Abstract
Reactivation of cytomegalovirus (CMV) remains a serious complication after allogeneic stem cell transplantation, but the role of γδ T cells is undefined. We have studied the immune reconstitution of Vδ2negative (Vδ2neg) γδ T cells, including Vδ1 and Vδ3 subsets and Vδ2positive (Vδ2pos) γδ T cells in 40 patients during the first 24 months after stem cell transplantation. Significant long-term expansions of Vδ2neg but not Vδ2pos γδ T cells were observed during CMV reactivation early after transplantation, suggesting direct involvement of γδ T cells in anti-CMV immune responses. Similarly, significantly higher numbers of Vδ2neg γδ T cells were detected in CMV-seropositive healthy persons compared with seronegative donors; the absolute numbers of Vδ2pos cells were not significantly different. The expansion of Vδ2neg γδ T cells appeared to be CMV-related because it was absent in CMV-negative/Epstein-Barr virus-positive patients. T-cell receptor-δ chain determining region 3 spectratyping of Vδ2neg γδ T cells in healthy subjects and patients showed restricted clonality. Polyclonal Vδ2neg cell lines generated from CMV-seropositive healthy donors and from a recipient of a graft from a CMV-positive donor lysed CMV-infected targets in all cases. Our study shows new evidence for role of γδ T cells in the immune response to CMV reactivation in transplantation recipients.
Introduction
Human cytomegalovirus (CMV) is a widespread β-herpes virus that infects more than half of the Western population. Primary infection induces a life-long latent infection in the immunocompetent host with asymptomatic episodes of viral replication, which are controlled by a vigorous immune response. CMV infection is responsible for increased morbidity and mortality after allogeneic stem cell transplantation (SCT) as either CMV reactivation in CMV-seropositive patients or primary infection in CMV-seronegative patients receiving grafts from seropositive donors. Several studies have demonstrated a significant decline in a overall survival in human leukocyte antigen-identical sibling transplantations or in unrelated transplantations where either the donor or the patient was CMV-seropositive.1,2
The central mechanism controlling CMV infection is mediated by antigen-specific CD8+ cytotoxic and CD4+ αβ T lymphocytes and natural killer (NK) cells.3-5 CMV-specific CD3+CD8+CD28−CD57+ αβ T cells are regarded as the principle effector cells controlling CMV reactivation.6,7 Both humoral and cellular immunity is involved in protective immune responses to CMV reactivation and CMV resolution.8
Recent interest has focused on γδ T cells and their role in immune responses during CMV infection. γδ T cells are often termed the “unconventional” T cells and represent a minor population of circulating T cells (< 5%) in humans but are present in large numbers in epithelial tissues.9-13 In contrast to αβ T cells, which recognize peptides bound to major histocompatibility complex (MHC) class I or class II molecules, most γδ T cells lack surface expression of CD4 or CD8 and display a non–MHC-restricted recognition.14,15
There are 2 major subsets of γδ T cells present in human peripheral blood: a subset of γδ T cells expressing a T-cell receptor (TCR) encoded by the Vδ2 and Vγ9 gene segments, which accounts for 50% to 90% of γδ T cells in adult peripheral blood and a minor Vδ1 subset more frequent at mucosal epithelium sites, such as skin and the intestine. An additional small subset of Vδ3 γδ T cells is also present in peripheral blood but represents a minor population of less than 0.1% of CD3+ T cells. The γδ T cells recognize ligands that are not seen by αβ T cells and provide additional means by which the immune system can maintain local immunosurveillance with immediate tumor defense, selective recognition of viral antigens, and bacterial metabolites.12,14
Vδ1 γδ T cells recognize ligands for an activating receptor NKG2D, such as MHC class I-related chain A (MICA and MICB) stress-induced antigens expressed on epithelial tumor cells, some leukemias, lymphomas, and the UL16-binding proteins.16-24 Vδ2 γδ T cells recognize low molecular weight nonpeptidic phosphoantigens, particularly intermediates of the nonmevalonate pathway of bacterial isoprenoid biosynthesis25 or isopenthenyl pyrophosphate and aminobiphosphonates in eukaryotic cells.26
Recent studies have shown expansion and cytotoxic function of CMV-reactive Vδ1 and Vδ3 γδ T cells in the peripheral blood of patients receiving renal and lung allografts.27-31
In the present study, we analyzed the relative numbers of the Vδ2neg (Vδ1 and Vδ3 γδ T cells) compared with Vδ2pos γδ T cells in CMV-seropositive healthy healthy donors and in CMV-seropositive patients with documented CMV reactivation after allogeneic SCT. We found major differences in frequencies, repertoire profiles, and cytotoxic effector function in response to CMV between the γδ T-cell subsets. Together, the results suggest a protective role of Vδ2neg γδ T cells during CMV reactivation in immunocompromised patients after SCT analogous to that seen in the previous studies of solid organ allografts.
Methods
Patients
A cohort of 40 patients undergoing allogeneic SCT at the Royal Free Hospital NHS Trust, London, United Kingdom was enrolled for this study after informed consent in accordance with the Declaration of Helsinki. All experiments involving patient samples were approved by the United Kingdom Home Office. Patient characteristics are listed in Table 1. Patients were divided by CMV serostatus of their donors into 3 groups; CMV-positive patients with CMV-positive donors (CMV+/+, n = 14), CMV-negative patients with CMV-negative donors (CMV−/−, n = 20) and CMV mismatched; patient-donor pair CMV+/− (n = 2) and CMV−/+ (n = 4). CMV serology of patients' and healthy donors was determined by the Architect i2000SR analyser (Abbott Diagnostic). Posttransplantation monitoring of CMV reactivation was performed by in-house TaqMan human CMV PCR as described previously.32 Peripheral blood samples were collected from patients every 3 months during the 24-month follow-up after transplantation.
Patient and donor characteristics (n = 40)
| Characteristic . | Value . |
|---|---|
| Median patient age, y (range) | 37 (7-63) |
| Median donor age, y (range) | 35 (6-63) |
| Patient sex, male/female | 23/17 |
| Donor sex, male/female | 27/13 |
| Diagnosis, no. (%) | |
| Chronic myeloid leukemia, chronic phase | 3 (7.5) |
| Acute myeloid leukemia | 10 (25) |
| Acute lymphoblastic leukemia | 7 (17.5) |
| Myelodysplasia | 3 (7.5) |
| Non-Hodgkin lymphoma | 6 (15) |
| Hodgkin lymphoma | 1 (2.5) |
| Multiple myeloma | 4 (10) |
| Aplastic anemia | 4 (10) |
| FasL deficiency | 1 (2.5) |
| β-Thalassemia | 1 (2.5) |
| Type of transplantation, no. (%) | |
| Matched sibling | 23 (57.5) |
| Matched unrelated | 15 (37.5) |
| Mismatched unrelated | 2 (5) |
| Reduced-intensity conditioning | 14 |
| T-cell depletion, ex vivo | 9 |
| Stem cell source, no. (%) | |
| Bone marrow | 17 (42.5) |
| Peripheral blood | 23 (57.5) |
| CMV serology, donor/patient, no. (%) | |
| D+/P− | 4 (10) |
| D+/P+ | 14 (35) |
| D−/P− | 20 (50) |
| D−/P+ | 2 (5) |
| Characteristic . | Value . |
|---|---|
| Median patient age, y (range) | 37 (7-63) |
| Median donor age, y (range) | 35 (6-63) |
| Patient sex, male/female | 23/17 |
| Donor sex, male/female | 27/13 |
| Diagnosis, no. (%) | |
| Chronic myeloid leukemia, chronic phase | 3 (7.5) |
| Acute myeloid leukemia | 10 (25) |
| Acute lymphoblastic leukemia | 7 (17.5) |
| Myelodysplasia | 3 (7.5) |
| Non-Hodgkin lymphoma | 6 (15) |
| Hodgkin lymphoma | 1 (2.5) |
| Multiple myeloma | 4 (10) |
| Aplastic anemia | 4 (10) |
| FasL deficiency | 1 (2.5) |
| β-Thalassemia | 1 (2.5) |
| Type of transplantation, no. (%) | |
| Matched sibling | 23 (57.5) |
| Matched unrelated | 15 (37.5) |
| Mismatched unrelated | 2 (5) |
| Reduced-intensity conditioning | 14 |
| T-cell depletion, ex vivo | 9 |
| Stem cell source, no. (%) | |
| Bone marrow | 17 (42.5) |
| Peripheral blood | 23 (57.5) |
| CMV serology, donor/patient, no. (%) | |
| D+/P− | 4 (10) |
| D+/P+ | 14 (35) |
| D−/P− | 20 (50) |
| D−/P+ | 2 (5) |
D indicates donor; and P, patient.
Healthy subjects
Peripheral blood samples from 45 age-matched healthy adult volunteers (31 females, 14 males) with median age 34 years (range, 21-53 years) were collected after obtaining informed consent. The cohort included CMV-seronegative (n = 22) and CMV-seropositive (n = 23) subjects. Peripheral blood mononuclear cells (PBMCs) were separated from heparinized whole blood using density gradient separation (Lymphoprep, Nycomed).
Antibodies
The following monoclonal antibodies (mAbs) were purchased from BD Biosciences: anti-TCR-γδ-1, anti-CD3, anti-CD11a, anti-CD27, anti-CD45RO, and anti-CD45RA. Antibody directed against anti-Vδ2 TCR was purchased from BD Biosciences PharMingen. Unlabeled antibodies anti-Vδ1 TCR were from Beckman Coulter, anti-CD3 (OKT3), anti-IgG2a (eBioscience), anti–TCR-γδ and anti-IgG1 (BD Biosciences), and anti-NKG2D (R&D Systems).
Flow cytometric analysis
Freshly isolated PBMCs (2.5 × 105) were labeled with specific mAbs at 4°C in the dark for 30 minutes and then washed twice in 2 mL of cold phosphate-buffered saline containing 2.5% of fetal calf serum (FCS, Invitrogen) and subsequently fixed in 100 μL of 1% (wt/vol) paraformaldehyde (Sigma-Aldrich). Samples were acquired within 4 hours on a FACSCalibur (BD Biosciences) flow cytometer using CellQuest Version 3.3 software (BD Biosciences) and analyzed with FlowJo Version 8.6 software (TreeStar). The total lymphocyte population was gated based on forward versus side scatter followed by gating on CD3+ and either Vδ2pos γδ T cell (the predominant population representing up to 3% circulating T cells; range, 0.32-14.10; median, 2.43) or the remaining Vδ2neg γδ T cells; consisting of Vδ1 and Vδ3 subsets (range, 0.02-7.56; median, 0.90). Because of a limited availability of specific Vδ1 and Vδ3 mAbs and very low frequencies present in the peripheral blood, the Vδ1 and Vδ3 subsets were combined as Vδ2neg γδ T cells. The absolute numbers of Vδ2pos or Vδ2neg γδ T cells were determined by multiplying their percentages within the CD3+ population by the absolute CD3 counts generated with staining of whole blood samples with TriTest (BD Biosciences) reagents in TruCount tubes. After red cell lysis, the samples were acquired within 30 minutes on a FACSCalibur and the absolute counts (cells/μL) were calculated using the MultiSET software (BD Biosciences).
TCRVδ 1, 2, 3-chains and TCRVγ8-chain CDR3 size spectratyping analysis
Total RNA was extracted from separated CD4−CD8− cell pellets using RNeasy kit (QIAGEN) and transcribed into complementary DNA (cDNA) according to the manufacturer's instructions (Promega). Each cDNA was amplified using primers for TCR Vδ-1, 2, 3-chain variable and constant segments published previously27 and primers for TCR Vγ8-chain.33 Aliquots of 2 μL of the PCR products were then amplified with the same forward primer for each Vδ-chain with Cδ-FAM and Vγ8-chain with Cγ-FAM–labeled constant primer for 10 cycles with the same PCR conditions. Separations of the 2-μL labeled products were performed using ABI 3130 DNA Sequencer (Applied Biosystems). Fluorescence intensity for each sample was analyzed with the GeneScan Version 3.0 software (Applied Biosystems).
Generation of polyclonal γδ T-cell lines
Fresh PBMCs were isolated from peripheral blood of healthy volunteers and γδ T cells were sorted from PBMCs using anti-Vδ1 (Beckman Coulter) or anti-Vδ2 (BD Biosciences PharMingen) mAbs and magnetic beads (Miltenyi Biotec). Sorted cells were expanded with modified protocol described previously.28 Polyclonal γδ T-cell cultures were established with 1 μg/mL PHA-L (Sigma-Aldrich), 200 IU/mL of human recombinant IL-2 (R&D Systems), and irradiated allogeneic PBMCs (35 Gy). After 3 to 4 weeks of culture, polyclonal lines were immunophenotyped and purity (routinely > 95%) determined by multicolor fluorescent staining.
Infection of MRC5 fibroblasts
Human fetal lung fibroblasts MRC5 were obtained from European Collection of Cell Cultures. Cells were cultured in MEM supplemented with 2mM l-glutamine, nonessential amino acids (all from Sigma-Aldrich), and 10% FCS. Fibroblasts were used between 32 and 40 passages and were maintained at 37°C in a humid atmosphere containing 5% CO2.
Both clinical CMV strains TB40/E and VHL/E were kindly provided by Dr Sinzger (University of Tubingen, Tubingen, Germany); HSV-1 strain was provided by Dr Milne (UCL, London). For infection, 5 × 105 MRC5 fibroblasts per well were seeded into 24-well plate 24 hours before adding the virus. Subconfluent monolayers of MRC5 fibroblasts were incubated with CMV (or HSV-1) suspension at multiplicity of infection of 1 to 5 for 2 hours at 37°C. After virus adsorption, cells were washed and cultured for 2 to 4 days. Infection was verified by microscopy to determine the cytopathic effect. Before the cocultures, cells were washed with PBS and used in cytotoxicity assays.
Cytotoxicity assays
Effector γδ polyclonal cell lines were recovered from culture or cryopreservation and washed in Hanks buffered saline solution (Invitrogen) to remove FCS and culture media. Cells were resuspended in PKH26 diluent (Sigma-Aldrich) and labeled with PKH26 red fluorescent dye (Sigma-Aldrich) as described previously.34 Labeled effector cells were coincubated at indicated effector/target ratios (E:Ts, 10:1 and 20:1) with CMV-infected fibroblasts or with noninfected fibroblasts as controls. Cytotoxicity was measured in duplicates after 4-hour coculture at 37°C. To-Pro-3 iodide (1μM in PBS; Invitrogen) was added immediately before the acquisition on the flow cytometer. At least 10 000 target cells were acquired with 1024 channel resolution after gating out the red fluorescence of PKH26 dye and the proportion of To-Pro-3 iodide-positive cells. Background target cell death was determined from the cells incubated in the absence of effector cells. For the blocking assay, the effector cells were preincubated for 1 hour with 10 μg/mL of anti–TCR-γδ or anti–TCR-Vδ1, 10 μg/mL of anti-CD3, 20 μg/mL of anti-NKG2D, or control mouse IgG. Effector γδ cells were coincubated for 4 hours at 10:1 E/T ratio with CMV-infected and uninfected fibroblasts.
IFN-γ detection
The γδ polyclonal cell lines were cocultured at 10:1 E/T ratio with CMV-infected and uninfected fibroblasts; and after 6 hours, cell-free supernatants were collected. Interferon-γ (IFN-γ) release was determined by BD Cytometric Bead Array (CBA) from BD Biosciences according to the manufacturer's instructions. Samples were acquired on a FACSAria, and data were analyzed using the FCAP Version 1.0 software (BD Biosciences).
Statistical analyses
The statistical differences between CMV+ and CMV− groups were determined using the unpaired nonparametric Mann-Whitney U test using GraphPad Prism Version 4.0 software. Statistical significance was achieved when P was less than .05.
Results
Vδ2neg γδ T cells are significantly expanded in CMV-seropositive but not CMV-seronegative transplant recipients
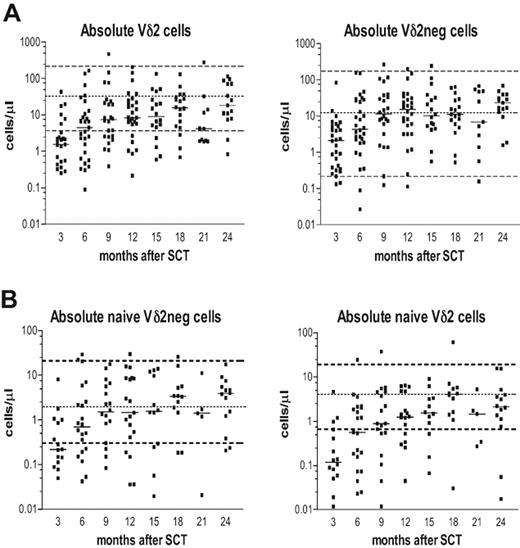
Posttransplantation immune reconstitution of Vδ2positive (Vδ2pos) and Vδ2negative (Vδ2neg) γδ T-cell subsets is shown in Figure 1A. The Vδ2neg γδ T cells showed a rapid reconstitution, reaching a plateau at the median of the normal range approximately 9 months after SCT. In contrast, the Vδ2pos subset showed a delayed reconstitution with patients failing to reach the median of the normal range during the 2-year follow-up. Next, we determined the immune reconstitution of naive γδ T-cell subsets in patients after SCT (Figure 1B), generally distinguished with CD27+CD45RA+ or CD45RO− phenotype5 or as CD27brightCD11adull.35 The absolute numbers of naive Vδ2neg γδ T cells reconstituted to normal levels within 6 months and were present at approximately the median counts from 9 months after transplantation. In contrast, the absolute numbers of naive Vδ2pos cells recovered slowly, reached only the lower normal range at 6 months and remained below the median values at 2 years after SCT.
The immune reconstitution of CD3+ Vδ2pos and CD3+ Vδ2neg γδ T cells in patients during the first 24 months after SCT. (A) Absolute numbers as cells/μL of Vδ2pos and Vδ2neg γδ T cells. (B) Absolute numbers of naive Vδ2pos and Vδ2neg γδ T cells were determined during 3 to 24 months after SCT. Isolated PBMCs were stained with anti-CD3, anti–TCR-γδ, and anti-Vδ2 antibodies and analyzed by flow cytometry. Samples were gated on live lymphocytes as side scatter versus forward scatter followed by gating on CD3+ T cells. Naive phenotype was defined as CD45RA+, CD27bright, and CD11adull. Dashed lines represent the upper and lower normal range generated from 45 healthy donors; the dotted line is the median value of the normal range. The horizontal bars represent the median value from patient samples for each time point.
The immune reconstitution of CD3+ Vδ2pos and CD3+ Vδ2neg γδ T cells in patients during the first 24 months after SCT. (A) Absolute numbers as cells/μL of Vδ2pos and Vδ2neg γδ T cells. (B) Absolute numbers of naive Vδ2pos and Vδ2neg γδ T cells were determined during 3 to 24 months after SCT. Isolated PBMCs were stained with anti-CD3, anti–TCR-γδ, and anti-Vδ2 antibodies and analyzed by flow cytometry. Samples were gated on live lymphocytes as side scatter versus forward scatter followed by gating on CD3+ T cells. Naive phenotype was defined as CD45RA+, CD27bright, and CD11adull. Dashed lines represent the upper and lower normal range generated from 45 healthy donors; the dotted line is the median value of the normal range. The horizontal bars represent the median value from patient samples for each time point.
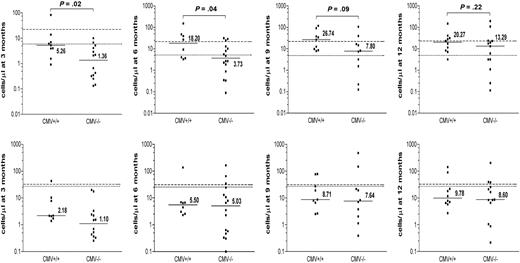
As patient-donor CMV status has been shown to have a profound impact on the immune reconstitution after SCT, we analyzed its effect on the reconstitution of γδ T-cell subsets. The absolute numbers of CD3+ Vδ2pos versus Vδ2neg γδ T cells were compared between CMV-seropositive and CMV-seronegative allogeneic transplantation recipients (Figure 2). During the first 6 months after transplantation, significantly higher absolute numbers of Vδ2neg γδ T cells were detected in CMV+/+ patient-donor pairs compared with the CMV−/− pairs (P = .02 and P = .04, respectively) or to the CMV mismatched group; CMV+/− (n = 2) and CMV−/+ (n = 4), (data not shown). These numbers were still elevated at 9 and 12 months after SCT. No significant differences were detected in the reconstitution of the Vδ2pos subset between CMV+/+ and CMV−/− patients. These results suggest that the expansion of Vδ2neg γδ T cells in lymphopenic patients is because of an initial or ongoing immune response to CMV infection in the early posttransplantation period when the immune reconstitution is incomplete.
The effect of patient-donor CMV status on immune reconstitution of Vδ2neg and Vδ2pos γδ T cells after SCT. The effect of the patient-donor CMV status on absolute counts of Vδ2neg (top row) and Vδ2pos γδ T-cell subsets (bottom row) was analyzed in patients during 3, 6, 9, and 12 months after transplantation. Patients were divided into 2 groups: 14 CMV+/+ and 20 CMV−/−. The horizontal bars represent the median values for each time point. The dashed line represents the median normal range generated from CMV+ healthy donors; and the dotted line, the median normal range generated from CMV− healthy donors. Available patient samples were analyzed using nonparametric Mann-Whitney test, and P values were determined. P values less than .05 were considered significant.
The effect of patient-donor CMV status on immune reconstitution of Vδ2neg and Vδ2pos γδ T cells after SCT. The effect of the patient-donor CMV status on absolute counts of Vδ2neg (top row) and Vδ2pos γδ T-cell subsets (bottom row) was analyzed in patients during 3, 6, 9, and 12 months after transplantation. Patients were divided into 2 groups: 14 CMV+/+ and 20 CMV−/−. The horizontal bars represent the median values for each time point. The dashed line represents the median normal range generated from CMV+ healthy donors; and the dotted line, the median normal range generated from CMV− healthy donors. Available patient samples were analyzed using nonparametric Mann-Whitney test, and P values were determined. P values less than .05 were considered significant.
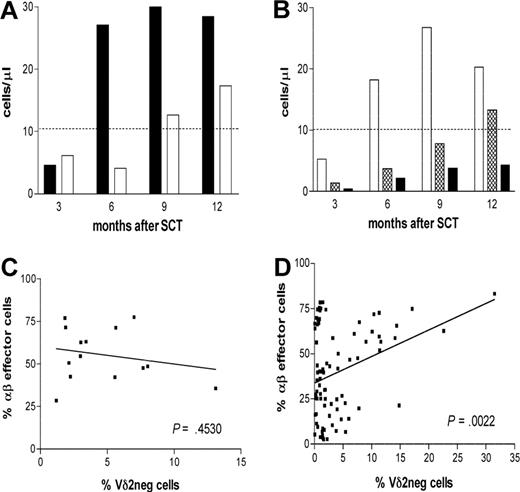
We then analyzed the effect of CMV reactivation on the reconstitution of the Vδ2neg γδ T cells during the first 12 months after SCT. Patients with an early CMV reactivation after transplantation (n = 6) showed large and long-lasting expansion of Vδ2neg γδ T cells compared with patients (n = 8) with no CMV reactivation after transplantation (Figure 3A). However, this difference was not statistically significant, and larger patient groups would need to be analyzed.
The expansion of Vδ2neg γδ T cells in patients with CMV reactivation during 3 to 12 months after SCT. (A) Expanded Vδ2neg γδ T cells were analyzed in patients divided into subgroups of patients with CMV reactivation (n = 6, ■) and patients without reactivation (n = 8, □). (B) Patients were divided according to CMV and EBV serology into CMV+/+ (n = 14, □), CMV−/− (n = 20,  ), and CMV−/EBV+ (n = 14, ■). The median values of absolute numbers of Vδ2neg γδ T cells were plotted. Dashed line represents the median normal range generated from 45 healthy persons. Samples were analyzed using nonparametric Mann-Whitney test, and P values were determined. P values less than .05 were considered significant. (C) Frequencies of CD3+ Vδ2neg γδ T cells and CD8 effector CD28−CD57+ αβ T cells were determined in patients reactivating CMV (left panel) and in patients with no reactivation (right panel).
), and CMV−/EBV+ (n = 14, ■). The median values of absolute numbers of Vδ2neg γδ T cells were plotted. Dashed line represents the median normal range generated from 45 healthy persons. Samples were analyzed using nonparametric Mann-Whitney test, and P values were determined. P values less than .05 were considered significant. (C) Frequencies of CD3+ Vδ2neg γδ T cells and CD8 effector CD28−CD57+ αβ T cells were determined in patients reactivating CMV (left panel) and in patients with no reactivation (right panel).
The expansion of Vδ2neg γδ T cells in patients with CMV reactivation during 3 to 12 months after SCT. (A) Expanded Vδ2neg γδ T cells were analyzed in patients divided into subgroups of patients with CMV reactivation (n = 6, ■) and patients without reactivation (n = 8, □). (B) Patients were divided according to CMV and EBV serology into CMV+/+ (n = 14, □), CMV−/− (n = 20,  ), and CMV−/EBV+ (n = 14, ■). The median values of absolute numbers of Vδ2neg γδ T cells were plotted. Dashed line represents the median normal range generated from 45 healthy persons. Samples were analyzed using nonparametric Mann-Whitney test, and P values were determined. P values less than .05 were considered significant. (C) Frequencies of CD3+ Vδ2neg γδ T cells and CD8 effector CD28−CD57+ αβ T cells were determined in patients reactivating CMV (left panel) and in patients with no reactivation (right panel).
), and CMV−/EBV+ (n = 14, ■). The median values of absolute numbers of Vδ2neg γδ T cells were plotted. Dashed line represents the median normal range generated from 45 healthy persons. Samples were analyzed using nonparametric Mann-Whitney test, and P values were determined. P values less than .05 were considered significant. (C) Frequencies of CD3+ Vδ2neg γδ T cells and CD8 effector CD28−CD57+ αβ T cells were determined in patients reactivating CMV (left panel) and in patients with no reactivation (right panel).
Analysis of the subgroup of CMV−/Epstein-Barr virus (EBV)+ patients (n = 14) showed no expansion of Vδ2neg γδ T cells during the posttransplantation follow-up (Figure 3B), suggesting that the Vδ2neg cell expansion is not simply a nonspecific response to chronic latent viral infections or to posttransplantation lymphopenia.
Frequencies of Vδ2neg γδ T cells and of CD8+CD28−CD57+ αβ T cells were monitored during the first 12 months after transplantation. No correlation between the aforementioned cell populations was found (P = .453) in patients with CMV reactivation early after transplantation (Figure 3C). These results suggest that the expansions of Vδ2neg γδ T cells respond to active CMV infection that occurs in the absence of the CMV-specific αβ T-cell response. In contrast, there was a significant correlation between CMV-reactive αβ T cells and Vδ2neg γδ T cells (P > .002) in patients without CMV reactivation (Figure 3D). This may reflect the protective effect of CD8+CD28−CD57+ αβ T cells and ongoing linkage between the innate and acquired immune response to CMV even in the presence of protective immunity.
Vδ2negative γδ T cells are increased in CMV-seropositive healthy subjects compared with CMV-seronegative persons
The absolute numbers of γδ T-cell subsets were measured and compared between CMV-seropositive (n = 23) and CMV-seronegative (n = 22) healthy persons (Figure 4A). Significantly increased absolute numbers of Vδ2neg cells were detected in CMV+ healthy persons compared with CMV− subject numbers (P > .001).
CMV seropositivity in healthy persons is associated with significantly higher absolute counts of Vδ2neg γδ T cells. (A) Healthy donors were divided by their CMV status into CMV+ (n = 23) and CMV− (n = 22) groups. Samples were gated on live lymphocytes as side scatter versus forward scatter followed by gating on CD3+ and Vδ2pos or Vδ2neg (left) γδ T-cell subset. Absolute counts of both cell subsets were compared between the groups using nonparametric Mann-Whitney test. The horizontal bars represent the median values. P values were determined, and those less than .05 were considered significant. (B) Positive correlation between frequencies and absolute numbers of CD3+ Vδ2neg γδ T cells and CD8+CD28−CD57+ αβ T cells was detected in the CMV+ group.
CMV seropositivity in healthy persons is associated with significantly higher absolute counts of Vδ2neg γδ T cells. (A) Healthy donors were divided by their CMV status into CMV+ (n = 23) and CMV− (n = 22) groups. Samples were gated on live lymphocytes as side scatter versus forward scatter followed by gating on CD3+ and Vδ2pos or Vδ2neg (left) γδ T-cell subset. Absolute counts of both cell subsets were compared between the groups using nonparametric Mann-Whitney test. The horizontal bars represent the median values. P values were determined, and those less than .05 were considered significant. (B) Positive correlation between frequencies and absolute numbers of CD3+ Vδ2neg γδ T cells and CD8+CD28−CD57+ αβ T cells was detected in the CMV+ group.
In contrast, absolute numbers of Vδ2pos cells were comparable between the 2 groups (P < .883). These results demonstrate the impact of CMV status on γδ T-cell subsets in anti-CMV immune responses in healthy immunocompetent donors. Although there was an apparent correlation between the frequencies and the absolute numbers of CMV-specific CD3+CD8+CD28−CD57+ αβ T cells and Vδ2neg γδ T cells in healthy CMV+ persons, it failed to reach statistical significance (P = .10 and P = .16, respectively; Figure 4B). Thus, the relationship between the anti-CMV αβ T cells and Vδ2neg γδ T cells seen early after transplantation is not so apparent in healthy CMV+ persons in whom the αβ T-cell response is better established and probably dominant.
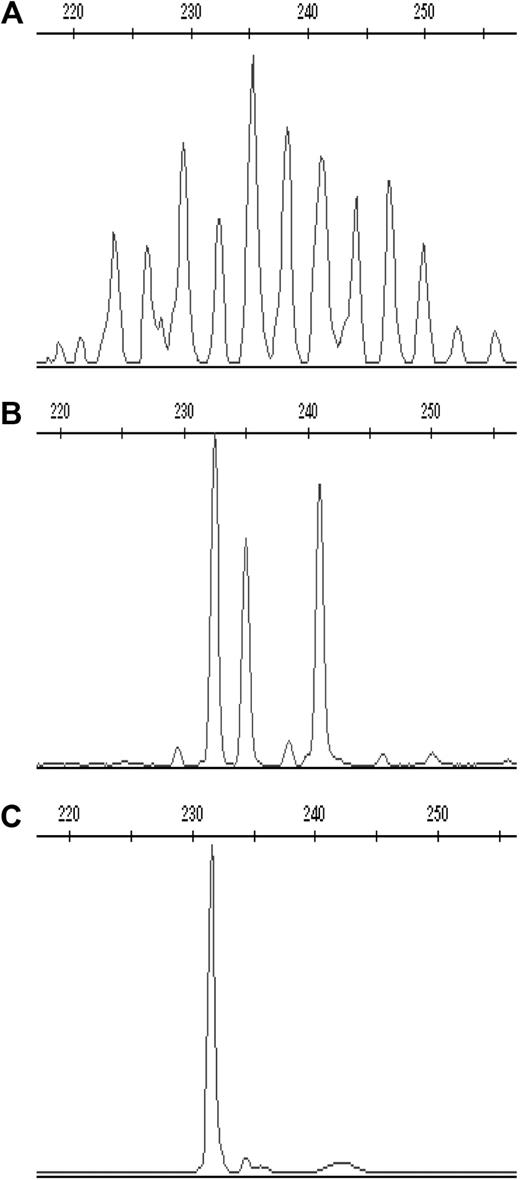
To determine whether the expansion of Vδ2neg γδ T cells in CMV+ healthy donors and patients is clonally restricted, we performed complementary-determining region (CDR3) spectratyping analysis of the TCR-δ chain repertoire. We scored the complexity of TCR-δ chains repertoire by counting the total number of peaks in each histogram. Healthy donors show Gaussian profiles of approximately 18 or more peaks representing different CDR3 length rearrangements, with dominant peaks above the normal distribution that identify expansions of one or several clones. Representative profiles of monoclonal (1 peak), oligoclonal (2-5 peaks), and polyclonal (8 and more peaks) distributions are shown in Figure 5. CDR3 profiles of the Vδ1, Vδ2, and Vδ3 subsets were generated in 13 CMV+ and 12 CMV− randomly selected healthy adults and in 8 CMV+/+ patients at multiple time points after transplantation, as shown in Figure 6.
Representative CDR3 spectratype profiles of the TCR-δ chain in one CMV-seropositive healthy donor. Profiles of (A) polyclonal, (B) oligoclonal, and (C) monoclonal CDR3 spectratype for Vδ3 cells are shown expressed as relative fluorescent intensity versus the CDR3 length size.
Representative CDR3 spectratype profiles of the TCR-δ chain in one CMV-seropositive healthy donor. Profiles of (A) polyclonal, (B) oligoclonal, and (C) monoclonal CDR3 spectratype for Vδ3 cells are shown expressed as relative fluorescent intensity versus the CDR3 length size.
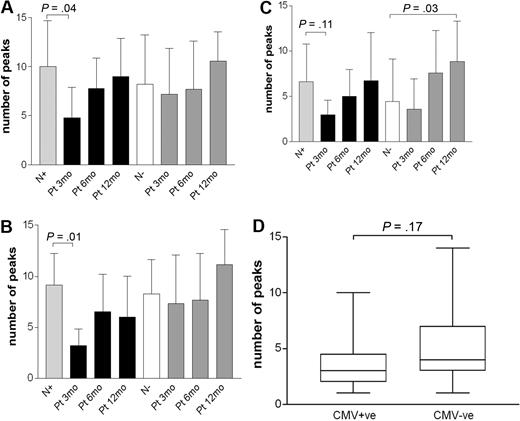
CDR3 spectratyping analysis of the TCR-δ chains in healthy adult donors and in patients after transplantation. Profiles of the CDR3 distribution of the Vδ1 (A), Vδ2 (B), and Vδ3 (C) subsets were generated from sorted CD4−CD8− cells from CMV+ (n = 13) and CMV− (n = 12) healthy donors. Longitudinal study of the γδ T-cell repertoire of the Vδ1, Vδ2, and Vδ3 subsets was carried out in patients during the first 12 months after SCT. (D) TCR Vγ8 chains profiles were generated from CMV+ (n = 19) and CMV− (n = 16) healthy donors. Results are presented as mean ± SD. N indicates healthy adult; Pt, patients; and 3/12, 6/12, and 12/12 indicate the 3-, 6-, and 12-month time points after transplantation, respectively. Samples were analyzed using nonparametric Mann-Whitney test, and P values were determined. P values less than .05 were considered significant.
CDR3 spectratyping analysis of the TCR-δ chains in healthy adult donors and in patients after transplantation. Profiles of the CDR3 distribution of the Vδ1 (A), Vδ2 (B), and Vδ3 (C) subsets were generated from sorted CD4−CD8− cells from CMV+ (n = 13) and CMV− (n = 12) healthy donors. Longitudinal study of the γδ T-cell repertoire of the Vδ1, Vδ2, and Vδ3 subsets was carried out in patients during the first 12 months after SCT. (D) TCR Vγ8 chains profiles were generated from CMV+ (n = 19) and CMV− (n = 16) healthy donors. Results are presented as mean ± SD. N indicates healthy adult; Pt, patients; and 3/12, 6/12, and 12/12 indicate the 3-, 6-, and 12-month time points after transplantation, respectively. Samples were analyzed using nonparametric Mann-Whitney test, and P values were determined. P values less than .05 were considered significant.
In the majority of healthy CMV+ donors, the Vδ1 cells showed oligoclonal profiles in addition to 2 monoclonal expansions; in comparison, CMV− donors showed equal numbers of oligoclonal and polyclonal Vδ1 repertoires. Interestingly, the Vδ3 profiles were predominantly oligoclonal regardless of CMV serostatus, and the decreased diversity was evident with only one of 13 CMV+ and 1/12 CMV− healthy donor showing polyclonal distributions. Overall, the majority of Vδ2pos γδ T cells displayed polyclonal TCR repertoires both in CMV+ and CMV− persons. Together, these results indicate that the peripheral expansions of the Vδ2neg γδ T cells in CMV infection in healthy donors are restricted to some degree but do not strictly correlate with CDR3-dependent selection.
The reconstituted TCR repertoire of Vδ1 and Vδ2 T cells in CMV+/+ patients showed significantly restricted CDR3 profiles at 3 months after transplantation (P = .04 and P = .01, respectively) regardless of whether or not the patients had CMV reactivation. Both subsets gradually increased their diversity at 6 months, resulting in polyclonal distribution at 12 months. TCR profiles of the Vδ3 subset remained mostly oligoclonal during the follow-up and showed significantly more polyclonal repertoire than healthy donors at 12 months. The reconstitution of the TCR repertoire of the Vδ1, Vδ2, and Vδ3 subsets in CMV− patients with CMV− donors showed a relatively wider range of oligoclonal and polyclonal profiles. Taken together, we could find no evidence of a CMV-driven clonal expansion of Vδ2neg γδ T cells in healthy persons or in transplantation recipients.
Recently, a public Vγ8Vδ1 TCR has been identified in CMV-infected newborns33 ; therefore, we analyzed Vγ8 profiles in 35 randomly selected healthy adults (CMV+, n = 19, CMV−, n = 16), as shown in Figure 6D. Oligoclonal Vγ8 repertoires were found in both groups regardless of CMV serostatus in the majority of donors, and no significant difference was detected between the groups.
Expanded Vδ2negative γδ T cells lyse CMV-infected cells in vitro
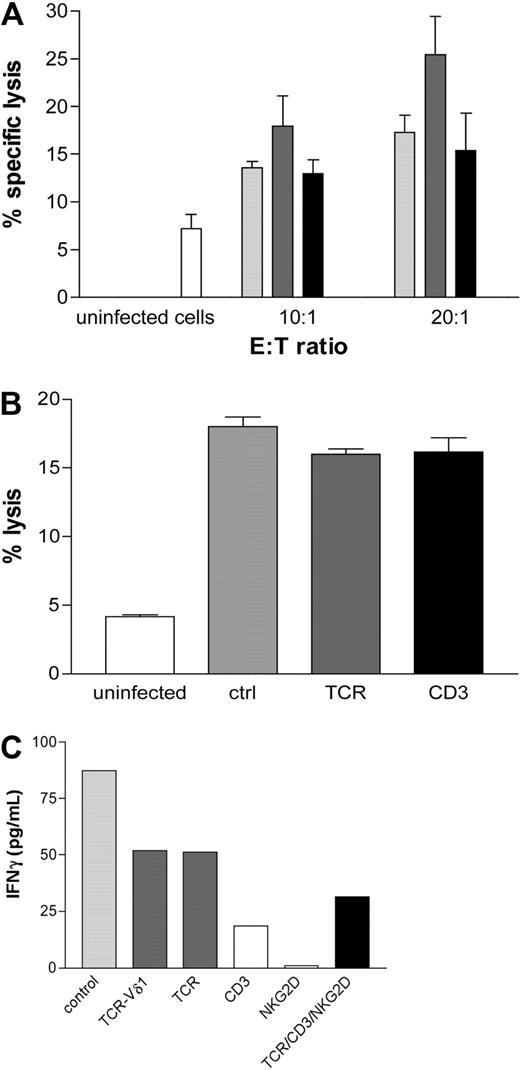
Polyclonal Vδ2neg γδ T-cell lines were generated from CMV-seropositive healthy donors (n = 8) and from a CMV+ patient who received a graft from a CMV+ donor at 2 separate early time points after transplantation. Monolayers of MRC5 fibroblasts infected with TB40E or VHLE clinical CMV strains were cocultured with Vδ2neg cell lines for 4 hours at indicated E:T ratios. Vδ2neg γδ T cells showed specific cytotoxicity by lysis of CMV-infected but not uninfected fibroblasts. Irrespective of the CMV serostatus of the allogeneic feeder cell donors, Vδ2neg cell lines were consistently able to lyse CMV-infected targets (Figure 7A). In contrast, Vδ2pos γδ T cells were unable to lyse CMV-infected fibroblasts (data not shown). The specific reactivity of Vδ2neg cells against CMV was supported by the lack of specific lysis of HSV-infected fibroblasts (data not shown).
Vδ2neg γδ T-cell lines are able to lyse CMV-infected cells in vitro. (A) Monolayers of uninfected MRC5 fibroblasts or infected with TB40E or VHLE clinical CMV strains were cocultured at indicated E:T ratios for 4 hours at 37°C with Vδ2neg γδ T polyclonal cell lines raised from CMV-seropositive healthy donors (n = 8,  ), from a CMV-seropositive transplantation recipient (■) at 2 early time points after transplantation and CMV-seronegative healthy donors (n = 9,
), from a CMV-seropositive transplantation recipient (■) at 2 early time points after transplantation and CMV-seronegative healthy donors (n = 9,  ). Lysis of uninfected cells as control is shown (□). Results are the mean of specific lysis of culture duplicates. CV was always less than 15%. (B) Vδ2neg γδ T-cell lines were cocultured with CMV-infected fibroblasts in the absence or presence of blocking anti-TCR-γδ and anti-CD3 antibodies. Uninfected cell lysis (white bar), control as lysis with no blocking (gray bar), TCR blocking (dark gray bar), and CD3 blocking (black bar). (C) IFN-γ secretion detected in the culture supernatants after 6-hour stimulation coculture of Vδ2neg γδ T-cell line with CMV-infected fibroblasts at 10:1 E:T in the absence (
). Lysis of uninfected cells as control is shown (□). Results are the mean of specific lysis of culture duplicates. CV was always less than 15%. (B) Vδ2neg γδ T-cell lines were cocultured with CMV-infected fibroblasts in the absence or presence of blocking anti-TCR-γδ and anti-CD3 antibodies. Uninfected cell lysis (white bar), control as lysis with no blocking (gray bar), TCR blocking (dark gray bar), and CD3 blocking (black bar). (C) IFN-γ secretion detected in the culture supernatants after 6-hour stimulation coculture of Vδ2neg γδ T-cell line with CMV-infected fibroblasts at 10:1 E:T in the absence ( ) or presence of anti-CD3 (□), anti-NKG2D, anti–TCR-γδ blocking (
) or presence of anti-CD3 (□), anti-NKG2D, anti–TCR-γδ blocking ( ), and TCR/CD3/NKG2D (■).
), and TCR/CD3/NKG2D (■).
Vδ2neg γδ T-cell lines are able to lyse CMV-infected cells in vitro. (A) Monolayers of uninfected MRC5 fibroblasts or infected with TB40E or VHLE clinical CMV strains were cocultured at indicated E:T ratios for 4 hours at 37°C with Vδ2neg γδ T polyclonal cell lines raised from CMV-seropositive healthy donors (n = 8,  ), from a CMV-seropositive transplantation recipient (■) at 2 early time points after transplantation and CMV-seronegative healthy donors (n = 9,
), from a CMV-seropositive transplantation recipient (■) at 2 early time points after transplantation and CMV-seronegative healthy donors (n = 9,  ). Lysis of uninfected cells as control is shown (□). Results are the mean of specific lysis of culture duplicates. CV was always less than 15%. (B) Vδ2neg γδ T-cell lines were cocultured with CMV-infected fibroblasts in the absence or presence of blocking anti-TCR-γδ and anti-CD3 antibodies. Uninfected cell lysis (white bar), control as lysis with no blocking (gray bar), TCR blocking (dark gray bar), and CD3 blocking (black bar). (C) IFN-γ secretion detected in the culture supernatants after 6-hour stimulation coculture of Vδ2neg γδ T-cell line with CMV-infected fibroblasts at 10:1 E:T in the absence (
). Lysis of uninfected cells as control is shown (□). Results are the mean of specific lysis of culture duplicates. CV was always less than 15%. (B) Vδ2neg γδ T-cell lines were cocultured with CMV-infected fibroblasts in the absence or presence of blocking anti-TCR-γδ and anti-CD3 antibodies. Uninfected cell lysis (white bar), control as lysis with no blocking (gray bar), TCR blocking (dark gray bar), and CD3 blocking (black bar). (C) IFN-γ secretion detected in the culture supernatants after 6-hour stimulation coculture of Vδ2neg γδ T-cell line with CMV-infected fibroblasts at 10:1 E:T in the absence ( ) or presence of anti-CD3 (□), anti-NKG2D, anti–TCR-γδ blocking (
) or presence of anti-CD3 (□), anti-NKG2D, anti–TCR-γδ blocking ( ), and TCR/CD3/NKG2D (■).
), and TCR/CD3/NKG2D (■).
Because γδ T cells represent part of the innate immune response, we investigated whether CMV-reactive Vδ2neg γδ T cells could be generated from CMV-seronegative healthy donors. Polyclonal Vδ2neg γδ T-cell lines were successfully raised from multiple CMV-seronegative donors (n = 9) and cocultured with CMV-infected or uninfected MRC5 fibroblasts and showed cytotoxicity (Figure 7A).
To assess the involvement of the TCR and CD3 in the cell lysis, Vδ2neg γδ T-cell lines were cocultured with CMV-infected fibroblasts in the absence or presence of blocking anti-TCR-γδ and anti-CD3 antibodies. Interestingly, we were unable to block the specific lysis of CMV-infected targets (Figure 7B). These results suggest that other antigens and cell interactions are involved in the target killing. However, blocking of CD3, TCR-γδ, and NKG2D separately or in combination inhibited the release of INFγ by T cells confirming that activation of Vδ2neg γδ T cell is mediated through the TCR/CD3 (Figure 7C).
Discussion
It has been known that human γδ T cells display potent antiviral reactivity against several different viruses.36 Elevated numbers of γδ T cells have been previously reported in patients with HIV,37-40 EBV,41,42 and CMV infections.27-30
This study demonstrates the impact of the patient-donor CMV status on the immune reconstitution of the γδ T-cell subsets in patients after SCT. Our results are the first to demonstrate that the expansion of Vδ2neg γδ T cells is the result of ongoing an active anti-CMV response in patients after allogeneic transplantations.
In line with the recent report by Pitard et al,30 we confirmed the finding of significantly increased numbers of Vδ2neg γδ T cells43 in CMV+ healthy volunteers compared with CMV− donors, suggesting an involvement of Vδ2neg γδ T cells in anti-CMV responses in healthy immunocompetent persons. The expansions of Vδ2neg γδ T cells were shown to be independent of those of CMV-specific αβ T cells in CMV+ healthy donors. Patients with episodes of CMV reactivation presented large and long-lasting expansions of Vδ2neg γδ T cells, suggesting a protective role in CMV infection. No correlation between Vδ2neg γδ T cells and the CMV-specific αβ T cells has been found in patients with CMV reactivation, suggesting similar functional properties of both cell populations against CMV.
Previous studies have reported expansion of γδ T cells during viral infections after allogeneic SCT. Cela et al43 documented high frequencies and numbers of total γδ T cells in patients with viral and fungal infections after T-cell depleted bone marrow transplantations, although the relative contribution of Vδ2neg γδ T cells was not reported. Another study described by Hirokawa et al44 reported the expansion of Vδ1 but not Vδ2 γδ T cells during the first 2 months after transplantation. In the patient cohort, all but one patient was CMV+ so the effect of CMV reactivation could not be assessed. In the follow-up paper, 61% of allogeneic SCT patients showed skewed Vδ1 TCR repertoire, but there was no association between Vδ1 TCR clonality and serologic status of CMV or EBV.45
Substantial expansions of Vδ2neg γδ T cells accounting for up to 30% of peripheral T cells have been reported in CMV+ recipients of renal allografts27 and have been associated with resolution of CMV infection.29 This report is the first to confirm this finding in the allogeneic SCT setting.
To analyze the impact of the CMV seropositivity on the repertoire of the γδ T cells, we used TCR-δ chain CDR3 spectratyping for the TCR-δ chains Vδ1, Vδ2, and Vδ3 subsets in patients after transplantation and in healthy donors. The majority of healthy CMV+ donors presented oligoclonal distribution of the Vδ1 profiles in addition to 2 monoclonal expansions. Vδ2pos cells displayed predominantly polyclonal TCR repertoire in both CMV+ and CMV− persons. Similar data have been documented recently.30 Highly restricted distributions of the Vδ3 profiles were observed in healthy adults and patients regardless of their CMV serostatus. Marked expansion of Vδ3 cells with a highly restricted TCR repertoire has been documented in CMV+ renal allograft recipients.27 Our results indicate that expanded Vδ2neg γδ T cells in CMV infection carry some degree of skewed γδ TCR rearrangements in healthy donors, and similar patterns of generally restricted oligoclonal distribution of the Vδ2neg γδ T cells were observed in CMV+/+ patients persisting during the first 12 months after transplantation. The fact that no significant differences in TCR repertoire diversity of expanded Vδ2neg γδ T cells were detected between patients with or without CMV reactivation suggests that the anti-CMV response of γδ T cells does not select unique monoclonal TCRs. This lack of clonal selection of the γδ TCR is similar to earlier reports showing CDR3 independent Vδ1 selection in HIV patients40,46 and in hepatitis C virus-infected patients.47 Of note, large expansions of Vδ2neg γδ T cells were detected also in 2 CMV−/− patients with recurrent infections. Dramatic expansion of Vδ1 γδ T cells in CMV− patient with a syndrome of recurrent fever and infection of unknown etiology has been reported.48 A recent report by Vermijlen et al described an expansion of Vγ8Vδ1 cells in CMV-infected newborns.33 We have analyzed a cohort of 35 healthy adults and found no significant difference between CMV+ and CMV− donors.
To address the question whether expanded Vδ2neg γδ T cells were CMV-reactive, polyclonal Vδ2neg γδ T-cell lines were raised from CMV-seropositive and also CMV-seronegative healthy donors, and both showed cytotoxicity toward CMV-infected MRC5 fibroblasts. In addition, Vδ2neg γδ T-cell lines raised from a CMV-seropositive patient at 2 different time points early after transplantation were also able to kill CMV-infected fibroblasts at the similar level to lines generated from healthy donors. These results represent the first evidence for CMV-reactive γδ T cells in recipient with CMV reactivation early postallogeneic transplantation.
In conclusion, this is the first report examining the role of γδ T cells in immune responses to CMV reactivation in patients after allogeneic SCT. The results in this study demonstrate the antiviral capacity of Vδ2neg γδ T cells directed toward CMV-infected cells, which could be potentially explored as a novel immunotherapy in allogeneic transplantation recipients. Reactivation of CMV infection, which usually occurs in more than 70% in immunocompromised CMV-seropositive patients, is being treated by adoptive cellular therapy developed and extended in our center consisting of infusion of CMV-specific αβ T lymphocytes after transplantation.49,50 Parallel to the cellular therapy, CMV-reactive Vδ2neg γδ T cells could be generated from CMV-seropositive donors and used adoptively in patients with CMV reactivation without a risk of graft-versus-host disease, in addition providing the beneficial GvL effect to patients' immune reconstitution postallogeneic SCT. CMV-reactive Vδ2neg γδ T cells could be also generated from CMV-seronegative donors for CMV-seropositive recipients who are lacking protective CMV immunity and are at greater risk of CMV reactivation. The novel observation of significant expansions of Vδ2neg γδ T cells in CMV-seropositive patients undergoing CMV reactivation after allogeneic SCT will require further investigations to fully determine the role of γδ T cells in immune responses to CMV infection. Additional studies are currently under way in our laboratory.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Julie Dechanet-Merville (Bordeaux, France) for advice on generating polyclonal cell lines, Dr Christian Sinzger (Tubingen, Germany) for CMV strains, Dr Richard Milne (University College London) for HSV-1 strain, Dr Lena Rai for assistance on in-house Vγ8 spectratyping, the BMT team and clinical staff at Crowley Ward at the Royal Free Hospital for help with recruiting patients and collecting blood samples, and all the patients who, by giving their consent, allowed this study to be performed.
This work was supported by the Anthony Nolan Trust. A.K. was supported by Leukaemia & Lymphoma Research.
Authorship
Contribution: A.K. performed research, analyzed data, and wrote the paper; S.G. collected patient blood samples and follow-up data; J.S. analyzed data; P.K. and S.M. discussed clinical data; P.J.T. and A.J.M. designed research and discussed data; and M.W.L. designed research, analyzed data, and contributed to paper writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for P.J.T. is MRC Centre for Regenerative Medicine, Edinburgh, United Kingdom.
Correspondence: Alejandro J. Madrigal, Anthony Nolan Research Institute, Fleet Rd, London NW3 2QG, United Kingdom; e-mail: a.madrigal@medsch.ucl.ac.uk.
References
Author notes
P.J.T. and M.W.L. contributed equally to this study and should be considered as joint senior authors.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal