Abstract
The adenosine monophosphate (AMP)–activated protein kinase (AMPK) is a regulator of energy balance at the cellular and whole-body levels, but little is known about the role of AMPK in platelet activation. We report that both the α1 and α2 AMPK isoforms are expressed by human and murine platelets and that thrombin elicits the phosphorylation of AMPKα as well as the upstream kinase, liver kinase B1 (LKB1). In human platelets, the kinase inhibitors iodotubercidin and compound C significantly inhibited thrombin-induced platelet aggregation and clot retraction without affecting the initial increase in [Ca2+]i. Clot retraction was also impaired in platelets from AMPKα2−/− mice but not from wild-type littermates or AMPKα1−/− mice. Moreover, rebleeding was more frequent in AMPKα2−/− mice, and the FeCl3-induced thrombi formed in AMPKα2−/− mice were unstable. Mechanistically, AMPKα2 was found to phosphorylate in vitro the Src-family kinase, Fyn, and isoform deletion resulted in the attenuated threonine phosphorylation of Fyn as well as the subsequent tyrosine phosphorylation of its substrate, β3 integrin. These data indicate that AMPKα2—by affecting Fyn phosphorylation and activity—plays a key role in platelet αIIbβ3 integrin signaling, leading to clot retraction and thrombus stability.
Introduction
The adenosine monophosphate (AMP)–activated protein kinase (AMPK) is a heterotrimeric serine/threonine protein kinase consisting of the catalytic subunit (α) and 2 regulatory subunits (β and γ) that exist as multiple isoforms and splice variants. Each subunit within the heterotrimeric AMPK complex has a distinct structure and function and their interaction is necessary for the modulation of kinase activity. As its name suggests, AMPK is activated in many different cell types by increased intracellular concentrations of AMP. It is generally referred to as a “metabolite-sensing kinase.” Indeed, AMPK is activated after heat shock, vigorous exercise, hypoxia/ischemia, and starvation. It appears to be a metabolic master switch, phosphorylating key target proteins that control flux through different metabolic pathways.1
Given that activity is clearly associated with the phosphorylation of the α subunit, it was initially assumed that an AMPK kinase (AMPKK), rather than AMPK itself, is activated by AMP. It now seems that AMP binds to the γ subunit allosterically activating it so that the α subunit can be phosphorylated at the same time, indirectly inhibiting dephosphorylation.2
There are several kinases that phosphorylate AMPKα subunits, including the constitutively active tumor suppressor gene product, liver kinase B1 (LKB1), and the Ca2+/calmodulin-dependent protein kinase, kinase β (CaMKKβ). The activation of the latter after cell stimulation with Ca2+ ionophores or Ca2+-elevating agonists results in the phosphorylation and activation of AMPK without detectable changes in the AMP/ATP ratio.3 There are 2 different AMPKα isoforms that are differentially expressed in tissue; the α1 isoform predominates in adipose tissue whereas AMPKα2, which determines whole-body insulin sensitivity,4 is expressed in skeletal muscle and to a lesser extent in cardiomyocytes.5 Whether AMPKα is a preferential substrate for one or more putative AMPKK remains to be clarified.
Platelets play a key role in thrombosis and hemostasis, and, although platelet activation is an energy-consuming process,6,7 surprisingly little is known about the consequences of platelet activation on AMPK or its role in the regulation of platelet function. In fact, only one study reports the presence of the kinase in washed human platelets and its activation by insulin.8 Therefore, the aim of the present study was to determine the role(s) of AMPKα1 and AMPKα2 in regulating human and murine platelet function.
Methods
Reagents
The anti-AMPKα1 antibody was generated by Eurogentec; antibodies against AMPK-α2 and phospho-Thr12-Fyn, Santa Cruz Biotechnology; antibody recognizing total Fyn antibody, Abcam; and antibodies recognizing phospho-Thr172 AMPK, phospho-Ser79 ACC, and phospho-Thr189 LKB1, Cell Signaling Technologies. The phospho-Tyr747 β3-integrin antibody was from Biosource; and β3-integrin antibody, Epitomics. The antibodies against acetyl-CoA carboxylase (ACC), LKB1, and phosphothreonine Fyn were from Upstate Biotechnology. Recombinant AMPKα1, AMPKα2, and STO-609 were from Calbiochem; recombinant Fyn, Upstate Biotechnology. Thrombin was from Hemochrom Diagnostica GmbH; 3,3′-dihexyloxacarbocyanine iodide (DIOC6), Molecular Probes. All other reagents were from Sigma-Aldrich.
Animals
AMPKα1−/− (mixed C57BL6 and SV-129 background) and AMPKα2−/− mice (C57BL6 background) were generated as described.4,9 Animals and their respective wild-type littermates (AMPKα1+/+ and AMPKα2+/+) were housed in conditions that conform to the Guide for the Care and Use of Laboratory Animals of the US National Institutes of Health (NIH; publication No. 85-23). Both the university animal care committee and the federal authority for animal research (Regierungspräsidium Darmstadt, Hessen, Germany) approved the study protocols.
Bleeding and rebleeding time
Mice were anesthetized by intraperitoneal injection of ketamine (80 mg/kg) and xylazine (5 mg/kg) and placed on a heated mat. A 1-mm section of the tail tip was cut, and the tail tip was immediately immersed in sterile saline at 37°C. The bleeding time (ie, the time between initial flow of blood and its cessation) was recorded. The mice were monitored for an additional 10 minutes. If tail bleeding restarted, the incident was recorded as rebleeding time.
Platelet isolation
Human platelets.
Platelets were obtained by centrifugation (900g, 7 minutes) of platelet-rich plasma, as described.10 The resulting pellet was washed in Ca2+-free HEPES buffer (NaCl, 136mM; KCl, 2.6mM; MgCl2, 0.93mM; NaH2PO4, 3.26mM; glucose, 5.5mM; HEPES, 3.7mM; pH 7.4 at 37°C), and samples were either lysed for Western blot analysis or resuspended in HEPES buffer to a density of 4 × 108 platelets/mL for the measurement of intracellular Ca2+ or platelet aggregation as described.8,10
Murine platelets.
Mice were anesthetized with isoflurane and blood was collected via cardiac puncture into a syringe containing 10% acidic citrate dextrose (120mM sodium citrate, 110mM glucose, 80mM citric acid) as anticoagulant. Blood was pooled from 3 or 4 mice in each genotype and platelets were prepared from whole blood by differential centrifugation and resuspended in Ca2+-free HEPES buffer to a density of 8 × 108 platelets/mL.
Clot retraction
Platelet-rich plasma (3 × 108 platelets/mL, human; 5 × 108 platelets/mL, murine, 300 μL) obtained by centrifugation of whole blood at 250g for 10 minutes was stimulated with thrombin (0.3 U/mL human; 1 U/mL murine) in the presence of CaCl2 (2mM) and 2 μL of erythrocytes to enhance the contrast of the clot. The clots were allowed to retract for up to 3 hours at 37°C and were photographed at different time points. The extent of retraction was quantified using TINA20 Version 2.9g software (Raytest GmbH).
Immunoblotting
Washed human or murine platelets were solubilized in Triton X-100 lysis buffer, and 50 μg of soluble protein (∼ 5 × 107 platelets) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and subjected to Western blotting as described.10 Proteins were visualized by enhanced chemiluminescence using a commercially available kit (Amersham).
Thrombus formation in a FeCl3-induced carotid artery model
Thrombus formation in vivo was assessed as described.11 Briefly, mice were anesthetized by intraperitoneal injection of ketamine and xylazine and placed on a heated mat. The fluorescent dye DiOC6 in 100μM solution (5 μL/g of body weight) was injected into the jugular vein to allow visualization of the thrombus. Thereafter, a segment of the right carotid artery was exposed and injury was induced by the topical application of FeCl3 for 2 minutes (Whatmann paper 1 mm2 soaked with 0.2 μL of 10% FeCl3). The artery was then rinsed with saline, and thrombus formation was monitored for 30 minutes by placing the carotid artery under a fluorescence microscope equipped with a camera (AxioScope; Carl Zeiss). Fluorescent images were acquired sequentially (1 image/second) and thrombus size was quantified using AxioVision 4.7 imaging software (Carl Zeiss).
In vitro kinase assay
AMPK activity was initiated by incubating recombinant AMPKα1 or AMPKα2 protein (100 ng) with recombinant Fyn (100 ng) in 30 μL of assay buffer (HEPES, 40mM, pH 7; NaCl, 80mM; MgCl2, 5mM; DTT, 2mM; EDTA, 0,8mM; and 8% glycerin, 200μM, 5′AMP, and 1μCi 32P-γATP) for 40 minutes at 37°C. The reaction was terminated with SDS sample buffer and samples were separated by SDS-PAGE. The gel was dried before being exposed to an X-ray film. Parallel experiments were performed in the presence of unlabeled ATP and Fyn phosphorylation determined by Western blotting.
Statistical analysis
Data are expressed as mean plus or minus SEM. Statistical evaluation was performed using Student t test for unpaired data, one-way analysis of variance (ANOVA), and a Bonferroni t test where appropriate. Values of P less than .05 were considered statistically significant.
Results
Thrombin induces AMPKα phosphorylation via the activation of LKB1
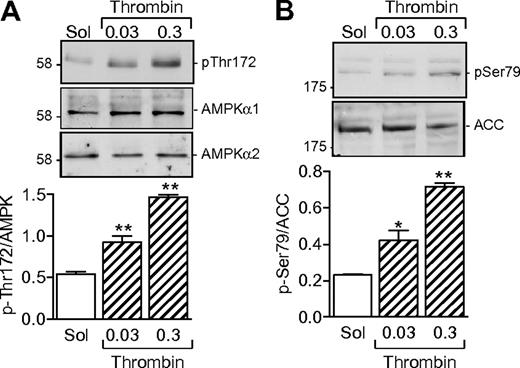
Washed human platelets expressed both the α1 and α2 subunits of AMPK (Figure 1A). A low level of AMPK phosphorylation was detectable in unstimulated platelets, but the addition of thrombin (0.03-0.3 U/mL) concentration dependently increased the phosphorylation of AMPK on Thr172. Phosphorylation is reported to be essential for AMPK activation.12 It was paralleled by an increase in kinase activity, as evidenced by the phosphorylation of the AMPK substrate, ACC (Figure 1B).
AMPKα subunit expression in human platelets and effect of thrombin. (A) Expression of AMPKα1 and AMPKα2 in washed human platelets and the effect of thrombin (0.03 or 0.3 U/mL, 10 minutes) on AMPK phosphorylation of Thr172. (B) Effect of thrombin on the phosphorylation of ACC on Ser79. The bar graphs summarize data from 4 independent experiments; *P < .05 and **P < .01 vs solvent (Sol)–stimulated platelets.
AMPKα subunit expression in human platelets and effect of thrombin. (A) Expression of AMPKα1 and AMPKα2 in washed human platelets and the effect of thrombin (0.03 or 0.3 U/mL, 10 minutes) on AMPK phosphorylation of Thr172. (B) Effect of thrombin on the phosphorylation of ACC on Ser79. The bar graphs summarize data from 4 independent experiments; *P < .05 and **P < .01 vs solvent (Sol)–stimulated platelets.
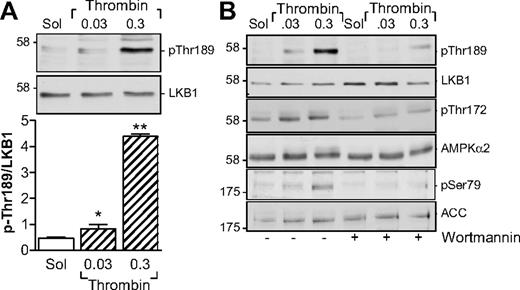
Several kinases are able to phosphorylate AMPKα subunits, including LKB1 and CaMKKβ.13 Although the thrombin-induced activation of AMPK in endothelial cells is dependent on the activation of CaMKKβ,14 the CaMKK inhibitor STO-609 did not inhibit but, in fact, tended to potentiate the thrombin-induced activation of AMPK as well as the thrombin-induced aggregation of human platelets (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). However, stimulation of washed human platelets with thrombin markedly increased the phosphorylation of LKB1, indicating that LKB1 is most probably the AMPKK under these conditions (Figure 2A). Because the phosphatidylinositol 3-kinase (PI3-K) is known to be activated by thrombin15 and has been reported as upstream of AMPK in insulin-stimulated platelets,8 we sought to determine whether PI3-K affects the LKB1-AMPK pathway. Inhibition of PI3-K using a low concentration of wortmannin (20nM) prevented the thrombin-induced phosphorylation of LKB1 as well as that of AMPK and ACC in human platelets (Figure 2B).
Parallel phosphorylation of LKB1 and AMPK in thrombin-stimulated human platelets. (A) Concentration-dependent effect of thrombin on the phosphorylation of LKB1 on Thr189. (B) Effect of wortmannin (20nM) on the thrombin-induced phosphorylation of LKB1, AMPK, and ACC. The bar graphs summarize data from 4 independent experiments; *P < .05 and **P < .01 vs solvent (Sol)–stimulated platelets.
Parallel phosphorylation of LKB1 and AMPK in thrombin-stimulated human platelets. (A) Concentration-dependent effect of thrombin on the phosphorylation of LKB1 on Thr189. (B) Effect of wortmannin (20nM) on the thrombin-induced phosphorylation of LKB1, AMPK, and ACC. The bar graphs summarize data from 4 independent experiments; *P < .05 and **P < .01 vs solvent (Sol)–stimulated platelets.
Role of AMPK in thrombin-induced aggregation and clot retraction
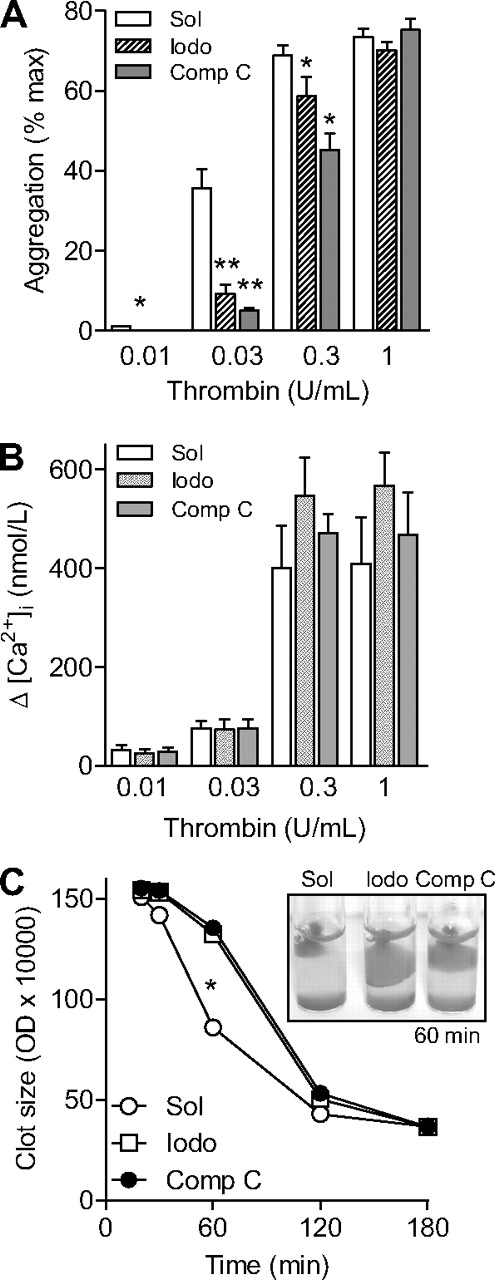
We next assessed the consequences of AMPK inhibition on thrombin-induced platelet aggregation and Ca2+ signaling. Stimulation of washed human platelets with thrombin (0.01-0.3 U/mL) led to a concentration-dependent aggregation (Figure 3A). Preincubation of platelets with either AMPK inhibitor, iodotubercidin (1μM) or compound C (10μM), significantly attenuated the aggregation induced by thrombin concentrations up to 0.3 U/mL (Figure 3A). The aggregation induced by the highest concentration of thrombin (1 U/mL) was, however, not affected by kinase inhibition. Neither iodotubercidin nor compound C affected the thrombin-induced increase in platelet [Ca2+]i (Figure 3B), suggesting that the involvement of AMPK in thrombin-induced aggregation is downstream of the increase in [Ca2+]i.
Effect of AMPK inhibition on platelet function. Human platelets were pretreated with either solvent (Sol), iodotubercidin (Iodo, 10μM) or compound C (Comp C, 10μM) and the effects of thrombin on aggregation (A), the peak increase in Ca2+ (B), and clot retraction (C) were assessed. The graphs summarize data from 6 independent experiments; *P < .05 and **P < .01 vs solvent (CTL)–stimulated platelets.
Effect of AMPK inhibition on platelet function. Human platelets were pretreated with either solvent (Sol), iodotubercidin (Iodo, 10μM) or compound C (Comp C, 10μM) and the effects of thrombin on aggregation (A), the peak increase in Ca2+ (B), and clot retraction (C) were assessed. The graphs summarize data from 6 independent experiments; *P < .05 and **P < .01 vs solvent (CTL)–stimulated platelets.
Stimulation of human platelet-rich plasma with thrombin (.3 U/mL) resulted in the formation of platelet clots and their time-dependent retraction. Although iodotubercidin and compound C did not affect clot formation, both compounds prevented clot retraction over the course of 60 minutes (Figure 3C).
AMPKα2 not AMPKα1 regulates clot retraction and thrombus stability
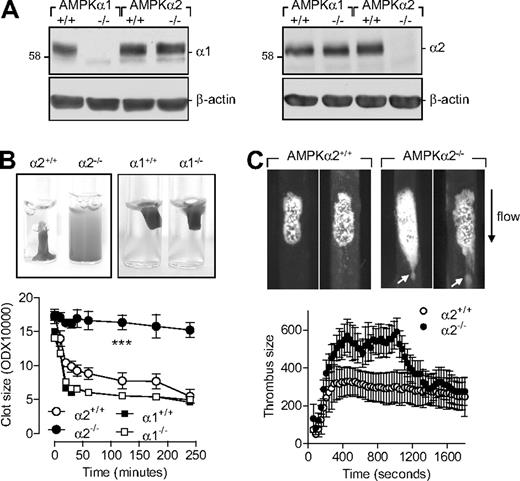
As the pharmacologic tools that target AMPK are not specific16,17 and there is no possibility of selectively inhibiting or down-regulating the different α subunits in platelets, we assessed the expression of AMPK in platelets from wild-type and AMPKα−/− mice. As with washed human platelets, platelets from wild-type mice expressed both AMPKα isoforms (Figure 4A). As expected, no AMPKα1 could be detected in platelets from AMPKα1−/− mice and no AMPKα2 could be detected in platelets from AMPKα2−/− mice. Consistent with the data obtained using human platelets, thrombin-induced aggregation was attenuated in platelets from AMPKα2−/− versus AMPKα2+/+ mice (supplemental Figure 2). Moreover, AMPKα2 activation during aggregation was not restricted to the signaling cascade activated by thrombin as collagen-induced aggregation was also significantly inhibited in platelets from AMPKα2−/− mice (supplemental Figure 2B). There was no significant difference in the thrombin-induced aggregation of platelets from AMPKα1+/+ and AMPKα1−/− mice (data not shown).
Role of AMPKα2 in clot retraction and thrombus stability. (A) Expression of AMPKα subunits in murine platelets from wild-type (α1+/+, α2+/+), AMPKα1−/−, and AMPKα2−/− mice, including (B) thrombin-induced clot retraction in platelet-rich plasma from the same animals. (C) Representative images and summary of FeCl3-induced thrombus formation in carotid arteries from AMPKα2+/+ and AMPKα2−/− mice. The arrows indicate emboli detaching from the thrombus. The graphs summarize data for 8 animals from each group; ###P < .001 vs AMPKα2+/+.
Role of AMPKα2 in clot retraction and thrombus stability. (A) Expression of AMPKα subunits in murine platelets from wild-type (α1+/+, α2+/+), AMPKα1−/−, and AMPKα2−/− mice, including (B) thrombin-induced clot retraction in platelet-rich plasma from the same animals. (C) Representative images and summary of FeCl3-induced thrombus formation in carotid arteries from AMPKα2+/+ and AMPKα2−/− mice. The arrows indicate emboli detaching from the thrombus. The graphs summarize data for 8 animals from each group; ###P < .001 vs AMPKα2+/+.
As with Ca2+ signaling in human platelets, which was unaffected by AMPK inhibition, platelet degranulation was also unaffected by AMPKα2 deletion. The thrombin-induced release of ATP (α2+/+: 34.2 ± 1.2 and α2−/−: 35.5 ± 1.8 μmol/μL platelet supernatant, 4 animals) and thromboxane B2 (α2+/+: 15.5 ± 1.1 and α2−/−: 14.0 ± 0.9 ng/mL platelet supernatant, 4 animals) were comparable.
Platelet-rich plasma from AMPKα1−/−, AMPKα2−/− mice and their respective wild-type (AMPKα+/+) littermates was stimulated with thrombin (0.3 U/mL). While maximal retraction was observed in platelet-rich plasma from wild-type and AMPKα1−/− animals (Figure 4B), clot retraction in platelet-rich plasma from AMPKα2−/−-deficient mice failed to occur.
There was no significant difference in the tail bleeding times in the animals studied; average bleeding times were 78 plus or minus 8 seconds and 118 plus or minus 28 seconds in AMPKα2+/+ and AMPKα2−/− mice, respectively (n = 11). However, rebleeding was more frequently observed in the latter group (6 of 10 vs 2 of 10), suggesting that AMPK is involved in stabilization and thrombus consolidation rather than formation of the primary platelet plug.
Therefore, we assessed thrombus formation and stability in vivo after FeCl3-induced carotid artery injury. While AMPKα2+/+ mice developed a dense thrombus covering the injured surface that attained maximum size approximately 5 minutes after injury, a less compact thrombus with a maximum size recorded after 15 minutes was formed in arteries from AMPKα2−/− mice (Figure 4C). Moreover, large emboli continuously detached from the thrombi formed in AMPKα2−/− mice, indicating clot instability and resulting in a decrease in thrombus size (Figure 4C and supplemental Videos 1-2). Thrombus development and stability were similar in AMPKα2+/+, AMPKα1+/+, and AMPKα1−/− mice (data not shown).
AMPK and the thrombin-induced phosphorylation of β3 integrin
The phosphorylation of the cytoplasmic domain of β3 integrin on Tyr747 is required for stable platelet aggregation and optimal clot retraction.18-20 Therefore, we sought to determine whether the altered function of platelets from AMPKα2−/− mice could be linked to changes in integrin phosphorylation.
In washed human platelets, thrombin elicited the concentration-dependent tyrosine phosphorylation of β3 integrin; a response markedly attenuated in the presence of compound C (Figure 5A). Thrombin also stimulated the tyrosine phosphorylation of β3 integrin in platelets from AMPKα2+/+ mice though it failed to elicit the same response in platelets from their AMPKα2−/− littermates. The thrombin-induced phosphorylation of β3 integrin was not significantly different in platelets from AMPKα1+/+ and α1−/− mice (Figure 5B). These data indicate that a β3-phosphorylating tyrosine kinase may be a substrate of AMPKα2 in platelets.
Role of AMPKα2 in the phosphorylation of β3 integrin. (A) Effect of AMPK inhibition (compound C [Comp C], 10μM) on the thrombin-induced phosphorylation (p-Tyr747) of β3 integrin in washed human platelets. (B) Thrombin-induced phosphorylation of β3 integrin in platelets from AMPKα2+/+, α2−/−, α1+/+, and α1−/− mice. The bar graphs summarize data from 6 different experiments; *P < .05 and **P < .01 vs solvent (Sol)–treated platelets.
Role of AMPKα2 in the phosphorylation of β3 integrin. (A) Effect of AMPK inhibition (compound C [Comp C], 10μM) on the thrombin-induced phosphorylation (p-Tyr747) of β3 integrin in washed human platelets. (B) Thrombin-induced phosphorylation of β3 integrin in platelets from AMPKα2+/+, α2−/−, α1+/+, and α1−/− mice. The bar graphs summarize data from 6 different experiments; *P < .05 and **P < .01 vs solvent (Sol)–treated platelets.
Several Src family kinases (SFK) can be detected in megakaryocytes and platelets, and are thought to be involved in regulating platelet function.21 Therefore, we sought to determine the consequences of SFK inhibition on β3 integrin phosphorylation.
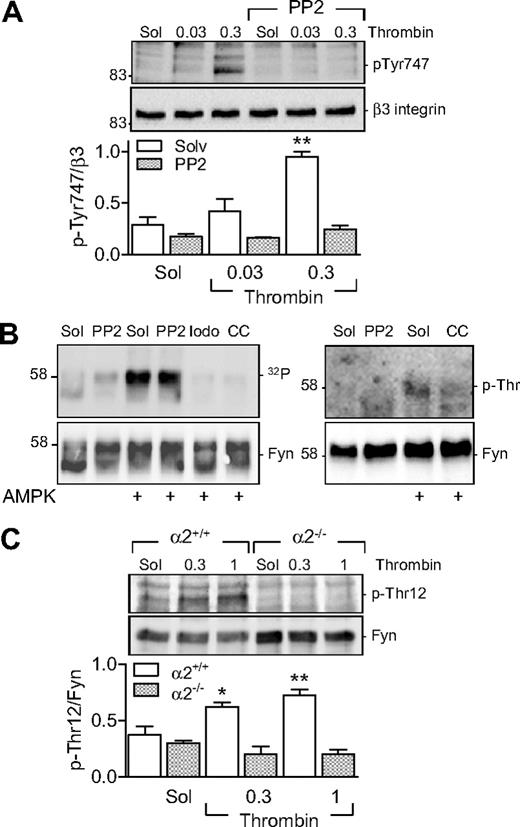
Pretreatment of human platelets with PP2 (30nM) attenuated the thrombin-induced phosphorylation of β3 integrin (Figure 6A) and aggregation. PP2 inhibited the aggregation induced by 0.03 U/mL thrombin by 50.52% (n = 4, P < .05) as well as that induced by 0.3 U/mL thrombin by 27.31% (n = 4, P < .05). PP2 did not affect the thrombin-induced phosphorylation of either AMPKα1 or AMPKα2 (data not shown). As Fyn can phosphorylate β3 integrin and Fyn is itself regulated by phosphorylation on Thr12,22 we next sought to determine whether a link exists between AMPKα2 and Fyn in platelets.
Role of AMPKα2 in the threonine phosphorylation of Fyn. (A) Effect of SKF inhibition (PP2, 30nM) on the thrombin-induced phosphorylation (p-Tyr747) of β3 integrin in washed human platelets. (B) In vitro kinase assays showing 32P incorporation into Fyn (left panel) and its threonine phosphorylation (p-Thr; right panel) in the presence of AMPK. Experiments were performed in the absence and presence of PP2 iodotubercidin (Iodo) and compound C (CC). (C) Thrombin-induced phosphorylation of Fyn on Thr12 in platelets from AMPKα2+/+ and α2−/− mice. The bar graphs summarize data from 6 different experiments; *P < .05 and **P < .01 vs solvent (Sol)-treated platelets in the absence of thrombin.
Role of AMPKα2 in the threonine phosphorylation of Fyn. (A) Effect of SKF inhibition (PP2, 30nM) on the thrombin-induced phosphorylation (p-Tyr747) of β3 integrin in washed human platelets. (B) In vitro kinase assays showing 32P incorporation into Fyn (left panel) and its threonine phosphorylation (p-Thr; right panel) in the presence of AMPK. Experiments were performed in the absence and presence of PP2 iodotubercidin (Iodo) and compound C (CC). (C) Thrombin-induced phosphorylation of Fyn on Thr12 in platelets from AMPKα2+/+ and α2−/− mice. The bar graphs summarize data from 6 different experiments; *P < .05 and **P < .01 vs solvent (Sol)-treated platelets in the absence of thrombin.
In an in vitro 32P kinase assay, we detected the autophosphorylation of Fyn in the absence of AMPK; but, in the presence of AMPKα2, we observed a marked increase in phosphorylation (Figure 6B). The latter effect was resistant to the SKF inhibitor PP2 but was abrogated by iodotubercidin and compound C, indicating the AMPK-dependent phosphorylation of Fyn—not the other way around. Experiments repeated in the absence of radioactive ATP using a phosphoselective threonine antibody confirmed the threonine phosphorylation of Fyn by AMPKα2 (Figure 6B). We were unable, however, to demonstrate the AMPK-mediated phosphorylation of a purified recombinant Src (data not shown).
Finally, we assessed the ability of thrombin to elicit the phosphorylation of Fyn on Thr12 in murine platelets. We found that, while thrombin induced the phosphorylation of Fyn on Thr12 in platelets from AMPKα2+/+ mice, there was no detectable phosphorylation of this residue in platelets from AMPKα2−/− mice (Figure 6C). No difference in Fyn phosphorylation was detected in AMPKα1+/+ or AMPKα1−/− platelets (data not shown).
Discussion
The results of the present study demonstrate that the α1 and α2 subunits of AMPK are expressed in human and murine platelets. Moreover, platelet activation by thrombin resulted in the phosphorylation of AMPKK, LKB1, AMPK, and the AMPK substrate, ACC. AMPK activation seems to be required for normal platelet responsiveness as pharmacologic inhibition of the kinase attenuated platelet aggregation and clot retraction. Moreover, based on studies in AMPKα1−/− and AMPKα2−/− mice, it seems that the α2 subunit plays a key role in the regulation of platelet function and thrombus stabilization by regulating the Fyn-mediated phosphorylation of β3 integrin.
Platelet activation is an energy-consuming process,6,7 which makes it logical to assume a potentially important role for AMPK in the regulation of platelet signaling and function. However, although AMPK is expressed in platelets, little is known about the predominance of the different α subunits or the mechanisms regulating AMPK activity.
We reported previously that platelet stimulation with insulin increased AMPK activity and that AMPK inhibition prevented the antiaggregatory effects of the hormone.8 More detailed analysis was hampered by the lack of selectivity of the available inhibitors and appropriate mouse models. We now report that both AMPKα1 and α2 subunits are expressed in human and murine platelets and that the kinase can be activated by cell stimulation with thrombin. Indeed, thrombin led to an increase in the phosphorylation of AMPK on Thr172 as well as that of its substrate ACC.
Several AMPKKs have been described. On the basis of the literature available, we initially speculated that CaMKKβ would be essential for the phosphorylation and activation of AMPK in platelets. Indeed, the thrombin-induced activation of AMPK in endothelial cells depends on CaMKKβ.14 However, we found that CaMKK inhibition failed to inhibit thrombin-induced AMPK phosphorylation in platelets although it was effective in endothelial cells. Thrombin-induced activation of AMPK was, however, paralleled by the phosphorylation of another kinase, LKB1, suggesting that it may act as an AMPKK under the conditions studied. The consequence of LKB1 phosphorylation for its activity is not clear. Indeed, LKB1 is assumed to be constitutively active, and increases in cellular AMP levels were reported to stimulate AMPK activity by decreasing its dephosphorylation by the phosphatase PP2C.2 In endothelial cells, however, stimuli such as fluid, shear stress,23 peroxynitrite,24 and the antidiabetic drug metformin,25 have all been reported to elicit phosphorylation and increase the activity of LKB1. Thus, it now seems that phosphorylation does, in fact, play a role in LKB1 activation. Unfortunately, due to the lack of an appropriate LKB1 inhibitor and an inability to apply small interfering RNA techniques to platelets, we are not able to provide direct evidence for the dependence of AMPK activity on the phosphorylation of LKB1.
What are the events upstream of LKB1 phosphorylation?
The phosphorylation of LKB1 as well as that of AMPK were both sensitive to low concentrations of wortmannin, indicating the upstream involvement of PI3-K. Although LKB1 is not known to be a direct substrate of PI3-K, activation of LKB1 by the downstream effector kinase, Akt, may explain the effects observed. Indeed, LKB1 has been shown to be required for the Akt-dependent phosphorylation of proapoptotic proteins in a cancer cell line.26 However, the interaction between AMPK and Akt is complex and controversial because Akt has been shown to inactivate AMPK in cardiac myocytes,27,28 and AMPK has been reported to negatively regulate Akt in breast cancer cells29 as well as endothelial cells exposed to flow.30
Platelet activation involves several initial steps, including the release of Ca2+ and the activation of PKC, which leads to granule secretion, platelet aggregation and integrin αIIbβ3 phosphorylation or “inside-out activation.” Thereafter, the active integrin binds fibrinogen and initiates the outside-in signaling that is crucial for clot retraction and thrombus consolidation.20 The results of the present investigation indicate that AMPK can influence processes involved in clot retraction. Indeed, although the inhibitors used affect the activity of several kinases, it was possible to show that the pharmacologic inhibition of thrombin-induced clot retraction and β3 integrin phosphorylation observed in human platelets was also evident in platelets from AMPKα2−/− mice. These in vitro observations were supported by the finding that thrombus formation and stability were unaffected by the deletion of the AMPKα1 subunit, but altered in AMPKα2−/− mice in the in vivo model of FeCl3-induced carotid artery injury. Taking all of this evidence together, it seems safe to conclude that the AMPKα2 subunit appears to be more important than α1 for the regulation of platelet function.
How could AMPK affect platelet aggregation and clot retraction?
One possibility is via interference with αIIbβ3-integrin signaling. There is indirect evidence suggesting such a link, as iodotubercidin has been reported to inhibit the phosphorylation of β3 integrin (on Thr753).31 However, attributing the latter effect to AMPK would be controversial given that phosphorylation was induced by the phosphatase inhibitor calyculin A and a second AMPK inhibitor, 9-β-D-arabinofuranoside, was without effect.
In the present study, we observed a clear kinase inhibitor–sensitive tyrosine phosphorylation of β3 integrin in human and murine platelets. Moreover, the thrombin-induced phosphorylation of β3 integrin was clearly inhibited in platelets from AMPKα2−/− mice. These data indicated that AMPKα2 may modulate platelet function by modulating the activity of a β3 integrin–phosphorylating tyrosine kinase. The most likely candidates to mediate the latter effect are the SFKs that are known to be activated by agonists such as thrombin,32 in addition to oxidative-signaling events.33 Several related kinases, including Src and Fyn, can be detected in megakaryocytes and platelets, and are thought to be involved in regulating platelet function21 via binding to distinct sites on the cytoplasmic domain of β3 integrin.34 Although we saw no evidence for a link between AMPKα2 and Src, we found that AMPKα2 phosphorylated Fyn on Thr12, a residue reported to be associated with kinase activation.22
In adipocytes, which predominantly express the AMPKα1 isoform,5 a link between Fyn and AMPK has already been suggested.35 However, in the latter study, Fyn was proposed to act as a negative regulator of AMPK activation; most probably by phosphorylating LKB 1, thus preventing its translocation from the nucleus to the cytoplasm and decreasing AMPK activation.36 We also observed that Fyn phosphorylated the recombinant AMPKα1, but we were unable to detect the phosphorylation of Fyn by AMPKα1 (V.R., unpublished observation, 2009)—findings that are in line with observations made in adipocytes.37 Moreover, our data also clearly indicate that AMPKα2, rather than AMPKα1, is linked with changes in platelet function and that it is able to phosphorylate Fyn on Thr12, and that this activated Fyn phosphorylates platelet β3 integrin. This sequence of events can account for the attenuated clot retraction in human platelets treated with iodotubercidin or compound C as well as the formation of unstable thrombi in AMPKα2−/− mice. These observations are certainly of physiologic and pathophysiologic relevance as the abnormalities in platelet function described in the present study are similar to the platelet phenotype of Fyn-deficient mice.34 Given that several diseases have been associated with altered AMPK activity1 and that the AMPKα2 subunit has been linked to whole-body insulin sensitivity,4 it will be interesting to determine whether changes in AMPK and/or Fyn activity can account for the altered platelet function observed in platelets from diabetic patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Isabel Winter, Katharina Bruch, and Mechtild Piepenbrock for expert technical assistance. They also thank Christian Gachet for technical help with the in vivo thrombosis model.
This work was supported in part by grants from the Deutsche Forschungsgemeinschaft (SFB 815/A16, SFB834/A5 and the Exzellenzcluster; 147 “Cardio-Pulmonary System”) and EICOSANOX (LSHM-CT-2004-005033 and LSHM-CT-2004-005272/exgenesis).
Authorship
Contribution: V.R. designed and performed research, analyzed data and wrote the paper; J.I. and T.F. performed research and assisted with data analysis; B.F. optimized research materials, contributed to the analytical tools and coordinated animal supply; B.V. contributed new reagents; K.T.P. performed phosphoprotein analyses and contributed to the experimental design; and I.F. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ingrid Fleming, PhD, Institute for Vascular Signalling, Centre for Molecular Medicine, Johann Wolfgang Goethe University, Theodor-Stern-Kai 7, D-60596 Frankfurt am Main, Germany; e-mail: fleming@em.uni-frankfurt.de.

![Figure 5. Role of AMPKα2 in the phosphorylation of β3 integrin. (A) Effect of AMPK inhibition (compound C [Comp C], 10μM) on the thrombin-induced phosphorylation (p-Tyr747) of β3 integrin in washed human platelets. (B) Thrombin-induced phosphorylation of β3 integrin in platelets from AMPKα2+/+, α2−/−, α1+/+, and α1−/− mice. The bar graphs summarize data from 6 different experiments; *P < .05 and **P < .01 vs solvent (Sol)–treated platelets.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/12/10.1182_blood-2010-04-279612/4/m_zh89991057640005.jpeg?Expires=1767697682&Signature=uE3URnFyd6H75HxG526-EYGWxUFf6m04Zl6LG4OZ70M9~5bGslmBrH-P1Z1OwwCv4j~s11UdtAgtLkRHCB9xO5pCQ3AEkv5RL-PV9a303bCU8pdHZLOkfrYdTKFtjatMWXn08VeoEFvIXz1lHliFRHGypm6O79QB8DCZv~o40EVedb9tiTG~~VxsCIy7icdxp--Rb8cdzhiwljBBmrVBqr-3NQ2HESuPOVvUuCU3evyPFH2-AFyASX0LlJtQkrqJJUa47-IIKXuS0ryHjK2Z3agTpmpkzdZ15h4dHbEy1VF9tRnfirqfIH-gMIcziWAf99KGMunN9C-H-ncQayakOQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal