developing additional types of therapies for CLL. Of particular interest are therapies that target signal transduction pathways essential to CLL cell survival mechanisms that are known to be aberrantly activated.
One such pathway is the phosphoinositide 3-kinase (PI3K) pathway. The PI3K pathway is acknowledged as a key component of cell survival in many cancers, including CLL. It is activated by receptors, or the small guanosine triphosphatase Ras, and is made up of various classes of PI3K isoforms.5 There are 3 classes of PI3K isoforms; however, only the class I isoforms phosphorylate inositol lipids to form second messenger phosphoinositides. Specifically, class I PI3K enzymes convert PtdIns(3,4)P2 into PtdIns(3,4,5)P3, in the cell membrane that recruit, via binding to the amino-terminal pleckstrin homology domain, downstream signaling proteins such as Tec kinases, phosphatidylinositol-dependent kinase, Akt, integrin-linked kinase, and Rac guanine exchange factor. Class I isoforms are made up of 2 subsets (IA and IB). Class IA encompasses p110α, p110β, and p110δ (catalytic domains), bound by p85, p50, or p55 (regulatory domains). Class IB is made up solely of the p110γ (catalytic domain) bound by the regulatory domain p101. The p110α and p110β isoforms are ubiquitously expressed, and knock-out mice for both are embryonic lethal.6 It is thought that this widespread functionality of PI3K signaling is at least partially responsible for the significant cellular toxicity associated with pan-PI3K inhibitors such as LY294002.7 However, in recent years it has been shown that the different class I isoforms, specifically the 4 catalytic subunits making up the 4 isoforms (p110α, p110β, p110δ, and p110γ), have nonredundant roles and different expression profiles in different cell types.8-11
The expression of PI3K-δ is generally restricted to hematopoietic cell types.12 Mice with deleted or mutated PI3K-δ exhibit a B-cell defect, with a lack of B1 lymphocytes, decreased mature B-cell numbers, and impaired antibody production.6,8,13 Biochemically, B cells derived from PI3K-δ knockout mice also show less AKT phosphorylation when activated and have decreased phosphatidylinositol 3,4,5-triphosphate levels and phosphopeptide activity.6 In contrast, PI3K-γ isoform knockout mice, although not embryonic lethal, have predominately a T-cell defect with no B-cell developmental or functional abnormalities.6 These mouse studies suggest that isoform-specific targeting of the PI3K-δ isoform may be cytotoxic to B cells with minimal toxicity to other hematopoietic cell types.
Forced expression of PI3K-δ was shown to be transforming in cell lines.14 Application of another specific PI3K-δ inhibitor in AML showed both preclinical activity and enhancement of the cytotoxic effect with chemotherapy in cells with active PI3K-δ.15,16 Previous studies have shown increased general activity of PI3K in the pathogenesis of CLL with convergence of CD40 ligand (CD40L), B cell–activating factor belonging to the tumor necrosis (TNF) family (BAFF), fibronectin, and B-cell receptor signaling through this pathway. However, only one study examining the influence of PI3K signaling on the microenvironment has addressed the relevance of specific PI3K isoform signaling. This study showed that both the cytotoxic effects of PI3K inhibitors and also microenvironmental protection were afforded predominately by inhibition of the PI3K-α isoform, but other isoforms also played a distinct role in these processes.17
Therapeutic targeting of a specific PI3K isoform expressed selectively in hematopoietic cell types represents a potentially promising approach for the treatment of CLL. However, until recently, no therapeutic agents that actively target specific PI3K isoforms have been available. CAL-101 is a potent and selective inhibitor of PI3K-δ isoform.18 To further justify transition of CAL-101 to clinical trials in CLL, we investigated this agent for both its direct therapeutic effect on CLL cells and also its ability to disrupt external survival stimuli provided by the microenvironment. These studies show that CAL-101 both directly promotes apoptosis in CLL cells and also disrupts multiple external survival pathways that contribute to CLL viability and proliferation in vivo. Collectively, these studies provide significant support for development of CAL-101 as a therapeutic agent for CLL.
Methods
Reagents and antibodies
CAL-101 and recombinant proteins for p110α, p110β, p110γ, and p110δ were provided by Calistoga Pharmaceuticals. Phycoerythrin (PE)–labeled isotype control mouse IgG1, CD19, CD3, CD56, fluorescein isothiocyanate–labeled annexin V, anti-CD28, and propidium iodide (PI) were purchased from BD PharMingen and used according to the manufacturer's instructions. LY294002 was purchased from BIOMOL Research Laboratories. Z-VAD-fmk (carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone), anti-caspase 3, human interleukin-6 (IL-6), IL-4, IL-10, TNF-α, and interferon-γ (INF-γ) Quantikine enzyme-linked immunoabsorbent assay (ELISA) kits, recombinant human IL-4 (rhIL-4), and rhBAFF were purchased from R&D Systems. PI3K ELISA assay was purchased from Echelon Biosciences. Anti-AKT, anti–phospho-AKT (Ser473), anti–phospho-STAT3 (signal transducer and activator of transcription 3; Tyr705), anti-STAT3, anti–phospho-GSK3β (glycogen synthase kinase 3 β; Ser9), anti-GSK3β, and anti-PARP [poly (adenosine diphosphate-ribose) polymerase] were purchased from Cell Signaling Technologies. Anti-actin, anti–mcl-1 and anti-p110δ were purchased from Santa Cruz Biotechnology. Anti–glyceraldehyde-3-phosphate dehydrogenase was purchased from Millipore. Recombinant human soluble CD40L was purchased from PeproTech. Amino trifluoromethyl coumarin (Ac-DEVD-AFC) was purchased from Enzyme Systems Products. Anti-CD3 T-cell activation plates and fibronectin-coated multiwell plates were purchased from BD Biosciences. TNF-α and 2-fluoroadenine-9-β-D-arabinofuranoside (fludarabine prodrug) were purchased from Sigma-Aldrich.
Patient sample processing and cell culture
Blood was obtained from patients with informed consent in accordance with the Declaration of Helsinki and under a protocol approved by the Institutional Review Board of The Ohio State University (OSU). All patients examined in this series had immunophenotypically defined CLL as outlined by the modified 1996 National Cancer Institute criteria.19 All of the patients had been without prior therapy for a minimum of 30 days at the time of collection. CLL B cells were isolated from freshly donated blood with Ficoll density gradient centrifugation (Ficoll-Plaque Plus, Amersham Biosciences). Enriched CLL fractions were prepared with the use of the “Rosette-Sep” kit from StemCell Technologies according to the manufacturer's instructions. Isolated cells were incubated in RPMI 1640 media supplemented with 10% heat-inactivated human serum (Valley Biomedical), 2mM l-glutamine (Invitrogen), and 100 U/mL penicillin/100 μg/mL streptomycin (Sigma-Aldrich) at 37°C in an atmosphere of 5% CO2. The purity of enriched populations of CLL was routinely checked with the use of CD19-PE staining by flow cytometry. Normal cells were obtained from Red Cross partial leukocyte preparations, and T cells or natural killer (NK) cells were negatively selected with the appropriate Rosette-Sep kits. The purity of enriched populations of normal cells was routinely checked with the use of CD19, CD3, and CD56-PE staining by flow cytometry. Normal samples were from anonymous donors as part of a second exemption protocol approved by the institutional review board at OSU. The HS-5 cell line was obtained from ATCC and cultured in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum.
Immunoblot analysis
Whole-cell lysates were prepared as previously described by our group with the addition of phosphatase inhibitor cocktail 1 and 2, protease inhibitor cocktail P8340, and 1mM phenylmethylsulfonyl fluoride (all from Sigma-Aldrich) to the lysis buffer.20 Equivalent amounts of protein (50 μg/lane) were separated on polyacrylamide gels and transferred onto nitrocellulose membranes. After antibody incubations, proteins were detected with chemiluminescent substrate (SuperSignal; Pierce).
PI3K assay
PI3K assay was preformed on whole-cell lysates from CLL or normal B cells. A PI3K ELISA assay was performed according to the manufacturer's instructions (Echelon Biosciences). Briefly, whole-cell extracts were added to a mixture of PI(4,5)P2 substrate and reaction buffer containing adenosine triphosphate (ATP) and allowed to incubate at room temperature. The reaction was stopped by adding PI(3,4,5)P3 detector mixed with EDTA (ethylenediaminetetraacetic acid) and allowed to incubate at room temperature for 1 hour. After this time, the mixture was transferred from each well to a PI3K ELISA plate and allowed to incubate 1 hour. Plates were washed and then incubated with secondary detector for 30 minutes. Plates were washed again, and 3,3′,5,5′-tetramethylbenzidine solution was added for 5 minutes at which time H2SO4 was added to stop all reactions. Plates were read at 450 nm on a Labsystems 96-well plate reader (Fisher Scientific).
Viability and flow cytometric studies
MTT (3′[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide) assays were performed to determine cytotoxicity. Briefly, 1 × 105 cells (CLL B cells or healthy volunteer T cells or NK cells) were incubated for 48 hours with different concentrations of CAL-101, 25μM LY294002, or vehicle control. MTT reagent (Sigma-Aldrich) was then added, and plates were incubated for an additional 20 hours before washing with protamine sulfate in phosphate-buffered saline. Dimethyl sulfoxide was added, and absorbance was measured by spectrophotometry at 540 nm in a Labsystems plate reader (Fisher Scientific). Cell viability was also measured at various time points with the use of annexin/PI flow cytometry (Beckman-Coulter). Data were analyzed with Expo-ADC32 software package (Beckman-Coulter). At least 10 000 cells were counted for each sample. Results were expressed as the percentage of total positive cells over untreated control. Experiments examining caspase-dependent apoptosis included the addition of 100μM Z-VAD. Experiments examining survival signals included the addition of 1 μg/mL CD40L, 800 U/mL IL-4, 50 ng/mL BAFF, 20 ng/mL TNF-α, or coculturing on fibronectin or stromal (HS-5 cell line) coated plates. Stromal coculture was done by plating a 75-cm2 flask (80%-100% confluent) per 6-well plate 24 hours before the addition of CLL cells.
Analysis of antibody-dependent cellular cytotoxicity
Antibody-dependent cellular cytotoxicity (ADCC) was determined by standard 4-hour 51Cr-release assay. 51Cr-labeled target cells (1 × 104 isolated CLL B cells) were incubated with media alone (vehicle only) or in the presence of various antibodies (alemtuzumab, rituximab, and trastuzumab [Herceptin; Genentech]) at a concentration of 10 μg/mL for 30 minutes at 37°C. Unbound antibody was washed off, and the cells were plated at 1 × 104 cells/well. Effector cells (negatively isolated NK cells from healthy donors) were pretreated with 10μM CAL-101 and then added to the plates at indicated effector-to-target ratios. After 4 hours of incubation, supernatants were removed, and the radioactivity was counted in a γ counter. The percentage of specific cell lysis was determined by the following formula: percentage of lysis = 100 × (ER − SR)/(MR − SR), where ER, SR, and MR represent experimental, spontaneous, and maximum 51Cr-release, respectively. Data were normalized to untreated control.
Detection of cytokine production by ELISA
Cytokine production was measured with the use of the Quantikine ELISAs (R&D Systems). CD3 stimulation was done with anti-CD3 T-cell activation plates (BD Biosciences). CD3+ T cells were drugged for 48 hours with media alone (vehicle only) and various doses of CAL-101. After drugging, 2 × 106 cells were added to each well of the anti-CD3 plate in duplicate. After 24 hours the supernatant from each well was collected, and an ELISA was performed according to the manufacturer's directions.
Quantitative real-time polymerase chain reaction
RNA was extracted from 2 × 107 cells with the use of TRIzol reagent (Invitrogen). cDNAs were prepared with the use of a SuperScript First-Strand Synthesis System (Invitrogen) as previously described.21 Real-time polymerase chain reaction was performed with predesigned TaqMan Gene Expression Assays and ABI Prism 7700 sequence detection system (Applied Biosystems).
Enzymatic caspase assay
The presence of active caspase enzymes was determined by the AFC assay, as previously described.22 Lysates containing approximately 3 × 106 cells were incubated with DEVD-AFC to determine the presence of active caspase-3 in a cyto-buffer (10% glycerol, 50mM PIPES [piperazine-1,4-bis(2-ethanesulfonic acid)], pH 7.0, 1mM EDTA [ethylenediaminetetraacetic acid]) containing 1mM dithiothreitol (DTT) and 20μM tetrapeptide substrate. Caspase-3 activity was measured immediately after addition of substrate. Release of free AFC was determined with the use of a Beckman Coulter DTX 880 multimode detector (Filters: excitation, 405/10 nm; emission, 535/25 nm).
Statistical analysis
All reported statistical evaluation was done by the Center for Biostatistics at OSU. Because many of the measurements used samples from the same patients, linear mixed effects models were used for analysis to take into consideration the dependency of these observations. To stabilize the variance, the raw Ct value of real-time polymerase chain reaction data was normalized to internal control, and the standardized data were analyzed with the linear mixed effects models. Holm procedure was used to correct for multiple comparisons when appropriate.23 Type I error is strongly controlled at α = 0.05 for single comparisons and after adjustment for multiple comparisons or multiple endpoints.
Results
PI3K-δ is expressed abundantly in CLL cells
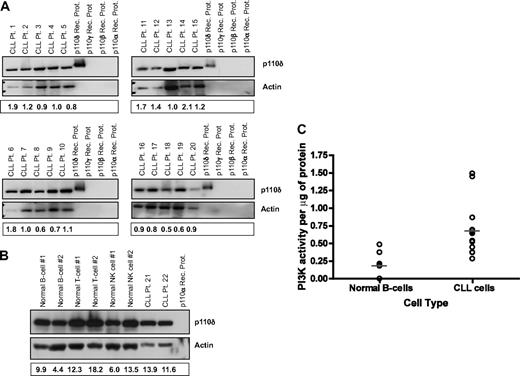
Given the broad importance of the PI3K-δ isoform in B-cell development, we sought to determine its expression profile in primary CLL cells. Expression of PI3K-δ was examined in cells from 20 separate patients with CLL. All CLL tumor cells showed expression of PI3K-δ, with relatively little variability among patients (Figure 1A). In addition to B cells from patients with CLL, we also evaluated the expression profile of PI3K-δ in normal hematopoietic cells. We found that all lymphoid cells expressed PI3K-δ with little variation across cell type (Fig 1B). Given the consistent expression of PI3K-δ in CLL cells and normal B cells, we next sought to determine whether the PI3K pathway was more active in tumor cells compared with normal B cells. As shown in Figure 1C, CLL cells overall have a statistically higher intrinsic PI3K activity compared with normal B cells (P = .006), as previously reported by others.24 These studies collectively confirm the presence of the PI3K-δ isoform in CLL cells and the activity of PI3K overall, thereby validating further exploration of specific inhibitors of this pathway.
p110δ is expressed abundantly in CLL cells. (A) CD19+ cells from patients with CLL (n = 20) were examined for p110δ expression by immunoblot. (B) CD19+ normal B cells, CD3+ normal T cells, CD56+ normal NK cells, and CD19+ cells from patients with CLL (n = 6 each) were examined for p110δ expression by immunoblot. (C) CD19+ cells from patients with CLL (n = 12) and from normal donors (n = 7) were examined for PI3K activity. Horizontal lines represent the mean. Results were calculated relative to microgram of protein.
p110δ is expressed abundantly in CLL cells. (A) CD19+ cells from patients with CLL (n = 20) were examined for p110δ expression by immunoblot. (B) CD19+ normal B cells, CD3+ normal T cells, CD56+ normal NK cells, and CD19+ cells from patients with CLL (n = 6 each) were examined for p110δ expression by immunoblot. (C) CD19+ cells from patients with CLL (n = 12) and from normal donors (n = 7) were examined for PI3K activity. Horizontal lines represent the mean. Results were calculated relative to microgram of protein.
CAL-101 induces selective cytotoxicity in CLL cells independent of IgVH mutational status or interphase cytogenetics
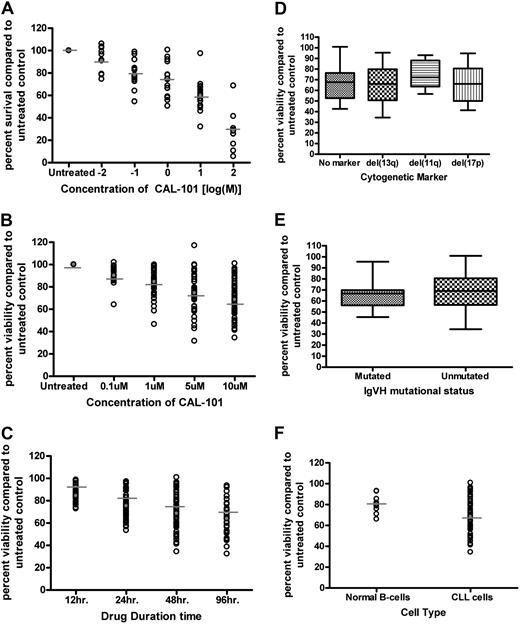
CAL-101 is a selective PI3K-δ inhibitor whose chemical structure and inhibitory properties have been previously described.18 To determine the potential in vitro activity of CAL-101 against CLL cells, B cells from 16 patients with CLL were treated with different concentrations of CAL-101 for 48 hours. We found that CAL-101 exhibited a dose-dependent induction of cytotoxicity in CLL cells (Figure 2A) as measured by MTT. Cell death induced by therapeutic agents in CLL can occur though caspase-dependent or -independent apoptosis or by necrosis. Studies that used annexin V/PI staining show evidence of both early (annexin V-positive only) and late (annexin V/PI both positive) apoptosis (data not shown). CAL-101–induced apoptosis of CLL cells was significant compared with vehicle treatment alone (P < .001) (Figure 2B). These results shown in Figure 2B provide support that the cytotoxicity induced by CAL-101 occurs by the induction of apoptosis and corroborate the MTT data (Figure 2A). Although cytotoxicity was initially measured at 48 hours, CAL-101–induced cell death increased in a dose- and time-dependent manner, with evidence of apoptosis observed as early as 12 hours (Figure 2B-C).
CAL-101 induces selective cytotoxicity in CLL cells independent of IgVH mutational status or interphase cytogenetics. (A) CD19+ cells from patients with CLL (n = 16) were incubated with or without CAL-101 (0.01-100μM) for 48 hours. Viability was determined by MTT assay and was calculated relative to time-matched untreated controls. (B) CD19+ cells from patients with CLL (n = 40) were incubated with or without CAL-101 (0.1-10μM) for 48 hours. Viability was determined by annexin/PI flow cytometry. (C) CD19+ cells from patients with CLL (n = 40) were incubated with or without 10μM CAL-101 for 12 to 96 hours. Horizontal lines represent the mean. (D) CD19+ cells from patients with CLL (n = 40; 10 per group) were incubated with or without 10μM CAL-101 for 48 hours. Cytogenetics was determined independently of our laboratory. (E) CD19+ cells from patients with CLL (n = 30; 15 per group) were incubated with or without 10μM CAL-101 for 48 hours. Mutational status was determined independently of our laboratory. Error bars represent the SD from the mean. (F) CD19+ cells from CLL patient cells (n = 40) and CD19+ cells from normal B cells (n = 9) were incubated with 10μM CAL-101 for 48 hours. (B-F) Viability was determined by annexin/PI flow cytometry and was calculated relative to time-matched untreated controls. Horizontal lines represent the mean.
CAL-101 induces selective cytotoxicity in CLL cells independent of IgVH mutational status or interphase cytogenetics. (A) CD19+ cells from patients with CLL (n = 16) were incubated with or without CAL-101 (0.01-100μM) for 48 hours. Viability was determined by MTT assay and was calculated relative to time-matched untreated controls. (B) CD19+ cells from patients with CLL (n = 40) were incubated with or without CAL-101 (0.1-10μM) for 48 hours. Viability was determined by annexin/PI flow cytometry. (C) CD19+ cells from patients with CLL (n = 40) were incubated with or without 10μM CAL-101 for 12 to 96 hours. Horizontal lines represent the mean. (D) CD19+ cells from patients with CLL (n = 40; 10 per group) were incubated with or without 10μM CAL-101 for 48 hours. Cytogenetics was determined independently of our laboratory. (E) CD19+ cells from patients with CLL (n = 30; 15 per group) were incubated with or without 10μM CAL-101 for 48 hours. Mutational status was determined independently of our laboratory. Error bars represent the SD from the mean. (F) CD19+ cells from CLL patient cells (n = 40) and CD19+ cells from normal B cells (n = 9) were incubated with 10μM CAL-101 for 48 hours. (B-F) Viability was determined by annexin/PI flow cytometry and was calculated relative to time-matched untreated controls. Horizontal lines represent the mean.
Chromosomal deletions of del(11q22.3) and del(17p13.1) are one of the strongest laboratory predictors of CLL response to chemotherapy.25 Similarly, IgVH gene mutational status influences the duration of remission to standard therapies used to treat CLL.26 Figure 2D shows there was no significant difference in CAL-101 sensitivity according to different interphase cytogenetic groups, including those with del(17p13.1) (P = .34). Furthermore, we observed no significant differences in CAL-101 cytotoxicity in patients with IgVH-mutated versus IgVH-unmutated CLL cells (P = .51; Figure 2E). Similar findings with lower doses of CAL-101 (1μM) and LY294002 (pan-PI3K inhibitor) were observed for both cytogenetic and mutational analysis comparisons (data not shown). In the same patient pools fludarabine was found to have a significant difference among cytogenetic and mutational status populations (data not shown). These studies suggest that CAL-101 may induce cell death independent of p53 function and other adverse prognostic factors associated with poor response to therapy in patients with CLL, which is in contrast to other available agents.
To examine the selectivity of CAL-101 on CLL cells compared with normal B cells, we evaluated the cytotoxicity of CAL-101 on CD19 selected normal B cells from healthy volunteers. At high concentration (10μM) we found that CAL-101 did produce modest apoptosis (95% CI, −12.1630 to −5.0370) in this cell population that was significantly increased (P < .001) compared with media control as shown in Figure 2F. The apoptosis noted in normal B cells was, however, significantly less than that observed in CLL cells (P < .001). These data provide support that CAL-101 induces cytotoxicity preferentially to CLL cells compared with normal B cells.
CAL-101 cytotoxicity against CLL cells partially depends on caspase activity
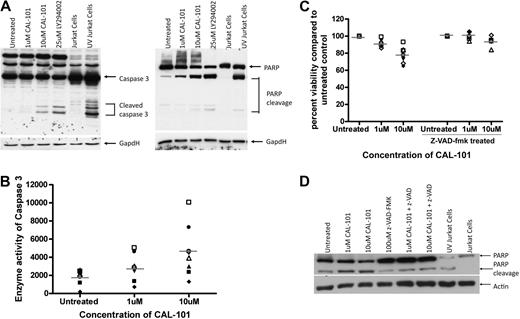
We next investigated the mechanism by which CAL-101 mediates cell death in CLL cells. Apoptosis with other cytotoxic agents in CLL occurs by caspase-dependent and -independent pathways.27-29 In an attempt to determine whether the cytotoxicity induced by CAL-101 is due to an increase in caspase-dependent apoptosis, CLL cells were incubated with CAL-101 for 12 hours, after which assessment of pro–caspase-3 processing to the active p20 caspase-3 cleavage product was assessed by immunoblot analysis. Treatment of CAL-101 resulted in an increase in active caspase-3 concurrent with a decrease in the pro-form (Figure 3A). Concurrently, the appearance of the cleaved product of the caspase-3 substrate PARP was detected (Figure 3A). Enzymatic activity of caspase-3 was also detected after 12 hours of CAL-101 treatment and increased after longer treatments (Figure 3B; data not shown). To determine whether apoptosis was occurring through a caspase-dependent mechanism we treated CLL cells with CAL-101 in the presence or absence of the pan-caspase inhibitor z-VAD-fmk. We found that the addition of z-VAD-fmk to CAL-101–treated samples significantly decreased the cytotoxicity observed with CAL-101 (Figure 3C; P = .004) and diminished the appearance of the cleaved product of PARP (Figure 3D). Collectively, these studies suggest that CAL-101 mediates cytotoxicity primarily through a caspase-dependent mechanism.
CAL-101 cytotoxicity against CLL cells is partially dependent on caspase activity. (A) CD19+ cells from patients with CLL (n = 4) were incubated with or without 1 or 10μM CAL-101 or 25μM LY294002 (pan-PI3K inhibitor) for 12 hours, and caspase-3 and PARP were assessed by immunoblot. Results are shown from 1 of 4 experiments. (B) CD19+ cells from patients with CLL (n = 7) were incubated with or without 1 or 10μM CAL-101 for 12 hours. Cells were lysed, and caspase activity was determined by the amino trifluoromethyl coumarin assay. Results were calculated relative to microgram of protein. Each symbol represents an individual patient. (C) CD19+ cells from patients with CLL (n = 6) were incubated with or without 1 or 10μM CAL-101 and 100μM z-VAD-fmk for 48 hours. Viability was determined by annexin/PI flow cytometry and is shown relative to time-matched untreated controls. Each symbol represents an individual patient. Horizontal lines represent the mean. (D) CD19+ cells from patients with CLL (n = 4) were incubated with or without CAL-101 (1-10μM) and 100μM z-VAD-fmk for 12 hours. PARP cleavage was assessed by immunoblot. Results are shown from 1 of 4 experiments.
CAL-101 cytotoxicity against CLL cells is partially dependent on caspase activity. (A) CD19+ cells from patients with CLL (n = 4) were incubated with or without 1 or 10μM CAL-101 or 25μM LY294002 (pan-PI3K inhibitor) for 12 hours, and caspase-3 and PARP were assessed by immunoblot. Results are shown from 1 of 4 experiments. (B) CD19+ cells from patients with CLL (n = 7) were incubated with or without 1 or 10μM CAL-101 for 12 hours. Cells were lysed, and caspase activity was determined by the amino trifluoromethyl coumarin assay. Results were calculated relative to microgram of protein. Each symbol represents an individual patient. (C) CD19+ cells from patients with CLL (n = 6) were incubated with or without 1 or 10μM CAL-101 and 100μM z-VAD-fmk for 48 hours. Viability was determined by annexin/PI flow cytometry and is shown relative to time-matched untreated controls. Each symbol represents an individual patient. Horizontal lines represent the mean. (D) CD19+ cells from patients with CLL (n = 4) were incubated with or without CAL-101 (1-10μM) and 100μM z-VAD-fmk for 12 hours. PARP cleavage was assessed by immunoblot. Results are shown from 1 of 4 experiments.
CAL-101 does not show cytotoxicity toward other normal immune cells
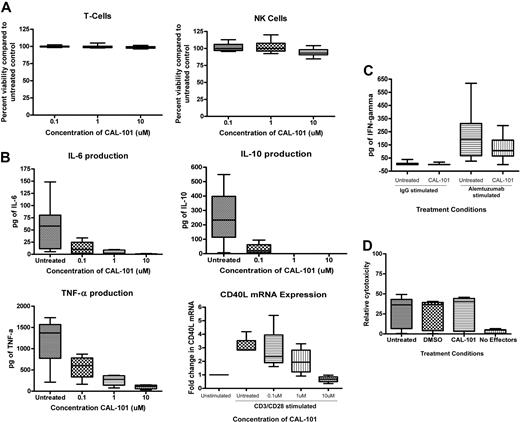
Treating normal NK cells or T cells with CAL-101 under conditions described in Figure 2, we did not observe significant cell death even at a dose of 100μM (P values for T cells and NK cells were .16 and .48, respectively; Figure 4A). This result suggests that CAL-101 lacks significant cytotoxicity toward normal T cells and NK cells compared with that observed with CLL cells.
CAL-101 does not show cytotoxicity toward other normal immune cells but alters cytokine production. (A) CD3+ T cells and CD56+ NK cells (n = 9; each) from healthy volunteers were incubated with or without CAL-101 (0.1-10μM) for 48 hours. Viability was determined by annexin/PI flow cytometry and was calculated relative to time-matched untreated controls. (B) CD3+ T cells (n = 12) from healthy volunteers were incubated with or without CAL-101 (0.1-10μM) for 48 hours. Cells were stimulated with an anti-CD3 T-cell activation plate for 24 hours, and IL-6, IL-10, and TNF-α production was measured by ELISA. For CD40L mRNA assay, CD4+ T cells from healthy volunteers (n = 4) were incubated with and without various doses of CAL-101 and 5 μg/mL CD28. Cells were then stimulated with an anti-CD3 T-cell activation plate for 48 hours. Real-time polymerase chain reaction analysis was done to determine quantities of CD40L mRNA. (C) CD56+ NK cells (n = 8) from healthy volunteers were incubated with or without alemtuzumab, CAL-101, or the combination for 4 hours. IFN-γ production was determined by ELISA. (D) CD56+ NK cells (n = 3) from healthy volunteers were used as effector cells for a CLL-cell ADCC assay. NK cells were left untreated or treated with 10μM CAL-101; whereas CLL effector cells were treated with alemtuzumab. IgG indicates immunoglobulin G; and DMSO, dimethyl sulfoxide. Error bars represent the SD from the mean.
CAL-101 does not show cytotoxicity toward other normal immune cells but alters cytokine production. (A) CD3+ T cells and CD56+ NK cells (n = 9; each) from healthy volunteers were incubated with or without CAL-101 (0.1-10μM) for 48 hours. Viability was determined by annexin/PI flow cytometry and was calculated relative to time-matched untreated controls. (B) CD3+ T cells (n = 12) from healthy volunteers were incubated with or without CAL-101 (0.1-10μM) for 48 hours. Cells were stimulated with an anti-CD3 T-cell activation plate for 24 hours, and IL-6, IL-10, and TNF-α production was measured by ELISA. For CD40L mRNA assay, CD4+ T cells from healthy volunteers (n = 4) were incubated with and without various doses of CAL-101 and 5 μg/mL CD28. Cells were then stimulated with an anti-CD3 T-cell activation plate for 48 hours. Real-time polymerase chain reaction analysis was done to determine quantities of CD40L mRNA. (C) CD56+ NK cells (n = 8) from healthy volunteers were incubated with or without alemtuzumab, CAL-101, or the combination for 4 hours. IFN-γ production was determined by ELISA. (D) CD56+ NK cells (n = 3) from healthy volunteers were used as effector cells for a CLL-cell ADCC assay. NK cells were left untreated or treated with 10μM CAL-101; whereas CLL effector cells were treated with alemtuzumab. IgG indicates immunoglobulin G; and DMSO, dimethyl sulfoxide. Error bars represent the SD from the mean.
CAL-101 alters cytokine production by T cells and NK cells
Although no significant cytotoxicity was seen in normal T or NK cells, we sought to determine whether CAL-101 affects the function of T or NK cells. Recent studies have shown that PI3K-δ is essential to cytokine production by immune effector cells.30,31 We, therefore, assessed the ability of CAL-101 to disrupt T-cell cytokine production by measuring IL-6, TNF-α, IL-10, and IL-4 production after anti-CD3 stimulation. We found that low doses of CAL-101 could inhibit normal T cells from producing IL-6, IL-10, and TNF-α (Figure 4B). Our assays failed to detect production of IL-4 in normal T cells. Similar results were seen with T cells isolated from patients with CLL for evaluated cytokines (IL-6, IL-4, and IL-10; data not shown). In addition to the aforementioned cytokines, activated T cells also produce CD40 ligand (CD40L or CD154). Figure 4B shows that treatment of CD3-ligated T cells costimulated with CD28 results in an increase of CD154 mRNA. The addition of CAL-101 to these activated cells resulted in a dose-dependent inhibition of CD154 mRNA induction. In a similar manner, we assessed the ability of CAL-101 to disrupt NK-cell cytokine production. NK cells were treated with or without CAL-101 and incubated with plate-immobilized alemtuzumab. As shown in Figure 4C, a modest but significant decrease in IFN-γ production was observed with CAL-101 treatment. Similar findings with lower doses of CAL-101 (1μM) and LY294002 were observed (data not shown). These studies show collectively that CAL-101 lacks direct cytotoxic potential to T cells and NK cells but that it can inhibit production of inflammatory cytokines, such as IL-6, IL-10, TNF-α, and IFN-γ, and activation-induced cytokines, such as CD40L, that others have shown promote proliferation or enhance survival of CLL cells.32-36
CAL-101 does not alter ADCC against CLL cells
Therapeutic antibodies such as rituximab and alemtuzumab are widely used for the treatment of CLL. The mechanism of cytotoxicity of these antibodies probably involves ADCC, complement-dependent cytotoxicity, and direct killing.37,38 Because rituximab and alemtuzumab are frequently used in combination therapies, we examined NK cell ADCC with these therapeutic agents after treatment with CAL-101. Target CLL cells were incubated with alemtuzumab, rituximab, or trastuzumab. As shown in Figure 4D, pretreatment of NK cells with CAL-101 did not significantly diminish ADCC of alemtuzumab-labeled CLL target cells. Similar results were observed with rituximab (data not shown).
CAL-101 induces apoptosis more selectively than pan-PI3K inhibitors
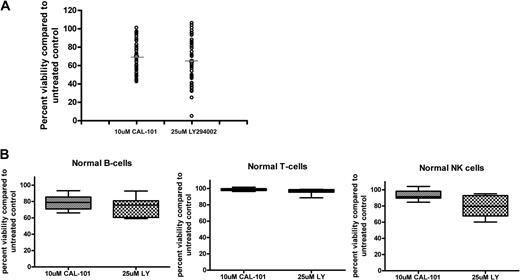
To determine whether inhibition of PI3K-δ induced apoptosis comparable with inhibition of all isoforms of PI3K, we compared CAL-101 to LY294002, a pan-PI3K inhibitor. We found that CAL-101 and LY294002 were not significantly different in their cytotoxic properties in B cells from patients with CLL (Figure 5A). We next sought to determine whether CAL-101 produced more selective cytotoxicity than LY294002 in other hematopoietic cells. We found that CAL-101 and LY294002 did not show significant differences in their cytotoxic properties in normal B or T cells; however, LY294002 produced significant cytotoxicity in normal NK cells (P = .004), which was in contrast to CAL-101 (Figure 5B). This suggests that inhibition of the PI3K-δ isoform alone, by CAL-101, is sufficient to induce apoptosis in CLL patient cells without producing cytotoxic effects in other hematopoietic cells.
CAL-101 induces apoptosis more selectively than pan-PI3K inhibitors. (A) CD19+ cells from patients with CLL (n = 49) were incubated with or without 10μM CAL-101 or 25μM LY294002 for 48 hours. Viability was determined by annexin/PI flow cytometry and is shown relative to time-matched untreated controls. Horizontal lines represent the mean. (B) CD19+ B cells, CD3+ T cells, and CD56+ NK cells (n = 10; each) were incubated with or without 10μM CAL-101 and 25μM LY294002 for 48 hours. Viability was determined by annexin/PI flow cytometry and is shown relative to time-matched untreated controls. Error bars represent the SD from the mean.
CAL-101 induces apoptosis more selectively than pan-PI3K inhibitors. (A) CD19+ cells from patients with CLL (n = 49) were incubated with or without 10μM CAL-101 or 25μM LY294002 for 48 hours. Viability was determined by annexin/PI flow cytometry and is shown relative to time-matched untreated controls. Horizontal lines represent the mean. (B) CD19+ B cells, CD3+ T cells, and CD56+ NK cells (n = 10; each) were incubated with or without 10μM CAL-101 and 25μM LY294002 for 48 hours. Viability was determined by annexin/PI flow cytometry and is shown relative to time-matched untreated controls. Error bars represent the SD from the mean.
CAL-101 antagonizes CD40L-mediated CLL cell survival
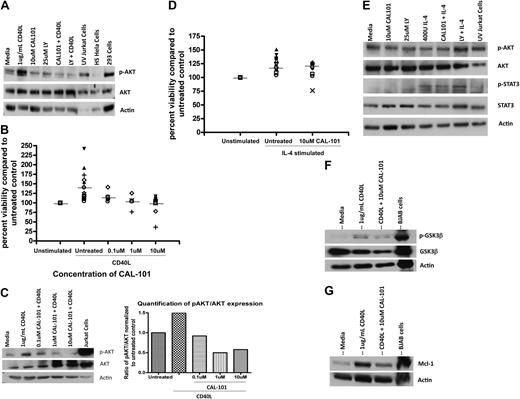
Cytokines such as CD40L (CD154) are produced by CD4+ T cells and also follicular dendritic cells and have been shown to promote survival of CLL cells in part through the PI3K pathway. Although our data with T cells show that CAL-101 can antagonize production of this cytokine, we hypothesized that it might also disrupt intracellular signaling in CLL cells. After treatment with CD40L, CLL cells show increased phosphorylation of AKT at the Ser473 site, a finding consistent with PI3K pathway activation (Figure 6A). In conjunction with CD40L treatment of CLL cells, we found significant protection from spontaneous apoptosis (Figure 6B; P < .001). Treatment of CLL cells with CAL-101 could decrease, although not completely prevent, the observed increase in AKT phosphorylation at the Ser473 site seen with CD40L (Figure 6A). This reversal of induced AKT phosphorylation by CD40L occurred in a dose-dependent manner with CAL-101 (Figure 6C). The CAL-101 antagonism of Ser473 AKT phosphorylation, although not complete, was sufficient to abrogate the protective effect provided by CD40L as shown by annexin/PI flow cytometry (P < .001; Figure 6B). In contrast to CD40L, IL-4–mediated prevention of spontaneous apoptosis of CLL cells was not reversible with CAL-101 treatment (Figure 6D). Furthermore, IL-4 did not increase basal AKT phosphorylation but rather activated the Janus kinase/STAT pathway by phosphorylation of STAT3 (Figure 6E), suggesting an alternative mechanism of protection for this cytokine. In addition to changes in the phosphorylation of AKT, we also found alteration of downstream proteins. CD40L induced phosphorylation of GSK3β at the Ser9 site. Similar to AKT, CAL-101 reversed the phosphorylation of GSK3β (Figure 6F). Along the same lines, we also saw an increase in Mcl-1 expression after CD40L that was reversible by CAL-101 treatment (Figure 6G). These findings support a role for PI3K-δ in the protective effect of CD40L on CLL cells and also the specificity for CAL-101 to this pathway.
CAL-101 antagonizes CD40-CD40L–mediated CLL cell survival. (A) CD19+ cells from patients with CLL (n = 4) were incubated with 10μM CAL-101 and 1μg/mL CD40L for 2 hours. AKT phosphorylation at Ser473 was assessed by immunoblot. Results are shown from 1 of 4 experiments. (B) CD19+ cells from patients with CLL (n = 5-18) were incubated with or without various doses of CAL-101 and 1μg/mL CD40L for 48 hours. Viability was determined by annexin/PI flow cytometry and is shown relative to time-matched untreated controls. Horizontal lines represent the mean. (C) CD19+ cells from CLL patients (n = 3) were incubated with various concentrations of CAL-101 and 1μg/mL CD40L for 2 hours. AKT phosphorylation at Ser473 was assessed by immunoblot. Results are shown from 1 of 3 experiments. Quantification was done with the Alpha Innotech FluorChemQ MultiImage III system. (D) CD19+ cells from patients with CLL (n = 20) were incubated with or without 10μM CAL-101 and 800U/mL IL-4 for 48 hours. Viability was determined by annexin/PI flow cytometry and is shown relative to time-matched untreated controls. Horizontal lines represent the mean. (E) CD19+ cells from patients with CLL (n = 4) were incubated with or without 10μM CAL-101 (or 25μM LY294002) and 800U IL-4 for 2 hours. Western blot analysis was done to detect activation of AKT (phosphorylation at Ser473) or STAT3 (phosphorylation at Tyr705). Results are shown from 1 of 4 experiments. (F) CD19+ cells from patients with CLL (n = 3) were incubated with 10μM CAL-101 and 1μg/mL CD40L for 2 hours. GSK3β phosphorylation at Ser9 was assessed by immunoblot. Results are shown from 1 of 3 experiments. (G) CD19+ cells from patients with CLL (n = 3) were incubated with 10μM CAL-101 and 1μg/mL CD40L for 2 hours. Mcl-1 expression was assessed by immunoblot. Results are shown from 1 of 3 experiments.
CAL-101 antagonizes CD40-CD40L–mediated CLL cell survival. (A) CD19+ cells from patients with CLL (n = 4) were incubated with 10μM CAL-101 and 1μg/mL CD40L for 2 hours. AKT phosphorylation at Ser473 was assessed by immunoblot. Results are shown from 1 of 4 experiments. (B) CD19+ cells from patients with CLL (n = 5-18) were incubated with or without various doses of CAL-101 and 1μg/mL CD40L for 48 hours. Viability was determined by annexin/PI flow cytometry and is shown relative to time-matched untreated controls. Horizontal lines represent the mean. (C) CD19+ cells from CLL patients (n = 3) were incubated with various concentrations of CAL-101 and 1μg/mL CD40L for 2 hours. AKT phosphorylation at Ser473 was assessed by immunoblot. Results are shown from 1 of 3 experiments. Quantification was done with the Alpha Innotech FluorChemQ MultiImage III system. (D) CD19+ cells from patients with CLL (n = 20) were incubated with or without 10μM CAL-101 and 800U/mL IL-4 for 48 hours. Viability was determined by annexin/PI flow cytometry and is shown relative to time-matched untreated controls. Horizontal lines represent the mean. (E) CD19+ cells from patients with CLL (n = 4) were incubated with or without 10μM CAL-101 (or 25μM LY294002) and 800U IL-4 for 2 hours. Western blot analysis was done to detect activation of AKT (phosphorylation at Ser473) or STAT3 (phosphorylation at Tyr705). Results are shown from 1 of 4 experiments. (F) CD19+ cells from patients with CLL (n = 3) were incubated with 10μM CAL-101 and 1μg/mL CD40L for 2 hours. GSK3β phosphorylation at Ser9 was assessed by immunoblot. Results are shown from 1 of 3 experiments. (G) CD19+ cells from patients with CLL (n = 3) were incubated with 10μM CAL-101 and 1μg/mL CD40L for 2 hours. Mcl-1 expression was assessed by immunoblot. Results are shown from 1 of 3 experiments.
CAL-101 antagonizes alternative microenvironment stimuli
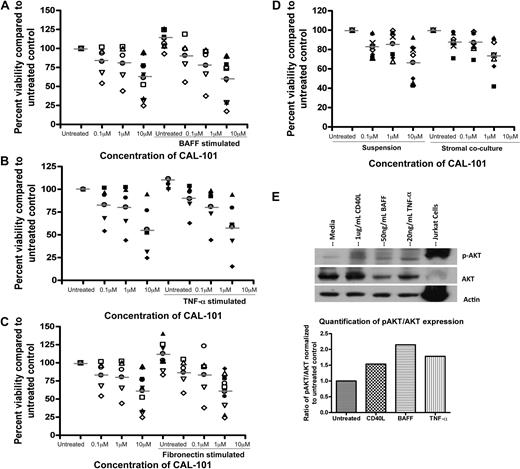
We next sought to further evaluate the role of CAL-101 in regulating alternative microenvironmental stimuli. The TNF family member BAFF has been shown in both mouse and human CLL to effectively prevent spontaneous apoptosis of CLL patient cells.39 Consistent with previous reports, BAFF ligation protected CLL cells from spontaneous apoptosis (Figure 7A). However, treatment with CAL-101 abrogated the protection induced by BAFF (Figure 7A). Similar to BAFF, TNF-α has been shown to protect CLL cells from spontaneous apoptosis, and TNF-α levels are frequently elevated in the plasma of patients with CLL.40 Because we showed previously that CAL-101 could inhibit the production of TNF-α from T cells, we assessed whether it could also prevent the direct effect of TNF-α on CLL cells. We found that TNF-α indeed protected CLL cells from spontaneous apoptosis and showed that treatment with CAL-101 abrogated this protection (Figure 7B). We next evaluated the effect of CAL-101 on fibronectin adhesion. Fibronectin has been shown to be a ligand for CD49d (α4β1 integrins/VLA4); thus, the protective effect elicited by fibronectin adhesion acts through a separate PI3K signaling cascade compared with ligation of TNF family members. We found that fibronectin adhesion also protected CLL cells from spontaneous apoptosis, and CAL-101 again completely abrogated the protective effect of fibronectin (Figure 7C). These findings further support the ability of CAL-101 for disrupting microenvironment signals. To confirm that the alterations in survival provided by these compounds were working through a PI3K-dependent pathway, we evaluated phosphorylation of AKT after stimulation. We found that BAFF, TNF-α, and fibronectin all lead to an increase in phosphorylation of AKT at the Ser473 site similarly to what was observed with CD40L treatment (Figure 7E; data not shown).
CAL-101 antagonizes alternative microenvironment stimuli activated by PI3K pathway. (A) CD19+ cells from patients with CLL (n = 5-10) were incubated with or without various doses of CAL-101 and 50 ng/mL BAFF for 48 hours. (B) CD19+ cells from patients with CLL (n = 5) were incubated with or without various doses of CAL-101 and 20ng/mL TNF-α for 48 hours. (C) CD19+ cells from patients with CLL (n = 5-10) were incubated with or without various doses of CAL-101 on and off fibronectin-coated plates for 48 hours. (D) CD19+ cells from patients with CLL (n = 7) were isolated from peripheral blood and incubated with or without 1 or 10μM CAL-101 in suspension or on an HS-5 cell layer for 48 hours. Viability was determined by annexin/PI flow cytometry and is shown relative to time-matched untreated controls for each group. Horizontal lines represent the mean. (E) CD19+ cells from patients with CLL (n = 4) were incubated with 1 μg/mL CD40L, 50 ng/mL BAFF, and 20 ng/mL TNF-α for 2 hours. AKT phosphorylation at Ser473 was assessed by immunoblot. Results are shown from 1 of 4 experiments.
CAL-101 antagonizes alternative microenvironment stimuli activated by PI3K pathway. (A) CD19+ cells from patients with CLL (n = 5-10) were incubated with or without various doses of CAL-101 and 50 ng/mL BAFF for 48 hours. (B) CD19+ cells from patients with CLL (n = 5) were incubated with or without various doses of CAL-101 and 20ng/mL TNF-α for 48 hours. (C) CD19+ cells from patients with CLL (n = 5-10) were incubated with or without various doses of CAL-101 on and off fibronectin-coated plates for 48 hours. (D) CD19+ cells from patients with CLL (n = 7) were isolated from peripheral blood and incubated with or without 1 or 10μM CAL-101 in suspension or on an HS-5 cell layer for 48 hours. Viability was determined by annexin/PI flow cytometry and is shown relative to time-matched untreated controls for each group. Horizontal lines represent the mean. (E) CD19+ cells from patients with CLL (n = 4) were incubated with 1 μg/mL CD40L, 50 ng/mL BAFF, and 20 ng/mL TNF-α for 2 hours. AKT phosphorylation at Ser473 was assessed by immunoblot. Results are shown from 1 of 4 experiments.
CLL viability in vivo is not only influenced by soluble factors but also by co-contact with a variety of cells that compose the bone marrow and lymph node microenvironment.41 This is becoming increasingly recognized as important because many drugs that are brought into the clinic have a lower in vivo effect than expected because of the cell survival-promoting mechanisms produced by the microenvironment.42 Because of this we sought to determine whether coincubation with CLL cells on a stromal cell line (HS-5) would affect the cytotoxic properties of CAL-101. We initially treated HS-5 cells with CAL-101 and determined it had no influence on stromal cell viability or structure (data not shown). Coculture of CLL cells on the HS-5 cell line showed diminished spontaneous apoptosis compared with cells cocultured in media alone (data not shown). However, CAL-101 treatment of CLL cells cocultured with HS-5 cells resulted in a similar proportion of cytotoxicity compared with treatment of CLL cells without coculture (Figure 7D; P = .499). These data suggest that CAL-101 has the potential to mediate cytotoxicity independent of the protective effect of contact with stromal cells.
Discussion
Herein, we have described a selective PI3K-δ inhibitor, CAL-101, that lacks off-target effects of other PI3K inhibitors (such as LY294002) but still induces apoptosis in primary CLL cells. All CLL patient B cells tested express PI3K-δ, and PI3K activity was higher in these cells than in B cells from healthy volunteers. Previous studies have shown that PI3K inhibitors induce apoptosis in a variety of cancer cells; however, these agents have shown global toxicities from off-target effects in nonhematopoietic cells. CAL-101 at low doses targets only the PI3K-δ isoform of PI3K, which is selectively expressed in hematopoietic cells. We have shown that CAL-101 has selective activity against B cells relative to T cells and NK cells as measured by cell death, although cytokine production of non-B cells is inhibited by CAL-101. The cytotoxic activity of CAL-101 in CLL occurred independently of the del(17p13.1) and IgVH mutation prognostic markers. Furthermore, this study suggests that CAL-101 mediates cytotoxicity both by directly inhibiting CLL cell PI3K signaling and also antagonizing extrinsic activation of this pathway by CD40L, BAFF, TNF-α, fibronectin, and stromal cells. Together, these data provide strong justification that CAL-101 will be beneficial in the treatment of CLL.
The importance of the PI3K signaling pathway to mature B cells has recently been established in a seminal study that showed its role in survival after B-cell stimulation.43 Similarly, a recent publication has shown that PI3K-δ is essential for the production of antibodies by nontransformed B cells.44 This study extends the importance of PI3K-δ signaling to transformed CLL cells in which we demonstrate that disruption of this signaling pathway directly promotes apoptosis and also antagonizes stromal cell interactions. The findings put forth by us contrast from a recent study by Niedermeier et al17 who suggest that inhibition of PI3K-α is the more essential isoform for effective disruption of microenvironmental signals and apoptosis. Although we demonstrate the PI3K-δ isoform is expressed in CLL cells, only minimal PI3K-α expression was shown (data not shown). CAL-101 is devoid of PI3K-α inhibitory activity, and yet apoptosis and pathway disruption from signaling by multiple cytokines were observed. In addition, treatment of CLL cells with pan-PI3K inhibitors such as LY294002 did not enhance apoptosis further over that observed with CAL-101. Given the complexities and potential problems of pan-PI3K inhibition from non–tumor-specific pathway inhibition, our findings and those reported also in normal B cells suggest that clinical exploration of PI3K-δ isoform–specific inhibitors could have significant benefit to patients with CLL and warrant future investigation.
In addition to demonstrating the effect of CAL-101 on primary CLL cells, herein, we were also able to demonstrate that this PI3K-δ–specific agent greatly diminishes production of several inflammatory cytokines, including TNF-α, CD40L, and IL-6 by T cells and IFN-γ by NK cells. Recent publications have shown the critical importance of PI3K-δ–specific signaling in T-cell45 and NK-cell30 cytokine production. Blocking production of these different cytokines in vivo in patients with CLL would potentially have the effect of antagonizing the survival effects of these cytokines on CLL cells. In addition, these cytokines are also produced after initial administration of several therapeutic antibodies used in CLL, including rituximab, alemtuzumab,and ofatumumab, and are associated with life-threatening infusion toxicity. Administration of CAL-101 with these therapeutic antibodies may diminish this infusion toxicity by abrogating production of these cytokines. In contrast, we demonstrate that CAL-101 does not interfere with antibody-mediated ADCC with rituximab and alemtuzumab. On the basis of the unique mechanism of action of CAL-101, exploration of such combination strategies with therapeutic antibodies is indicated.
In CLL primary cells we demonstrate low levels of Ser473 AKT phosphorylation in most patients examined, which is increased after CD40 ligation and stromal contact, which in turn protects CLL cells from spontaneous apoptosis in vitro.46 We also found that other microenvironmental stimuli (such as BAFF, TNF-α, fibronectin, and IL-6) act in a similar manner to promote phosphorylation of downstream targets of PI3K and thus prevent spontaneous apoptosis in vitro. At submicromolar concentrations of CAL-101 we observe significant antagonism of these survival signals. This key finding suggests that CAL-101 may inhibit downstream phosphorylation events initiated by multiple survival factors in a fashion similar to what we have shown with CD40L-mediated signaling. Collectively, these findings suggest that CAL-101 may effectively antagonize multiple mechanisms of CLL survival provided by nodal and bone marrow stromal cells. Finally, the ability of CAL-101 to induce apoptosis and to antagonize multiple environmental stimuli shows true potential for the treatment of CLL and related lymphoid malignancies. Other compounds targeting proximal or distal signaling involved in PI3K have shown clinical activity in patients with CLL, including the Syk inhibitor fostamatinib disodium47 and the mTOR (mammalian target of rapamycin) inhibitor RAD001,48,49 providing strong rational for targeting this pathway. The data put forward here from our laboratory data clearly justify clinical development of CAL-101 in CLL and related lymphoid malignancies, which is now well under way.50
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Leukemia & Lymphoma Society (grants P50-CA140158, PO1-CA95426, PO1 CA81534, 1K12 CA133250), the D. Warren Brown Foundation, and Calistoga Pharmaceuticals. A.J.J. is a Paul Calabresi Scholar.
National Institutes of Health
Authorship
Contribution: S.E.M.H. planned the research, performed experiments, analyzed data, drafted the first and subsequent drafts of the paper, and approved the final version of the paper; A.L.G. and A.J.W. were involved in planning components of the research, performing experiments, reviewed drafts, and approved the final version of the paper; N.A.H. and W.Z. were involved in determining cytogenetics or mutational status of patient samples, reviewed drafts, and approved the final version of the paper; J.M.F., L.A., and J.J. accrued patients to the CLL clinical trial, reviewed drafts of the paper, and approved the final version of the paper; X.Z. and L.W. were involved in planning components of the research, did all the statistical analysis, reviewed drafts, and approved the final version of the paper; B.J.L., K.D.P., and N.A.G. were involved in planning components of the research, provided necessary reagents essential to the hypothesis of this paper, reviewed drafts, and approved the final version of the paper; and J.C.B. and A.J.J. planned every aspect of the proposal, supervised the research, analyzed data, reviewed drafts, obtained funding for the research work, and approved the final version of the paper.
Conflict-of-interest disclosure: B.J.L., K.D.P., and N.A.G. are employees of Calistoga Pharmaceuticals and have financial interests in CAL-101 development; J.C.B. has been a consultant to Calistoga and has financial interests in CAL-101 development. The remaining authors declare no competing financial interests.
Correspondence: Amy J. Johnson, OSUCCC Bldg, Rm 455C, The Ohio State University, 410 W 12th Ave, Columbus, OH 43210; e-mail: amy.johnson@osumc.edu.