Abstract
Despite promising clinical results from imatinib mesylate and second-generation ABL tyrosine kinase inhibitors (TKIs) for most BCR-ABL+ leukemia, BCR-ABL harboring the mutation of threonine 315 to isoleucine (BCR-ABL/T315I) is not targeted by any of these agents. We describe the in vitro and in vivo effects of AT9283 (1-cyclopropyl-3[5-morpholin-4yl methyl-1H-benzomidazol-2-yl]-urea), a potent inhibitor of several protein kinases, including Aurora A, Aurora B, Janus kinase 2 (JAK2), JAK3, and ABL on diverse imatinib-resistant BCR-ABL+ cells. AT9283 showed potent antiproliferative activity on cells transformed by wild-type BCR-ABL and BCR-ABL/T315I. AT9283 inhibited proliferation in a panel of BaF3 and human BCR-ABL+ cell lines both sensitive and resistant to imatinib because of a variety of mechanisms. In BCR-ABL+ cells, we confirmed inhibition of substrates of both BCR-ABL (signal transducer and activator of transcription-5) and Aurora B (histone H3) at physiologically achievable concentrations. The in vivo effects of AT9283 were examined in several mouse models engrafted either subcutaneously or intravenously with BaF3/BCR-ABL, human BCR-ABL+ cell lines, or primary patient samples expressing BCR-ABL/T315I or glutamic acid 255 to lysine, another imatinib-resistant mutation. These data together support further clinical investigation of AT9283 in patients with imatinib- and second-generation ABL TKI-resistant BCR-ABL+ cells, including T315I.
Introduction
Philadelphia (Ph) chromosome results from a reciprocal translocation between chromosomes 9 and 22 and generates the BCR-ABL chimera protein, the cause of chronic myeloid leukemia (CML) and Ph+ acute lymphoid leukemia (ALL). The ABL tyrosine kinase inhibitor (TKI), imatinib mesylate, has dramatically changed the first-line therapy of CML.1 Most patients with newly diagnosed CML with chronic phase, when treated with imatinib, achieve durable responses. However, emergence of refractory disease and relapse have frequently been reported, particularly in patients with CML with advanced-stage disease and patients with Ph+ ALL.2,3 Among several mechanisms of resistance, point mutations within the ABL kinase domain that interfere with imatinib binding are the most critical cause of imatinib resistance.4,5
To overcome these imatinib resistance mechanisms, 4 second-generation ABL TKIs have been developed: dasatinib,6 nilotinib,7 bosutinib8 and bafetinib (formerly INNO-406).9,10 Despite promising clinical results from these second-generation ABL TKIs for most patients with imatinib-resistant BCR-ABL+ leukemia, the mutation of threonine 315 to isoleucine (T315I) confers resistance to all these TKIs.11,12 Thus, identification of novel agents for the effective treatment of patients with CML with T315I is an important and challenging task.13 Aurora kinases A and B are a family of serine/threonine kinases involved in many cellular functions.14-16 Inappropriate expression of these enzymes in certain cancers may result in aneuploidy and carcinogenesis.17 Consequently, the potential therapeutic value of targeting Aurora kinases has become a focus of anticancer therapy.14
Recently, we identified an Aurora kinase inhibitor, AT9283 (1-cyclopropyl-3[5-morpholin-4yl methyl-1H-benzomidazol-2-yl]-urea) by way of structure-based optimization of a ligand-efficient pyrazole-benzimidazole fragment. X-ray crystallographic structures were generated with the use of a novel soakable form of Aurora A and were used to drive the optimization toward potent (half-maximal inhibition constant [IC50] < 3nM) dual Aurora A/B inhibitor. AT9283 that also potently inhibits several kinases, including Janus kinase-2 (JAK2) and JAK3 (1.2 and 1.1nM, respectively), c-ABL (110nM), and ABL/T315I (4nM), is currently under evaluation in phase 1 clinical trials for metastatic solid tumors and hematologic malignancies.18,19
Here, we report the putative mechanism by which AT9283 binds to BCR-ABL/T315I and its activity against imatinib-resistant BCR-ABL+ leukemic cells, including those with the T315I mutation.
Methods
Reagent and cell lines
AT9283 was synthesized by Astex Therapeutics Ltd (Figure 1A).18 Human CML cell lines (K562, MEG-01, BV173, KU812, MYL, KT-1, and KBM-5), human acute myeloid leukemia cell lines (HL60, KG1a), human ALL (Jurkat, Nalm6), and mouse pro-B cell line (BaF3) were used. KBM-5/STIR, which was the subclone of KBM-5 with T315I, and BaF3/wild type (wt) BCR-ABLp190 were also used. Source of all cell lines used are outlined in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). BaF3/wt-BCR-ABLp210 and BaF3 cells harboring imatinib-resistant mutations (glycine 250 to glutamic acid, glutamine 252 to histidine, tyrosine 253 to phenylalanine, glutamic acid 255 to lysine [E255K]), methionine 294 to valine, T315I, threonine 315 to alanine, phenylalanine 317 to leucine, phenylalanine 317 to valine, methionine 351 to threonine, and histidine 396 to proline) in the BCR-ABLp210 kinase domain were established as previously described.9 All cell lines were cultured in RPMI 1640 (Nissui) with 2mM l-glutamine (Nacalai Tesque) and 10% fetal bovine serum (Vitromex) and were maintained at 37°C in a fully humidified atmosphere of 5% CO2. For the culture of parental BaF3 cells, 1 ng/mL murine interleukin-3 (Sigma-Aldrich) was added to the medium. Cells undergoing exponential growth were used in the experiments.
Bone marrow cells from healthy donors and patients with CML in chronic phase were collected with written informed consents according to the Declaration of Helsinki. Primary leukemic cells were obtained from 2 patients with Ph+ ALL who relapsed after imatinib mesylate treatment because of E255K or T315I.20 These patients' peripheral blood contained more than 90% leukemic cells. Approval for primary leukemic cells was obtained from the institutional review board at Frankfurt University Hospital.
Docking model of AT9283 bound within the active site of c-ABL/T315I
A model of c-ABL/T315I was prepared in QUANTA (Accelrys Inc) by simple Thr>Ile mutation of residue 315 in an inhouse DFG-in, wt c-ABL structure. Docking of AT9283 into the active site of the c-ABL/T315I model was carried out with the use of a proprietary version of GOLD,21 using the Chemscore scoring function.22
Proliferation assays
Proliferation of the cell lines was determined with a modified 3-(4,5-dimethylthiazol -2-yl)-2,5-diphenyl-2H tetrazolium bromide assay with the SF reagent (Nacalai Tesque) as previously described.23 Leukemic cell lines were cultivated in a flat-bottomed 96-well plate (Greiner Labortechnik) at 1 × 104 (BaF3 series), 5 × 104 (BV173, KBM-5, KBM-5/STIR), or 1 × 105 (MEG01, KU812, HL60) cells per well in 100 μL of medium and incubated with various concentrations of AT9283 for 72 hours. The means of 5 data values for each treatment were calculated. IC50s were determined with the use of the nonlinear regression program CalcuSyn (Biosoft).24
Western blotting
BCR-ABL+ or BCR-ABL− cell lines were seeded at a concentration of 1 × 106/mL media onto 6-well tissue culture plates and allowed to recover for 16 hours. AT9283, at the indicated concentration, or vehicle control (0.1% dimethyl sulfoxide) were added for 24 hours. Cells were harvested and lysed in 100 μL of ice-cold Triton lysis buffer (0.1% vol/vol Tx-100). Lysates were cleared by centrifugation, and a sample of the supernatant was removed for protein determination. Equivalent amounts of protein lysate had sodium dodecylsulfate sample buffer added and were boiled for 5 minutes. Samples were resolved by sodium dodecylsulfate–polyacrylamide gel electrophoresis (NuPage system; Invitrogen) and blotted onto polyvinylidene difluoride filters. Immunoblotting was performed with specific antibodies for phospho (p) histone H3 (HH3)(Ser10) and total (t)HH3, p signal transducer and activator of transcription-5 (STAT5)(Tyr694), tSTAT5, pCrkL(Tyr207), tCrkL, p extracellular signal-regulated kinase (ERK)(Thr202/Tyr204), tERK, pAKT(Thr308), tAKT, pBCR-ABL(Tyr177), tBCR-ABL, pAurora A(Thr288), tAurora A, pAuroa B(Thr232), and tAurora B. All antibodies were obtained from Cell Signaling Technology. Detection was achieved with the use of Odyssey Infra Red Imaging, IR Dye secondary antibodies, and a Licor Odyssey Imager (Li-Cor Bioscience Ltd).
In vivo activity of AT9283 in mice bearing BCR-ABL+ leukemic cells
BaF3/wt-BCR-ABLp210, BaF3/T315I, or human CML K562 xenografts used male BALB/c nu/nu mice and were performed according to the United Kingdom Animals (Scientific Procedures) Act 1986. Animals were purchased from Harlan UK Ltd and housed in pathogen-free conditions. Six- to 8-week-old male BALB/c Hsd:athymic nude-Foxn1nu mice were implanted subcutaneously with 1 × 107 BaF3/wt-BCR-ABL, 1 × 107 BaF3/T315I, or 1 × 107 K562 cells per mouse into the right flank. Five days after implantation mice were arranged into groups of 8 according to tumor volume with a mean volume of 100 mm3. AT9283 was prepared in a vehicle of 10% dimethyl sulfoxide, 20% water, and 70% 2-(hydroxypropyl)-beta-cyclodextrin (25% wt/vol). Mice were then dosed according to schedule. Tumor volume was measured every 2 to 3 days. In each case a statistically significant slowing of increase in xenograft volume or regression of tumor volume over time compared with a matched control group was used to characterize efficacy. A complete regression was defined as a decrease in tumor volume to an undetectable size, taken as measurements of less than 3 mm in any dimension. Tolerability was estimated by standard criteria of behavioral observations, body weight loss less than 5%, and survival over the course of the study.
For the experiments to investigate the effects of AT9283 on the primary patient samples with BCR-ABL harboring E255K or T315I, nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice were used with the approval from the institutional review board at Kyoto University Hospital. Male NOD/SCID mice 4 to 6 weeks of age (Japan Clea) were individually sublethally irradiated (2 Gy) and inoculated intravenously with 5.0 × 105 primary leukemic cells harboring either BCR-ABL/E255K or T315I as previously described.20 Briefly, engraftments of inoculated primary human BCR-ABL+ cells at day 7 after transplantation were confirmed by polymerase chain reaction analysis, and treatments with AT9283 were initiated on day 7. Furthermore, the numbers of engrafted BCR-ABL+ cells were monitored by analysis of the percentage of human leukemic cells in mouse peripheral blood by flow cytometry as previously described.20,23 The mice were randomized into the following groups: E255K [each group, n = 7, as follows: (1) untreated mice, (2) mice treated with 6.25 mg/kg AT9283 twice daily] and T315I [each group, n = 5, as follows: (1) untreated mice, (2) mice treated with 10 mg/kg AT9283 daily, and (3) mice treated with 15 mg/kg AT9283 daily]. At day 7, treatment was administered intraperitoneally either twice daily 5 days on and 2 days off for 4 weeks at the doses of 6.25 mg/kg (E255K) or daily 4 days on and 3 days off continuously at the doses of 10 mg/kg and 15 mg/kg (T315I). For survival analysis, death was determined either by spontaneous death or elective killing because of pain or suffering according to established criteria.
Results
Identification and in vitro activity of AT9283
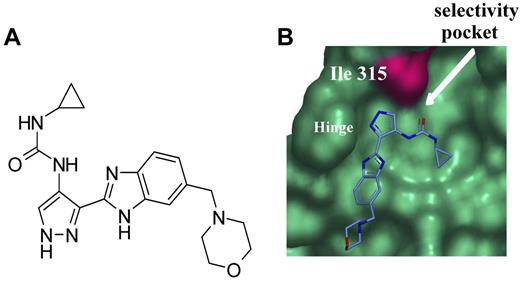
By analogy with the binding mode of AT9283 with Aurora A,18 it is predicted that AT9283 does not form a hydrogen bond with T315 in BCR-ABL in the way that inhibitors, including imatinib, do. Binding of AT9283 is therefore less likely to be affected by the T315I mutation (supplemental Figure 1A). Furthermore, AT9283 does not bind within the selectivity pocket behind the gatekeeper residue, meaning that its potency for ABL kinase is not abrogated by mutation of this residue to the bulkier isoleucine. Imatinib binding is sterically hindered by the presence of isoleucine at amino acid 315, whereas AT9283 is not (Figure 1B).
Chemical structure of AT9283 and model of AT9283 bound with ABL. (A) Chemical structure of AT9283. (B) AT9283 does not make a hydrogen bond interaction with T315 in the same way other kinase inhibitors in the class do. The pocket behind the T315 gatekeeper is not occupied by AT9283. Its potency for ABL kinase is not abrogated by mutation of this residue to the bulkier isoleucine (T315I).
Chemical structure of AT9283 and model of AT9283 bound with ABL. (A) Chemical structure of AT9283. (B) AT9283 does not make a hydrogen bond interaction with T315 in the same way other kinase inhibitors in the class do. The pocket behind the T315 gatekeeper is not occupied by AT9283. Its potency for ABL kinase is not abrogated by mutation of this residue to the bulkier isoleucine (T315I).
Cell-based activity of AT9283 in BCR-ABL–dependent cell lines
AT9283 inhibited proliferation of a panel of BaF3 cell lines transformed with either wt-BCR-ABL fusions or a variety of mutant forms, including T315I (Table 1). AT9283 inhibited all lines with similar potency in the range of 10 to 21nM. The IC50 of AT9283 for the BCR-ABL− parental BaF3 was 103nM, around 5 times higher than that for BaF3 cells harboring wt or mutated BCR-ABL. In addition, we observed that, although multinucleated cells, the hallmark of Aurora B inhibition, were visible microscopically (data not shown), this phenotype did not dominate, and we were able to generate cytotoxic IC50 values in the absence of intervention of the polyploid phenotype that maintains cells in a viable state for a longer period of time. These data suggest that additional activities of AT9283, which most likely include BCR-ABL inhibition, are responsible for some of the activity of the compound in vitro.
Antiproliferative activity of AT9283 in a panel of BaF3 BCR-ABL cell lines
| BaF3 . | AT9283 IC50, nM . |
|---|---|
| Parental (BCR-ABL−) | 103 |
| wt p190 | 16 |
| wt p210 | 13 |
| Y253F | 16 |
| T315I | 11 |
| T315A | 10 |
| Q252H | 21 |
| M351T | 18 |
| M294V | 18 |
| H396P | 21 |
| G250E | 12 |
| F317V | 14 |
| F317L | 15 |
| E255K | 13 |
| BaF3 . | AT9283 IC50, nM . |
|---|---|
| Parental (BCR-ABL−) | 103 |
| wt p190 | 16 |
| wt p210 | 13 |
| Y253F | 16 |
| T315I | 11 |
| T315A | 10 |
| Q252H | 21 |
| M351T | 18 |
| M294V | 18 |
| H396P | 21 |
| G250E | 12 |
| F317V | 14 |
| F317L | 15 |
| E255K | 13 |
Y253F indicates tyrosine 253 to phenylalanine; T315A, threonine 315 to alanine; Q252H, glutamine 252 to histidine; M351T, methionine 351 to threonine; M294V, methionine 294 to valine; H396P, histidine 396 to proline; G250E, glycine 250 to glutamic acid; F317V, phenylalanine 317 to valine; and F317L, phenylalanine 317 to leucine.
Similar observations were made in human imatinib-sensitive CML cell lines. These observations are again consistent with the BCR-ABL inhibitory activity of AT9283 contributing to the activity of the compound in CML cell lines harboring the translocation. We observed the dominant Aurora B phenotype of polyploidy in the K562 CML cell line and BCR-ABL− cell lines such as HL-60, Jurkat, Nalm6, and KG1a. In these cell lines, the dominant phenotype of Aurora B inhibition (polyploidy) was observed in contrast to other CML lines tested. AT9283 was active not only in BaF3/T315I, which was artificially generated, but also in the human CML subclone, KBM-5/STIR, which harbored the T315I mutation (Table 2).
Antiproliferative activity of AT9283 in a panel of human CML cell lines
| Cell line . | BCR-ABL status . | Additional characteristics . | IC50, nM . |
|---|---|---|---|
| Imatinib sensitive | |||
| BV173 | + | 55 | |
| KU812 | + | 26 | |
| MYL | + | 21 | |
| KT-1 | + | 81 | |
| KBM-5 | + | 84 | |
| MEG-01 | + | 31 | |
| K562 | + | Polyploidy at 100 | |
| Imatinib resistant | |||
| KBM-5/STIR | + | T315I mutation | 16 |
| BV173/shBim | + | Bim knockdown | 12 |
| HL60 | − | Polyploidy at 30 | |
| Jurkat | − | Polyploidy at 500 | |
| Nalm6 | − | Polyploidy at 140 | |
| KG1a | − | Polyploidy at 55 |
| Cell line . | BCR-ABL status . | Additional characteristics . | IC50, nM . |
|---|---|---|---|
| Imatinib sensitive | |||
| BV173 | + | 55 | |
| KU812 | + | 26 | |
| MYL | + | 21 | |
| KT-1 | + | 81 | |
| KBM-5 | + | 84 | |
| MEG-01 | + | 31 | |
| K562 | + | Polyploidy at 100 | |
| Imatinib resistant | |||
| KBM-5/STIR | + | T315I mutation | 16 |
| BV173/shBim | + | Bim knockdown | 12 |
| HL60 | − | Polyploidy at 30 | |
| Jurkat | − | Polyploidy at 500 | |
| Nalm6 | − | Polyploidy at 140 | |
| KG1a | − | Polyploidy at 55 |
Induction of apoptosis and alteration of cell cycle by AT9283
Apoptosis was dose-dependently induced in all cell lines tested, including BaF3/wt-BCR-ABLp210, BaF3/T315I, BaF3/E255K, K562, and BV173. AT9283 induced fewer apoptotic cells in K562 than the other 4 CML cell lines (supplemental Figure 2). Exposure of K562 cells to cytotoxic IC50 concentrations (100nM) of AT9283 resulted in the appearance of a large multinucleated cell population. Interestingly, exposure of the other 4 cell lines tested to their respective cytotoxic IC50 concentrations of AT9283 did not result in the appearance of polyploid cells. The dominant effect in 7 BCR-ABL+ cell lines except K562 was the induction of apoptosis as indicated by a significantly larger increase in subG1 DNA compared with 3 BCR-ABL− cell lines at 24 hours and 48 hours (supplemental Figures 3-4). If the concentration of AT9283 was increased to 3 times their respective IC50 concentrations, a small polyploid population could be observed in the BaF3 and BV173 cells, but the dominant phenotype remained an increase in the apoptotic population. These observations are consistent with the Aurora inhibitory activity of the compound dominating in BCR-ABL lines and K562 cells, resulting in apoptosis only after an increased exposure time and several rounds of endo-reduplication. In the BaF3/BCR-ABL and in other human CML cell lines, AT9283 results in an early apoptotic response in the absence of the classical Aurora inhibitory phenotype. This response is most likely due to the contribution of ABL inhibition in these cells (supplemental Figures 3-4).
Mechanism of action of AT9283 in BCR-ABL+ cell lines
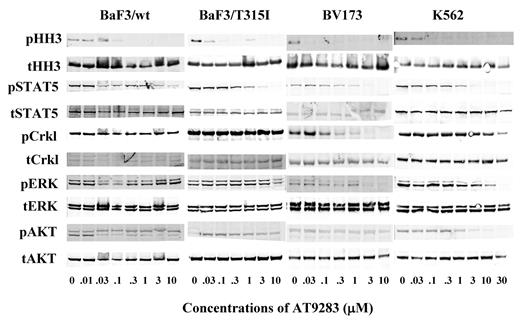
After 24 hours of incubation of BaF3/BCR-ABL wt or T315I cells with AT9283, inhibition of the signaling pathway downstream of Aurora B and BCR-ABL were examined. Phosphorylation of the Aurora B substrate, pHH3 was inhibited by AT9283 at concentrations greater than 30nM in all cell lines examined (Figure 2). Activity of the BCR-ABL fusion was determined by phosphorylation of the substrate STAT5. AT9283 inhibited phosphorylation of STAT5 at concentrations above 300nM in both BaF3/wt-BCR-ABLp210 and BaF3/T315I (Figure 2). Similar data were obtained in the human CML cell lines BV173 and K562 above 300nM and 1000nM, respectively. AT9283 inhibited phosphorylation of the BCR-ABL substrate CrkL only in BV173 at concentrations consistent with inhibition of pSTAT5. Incomplete inhibition of phosphorylation of AKT was observed in all cell lines with the most striking effects observed in BV173 cells and K562 cells above 100nM. Phospho-ERK inhibition was observed in BV173 and K562 cells but only at concentrations above 3000nM (Figure 2). In addition to the analysis of downstream signaling of AT9283 kinase targets, we also examined the effects of AT9283 on the phosphorylation status of BCR-ABL, Aurora A, and Aurora B themselves. AT9283 inhibited phosphorylation of BCR-ABL, Aurora A, and Aurora B in BV173, BCR-ABL+ cells, at 1.0, 0.1, and 0.03μM, respectively (supplemental Figure 5). In Jurkat, BCR-ABL− cells, AT9283 also inhibited phosphorylation of Aurora A and Aurora B at 0.3 and 0.3μM, respectively (data not shown). AT9283 inhibited the phosphorylation of HH3 and ERK but AKT in 3 BCR-ABL− cell lines (supplemental Figure 6). These data suggest that of the signaling pathways studied AT9283 inhibits both the Aurora kinases and BCR-ABL/STAT5 signaling pathways. Inhibition of pCrkL was also observed in the human CML cell line BV173. In other cell lines tested this was less clear and probably reflects the fact that CrkL signaling is regulated by different or additional kinases in BaF3/BCR-ABL lines and in the CML line K562. The latter responds to AT9283 in a manner atypical with respect to the other human CML lines tested, perhaps indicating that it harbors additional mutations or signaling aberrations that impinge on its survival and overall response to AT9283.
Mechanism of action of AT9283 in BaF3 BCR-ABL cells and human CML cell lines. BaF3/wt-BCR-ABL, BaF3/T315I, BV173, and K562 were incubated with the indicated concentration of AT9283, or vehicle control, for 24 hours before preparation for immunoblotting with the indicated antibodies. The blots shown are representative of at least 2 independent experiments in each cell line.
Mechanism of action of AT9283 in BaF3 BCR-ABL cells and human CML cell lines. BaF3/wt-BCR-ABL, BaF3/T315I, BV173, and K562 were incubated with the indicated concentration of AT9283, or vehicle control, for 24 hours before preparation for immunoblotting with the indicated antibodies. The blots shown are representative of at least 2 independent experiments in each cell line.
Effects of AT9283 in normal hematopoietic progenitors and primary CML cells
The number of colony forming units (CFUs) observed after AT9283 treatment of cells derived from 3 healthy individual donors and 3 patients with CML were examined by colony assay at day 14 to day 16. When normal progenitors were treated with 10, 30, 50, 70, and 100nM AT9283, the CFUs were 0.98% (± 0.06%), 0.62% (± 0.01%), 0.35% (± 0.01%), 0.18% (± 0.01%), 0.11% (± 0.1%), and 0% (± 0%) of the control, respectively. Although when primary CML cells were treated with 10, 30, 50, 70, and 100nM AT9283, the CFUs were 0.45% (± 0.1%), 0.23% (± 0.02%), 0.06% (± 0.02%), 0% (± 0%), 0% (± 0%), and 0% (± 0%) of the control, respectively (supplemental Figure 7). These percentages are the mean plus or minus SE between the 3 persons. These findings indicate that AT9283 was approximately 5 times more effective at inhibiting colony formation with cells derived from patients with CML than from healthy volunteers.
In vivo efficacy of AT9283 in subcutaneous BaF3 BCR-ABL xenograft models
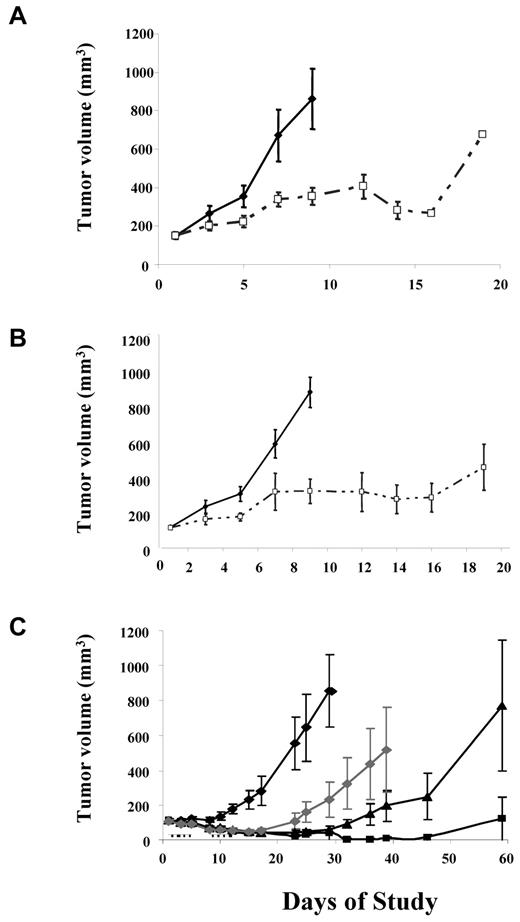
Two cycles of 12.5 mg/kg AT9283 daily for 5 days followed by a 2-day break inhibited tumor growth in subcutaneous xenograft models with BaF3 cells transfected with either BCR-ABL wt (Figure 3A) or T315I (Figure 3B) without obvious adverse effects. Moreover, AT9283 inhibited the growth of human CML cell line K562 xenografts in a dose-dependent fashion after twice daily dosing for 5 days in a 7-day period. Tumor regressions were observed at 12.5 mg/kg, the highest dose, with 4 of 8 mice remaining tumor free at 90 days after initiation of treatment (Figure 3C). This dose schedule was optimized in solid tumor xenograft models.19
In vivo efficacy of AT9283 in BCR-ABL+ cell line xenograft. Nude mice bearing either BaF3/wt-BCR-ABLp210 (A) or BaF3/T315I (B) xenografts were administered the indicated doses of AT9283 by the intraperitoneal route. Vehicle (♦) and 12.5 mg/kg AT9283 (□) was dosed twice daily for 5 days followed by a 2-day break. The dose cycle was repeated twice in each case. Nude mice bearing human CML cells, K562 (C) xenografts were also administered the indicated doses of AT9283 by the intraperitoneal route. Vehicle (♦), 12.5 mg/kg (■), 10 mg/kg (□), and 7.5 mg/kg (♦) AT9283 was dosed twice daily for 5 days followed by a 2-day break. The dose cycle was repeated twice in each case. Mean growth curves ± SEs are shown for groups of 8 mice in each instance.
In vivo efficacy of AT9283 in BCR-ABL+ cell line xenograft. Nude mice bearing either BaF3/wt-BCR-ABLp210 (A) or BaF3/T315I (B) xenografts were administered the indicated doses of AT9283 by the intraperitoneal route. Vehicle (♦) and 12.5 mg/kg AT9283 (□) was dosed twice daily for 5 days followed by a 2-day break. The dose cycle was repeated twice in each case. Nude mice bearing human CML cells, K562 (C) xenografts were also administered the indicated doses of AT9283 by the intraperitoneal route. Vehicle (♦), 12.5 mg/kg (■), 10 mg/kg (□), and 7.5 mg/kg (♦) AT9283 was dosed twice daily for 5 days followed by a 2-day break. The dose cycle was repeated twice in each case. Mean growth curves ± SEs are shown for groups of 8 mice in each instance.
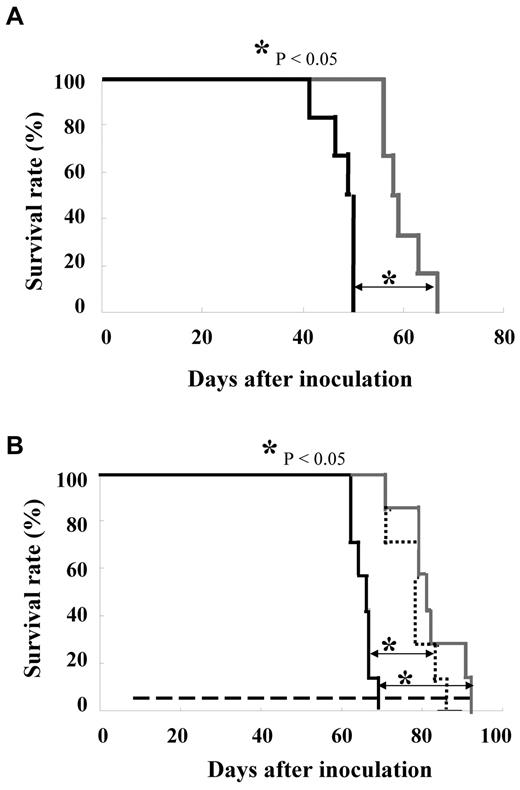
The activity of AT9283 was investigated in intravenously transplanted models with the use of primary BCR-ABL+ blasts isolated from patients with Ph+ ALL who showed imatinib acquired resistance by harboring 2 of the mutations most resistant to common kinase inhibitor therapies, E255K and T315I. Blasts isolated from patients were injected into the tail vein of NOD/SCID mice and allowed to engraft for a period of 7 days. In the case of the BCR-ABL/E255K model animals were dosed with either vehicle or 6.25 mg/kg AT9283 twice daily for 5 days followed by a 2-day break. This schedule was repeated for 4 cycles and resulted in a significant survival advantage of 17 days over control (Figure 4A). Initially a twice daily schedule was used on the basis of past experience with AT9283 and the optimum schedule in nude mice bearing human tumor xenografts derived from solid tumors. In the course of the studies, we determined that a once daily schedule at the indicated doses allowed indefinite continuous dosing with an improved tolerability profile in NOD/SCID mice and at least as good if not better efficacy in the models described. Thus, for the T315I model, animals were dosed once per day at either 10 or 15 mg/kg. In this case animals continued dosing through to the end of the experiment. In this model a significant survival advantage (P < .01) of 17 and 23 days for the 10- and 15-mg/kg groups, respectively, was obtained (Figure 4B). Fluorescence-activated cell sorting analysis, performed on the peripheral blood of mice inoculated with leukemic cells, showed that a number of these cells remained even after dosing with AT9283 (data not shown). The numbers of leukemic cells observed was fewer than that in vehicle-treated mice, consistent with the slower progression of the disease in these animals. In addition, we confirmed the existence of T315I clones both in peripheral blood of AT9283-treated and vehicle-dosed mice by the modified guanine quenching probe method (supplemental Figure 8). In several mice we performed postmortem examinations for the presence of leukemic cells in liver and spleen with the use of the same method (data not shown). Tissue samples from both vehicle- and AT9283-treated mice were shown to be positive for the T315I clone, showing that, although AT9283 significantly slowed the proliferation of leukemic cells and prolonged the survival of the mice, it could not completely suppress the growth of leukemic cells in this model.
In vivo efficacy of AT9283 in primary samples. Nonlethally irradiated NOD/SCID mice were intravenously inoculated with cells taken from patients with CML harboring BCR-ABL E255K (A) or BCR-ABL T315I (B). Either 6.25 mg/kg AT9283 (gray) or vehicle (black) controlled was administered twice daily (A) on the indicated schedules (- - -). We administered 15 mg/kg AT9283 (gray), 10 mg/kg At9283 (black dot), or vehicle (black) controlled once daily (B) on the indicated schedules (- - -). Kaplan-Meier survival curves show that treatment with 6.25 mg/kg AT9283 twice daily resulted in a significant survival advantage (P = .008) over vehicle-treated animals of 17 days in the E255K model. Similarly, in the T315I model 10 mg/kg per day or 15 mg/kg per day AT9283 resulted in a marked survival advantage (P = .002).
In vivo efficacy of AT9283 in primary samples. Nonlethally irradiated NOD/SCID mice were intravenously inoculated with cells taken from patients with CML harboring BCR-ABL E255K (A) or BCR-ABL T315I (B). Either 6.25 mg/kg AT9283 (gray) or vehicle (black) controlled was administered twice daily (A) on the indicated schedules (- - -). We administered 15 mg/kg AT9283 (gray), 10 mg/kg At9283 (black dot), or vehicle (black) controlled once daily (B) on the indicated schedules (- - -). Kaplan-Meier survival curves show that treatment with 6.25 mg/kg AT9283 twice daily resulted in a significant survival advantage (P = .008) over vehicle-treated animals of 17 days in the E255K model. Similarly, in the T315I model 10 mg/kg per day or 15 mg/kg per day AT9283 resulted in a marked survival advantage (P = .002).
Discussion
To date, at least 90 different point mutations in the BCR-ABL kinase domain have been isolated from patients with BCR-ABL+ leukemia who are resistant to imatinib.25,26 In addition to imatinib, other novel ABL TKIs are ineffective against the T315I mutant clone. This suggests that more patients with the T315I clone will emerge now that treatment with TKIs has become the standard of care in CML. The observation that T315I mutations were most frequently observed in dasatinib-resistant patients appears to support this observation.27 Some studies have suggested that patients with T315I have a poor prognosis, with a median survival of 12.6 months from the start of imatinib therapy.28,29 Therefore, there is much interest in developing novel agents effective against BCR-ABL/T315I clone.
To understand the activity of AT9283 versus BCR-ABL/T315I, we must first understand the mechanisms of resistance to existing agents in the adenosine triphosphate–binding pocket of BCR-ABL/T315I (Figure 1; supplemental Figure 1). As the explanations of resistance of ABL/T315I to imatinib, there are 3 hypotheses such as alteration of the 3-dimensional structure of the adenosine triphosphate pocket,30,31 the consequence of a conformational readjustment necessary to accommodate the mutant residue,32 and the breakdown of interactions between imatinib and both E286 and M290.33 In the case of AT9283, the simplest explanation for the tolerance of the T315I is 2-fold: the lack of any hydrogen bond formed by this compound with the side-chain of T315, and the compound's avoidance of the selectivity pocket behind the gatekeeper residue. Put simply, the binding mode and key interactions formed by AT9283 are probably largely independent of the nature of the residue at position 315 (Figure 1B; supplemental Figure 1).
Note that the structure of the kinase domain of c-ABL in complex with a T315I-sensitive Aurora kinase inhibitor, MK-0457 (formerly VX-680), has already been reported.34 The inhibition by both MK-0457 and PHA-739358 of the T315I mutant also arises because of their particular binding modes, which also avoid the gatekeeper region.35 MK-0457 was found to be active against BCR-ABL/T315I,34 and a phase 2 trial on MK-0457 for patients with CML with T315I showed some efficacy.36 Unfortunately, the development of MK-0457 was halted for commercial reasons. QTc prolongation37 or any adverse events was not involved in the closing of the phase 2 study, which confirmed significant activity in patients with T315I. PHA-739358 also has been reported to have strong antiproliferative and proapoptotic activity against BCR-ABL including T315I.38 A phase 2 trial of PHA-739358 in patients with CML who have relapsed after BCR-ABL therapy is ongoing.
Because AT9283 is a multitargeted inhibitor with activities that include ABL and the Aurora kinases, it is important to understand which activities are key in driving the effects in CML cells. AT9283 inhibited both BCR-ABL and Aurora signaling (Figure 2; supplemental Figures 5-6). In addition, cell death observed in BCR-ABL+ cell lines, treated with AT9283, is consistent with inhibition of BCR-ABL activity and is observed in the absence of the accumulation of large numbers of polyploid cells normally associated with Aurora inhibition in other cell lines. However, we do observe small numbers of multinucleated cells, suggesting that the Aurora activity of AT9283 is manifest in the background (Table 2; supplemental Figures 3-4). AT9283 is multitargeted in nature, and no single activity is the sole driver of its effect. We show potent inhibition of Aurora A and B; however, the BCR-ABL+ cell lines do exhibit a different phenotype to the classic Aurora phenotype described in our previous study.14 It is this combinatorial nature of the effect that suggests we may have a beneficial effect in the patient population with T315I for which there is at present no effective treatment.
In vivo models of CML have shown activity of AT9283 in a cell line grown as a subcutaneous xenograft (K562; Figure 3) and in models in which leukemic cells harboring E255K or T315I from patients were intravenously inoculated into NOD/SCID mice (Figure 4). In these models, 2 dosing regimens, twice daily and once daily, were used. In the nude mouse, there was no significant difference between twice daily and once daily dosing on tumor growth inhibition or tolerability (data not shown). In primary BCR-ABL+ tumor models a larger AT9283 dose administered once daily was tolerated better than a lower dose administered twice daily with no loss of efficacy. This could relate to the mechanism of action of AT9283 in these CML cells, perhaps indicating that transient, yet complete, inhibition of BCR-ABL signaling is sufficient to drive efficacy. Similar observations have been made for dasatinib.39,40
Because of the aggressive nature of the cell growth in these animal models, it is usually not possible for any ABL TKI to suppress completely the growth of leukemic cells engrafted in this manner. However, the survival advantage shown here, although small, is significant and offers hope for AT9283 and its therapeutic potential. For example, an ABL/LYN inhibitor INNO-406 (NS-187) prolonged the survival of mice engrafted with BCR-ABL+ cells in almost the same setting to a similar small yet significant extent showed responses in several imatinib-resistant patients in clinical trials.41,42 Thus, it is our opinion that the presented survival difference, afterAT9283 treatment in these models, has the potential to reflect clinical benefit.
We show that the colony formation capacity of primary CML cells was inhibited by AT9283 at IC50 concentrations that were approximately 5-fold lower than those required to produce the same effect in progenitors from healthy volunteers. In addition, IC50 value of AT9283 for parental BaF3 cells was much higher than BCR-ABL+ BaF3 cells, and IC50 values of AT9283 for most human CML cell lines, including imatinib-resistant cell lines. In clinical studies we have defined maximum-tolerated doses in patients with refractory solid tumor and patients with leukemia. Pharmacokinetic studies have shown that concentrations of AT9283 achieved in the plasma are consistent with the concentrations required to inhibit BCR-ABL+ cell growth in the studies presented here.43 In addition, biomarker modulations of a number of targets of AT9283 have been shown in samples taken during the course of these studies. These observations suggest that therapeutically relevant concentrations can be achieved at well-tolerated doses.43
On the basis of previous preclinical studies,18,19 we have already treated 2 patients with TKI-refractory CML in accelerated phase as part of a dose-finding study of AT9283. Although the ABL mutational statuses of these patients were unknown, both patients achieved a hematologic response after treatment with AT9283. The present study strongly suggests that AT9283 has the potential to significantly benefit patients with CML or with Ph+ ALL that is resistant to current forms of therapy. Moreover, AT9283 could be useful for patients bearing T315I clones. Therefore, the efficacy and safety of AT9283 for BCR-ABL+ leukemias warrants further investigation in a clinical setting.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Darcey Miller, Jayne Curry, and Kirsty Mallet for the provision of supporting data for these studies and Tom Davies and Valerio Berdini for discussion and interpretation of the BCR-ABL T315I modeling data.
This work was partly supported by Grant-in-Aids for Scientific Research and for JSPS fellows, Global COE Program “Center for Frontier Medicine” from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, and by Management Expenses Grants from the Government to the National Cancer Center of Japan.
Authorship
Contribution: R.T. performed research and analyzed data; M.S.S., and S.K. designed and performed research, and wrote the paper; A.Y., R.N., T.Y., M.T., H.Y., M.R., and E.A. performed research; T.S. designed research; J.F.L., N.T.T., and T.M. designed research and wrote the paper; and O.G.O. collected samples and performed research.
Conflict-of-interest disclosure: M.S.S., M.R., T.S., J.F.L., and N.T.T. are employees of Astex Therapeutics Ltd. The remaining authors declare no competing financial interests.
The current affiliation of M.R. is KGaA Germany, Merck Serono Research, Darmstadt, Germany.
Correspondence: Shinya Kimura, Division of Hematology, Respiratory Medicine and Oncology, Department of Internal Medicine, Faculty of Medicine, Saga University, 5-1-1 Nabeshima, Saga 849-8501, Japan; e-mail: shkimu@kuhp.kyoto-u.ac.jp.
References
Author notes
R.T. and M.S.S. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal