Abstract
Survival of pediatric acute myeloid leukemia (AML) has improved considerably over the past decades. Since 1985, allogeneic stem cell transplantation (allo-SCT) is widely recommended for patients who have a matched sibling donor. However, it remains controversial whether allo-SCT is superior to chemotherapy for children with newly diagnosed AML. This review summarizes phase 3 clinical trials that compared allo-SCT with chemotherapy (including autologous SCT) in pediatric AML, excluding studies that did not use the intention-to-treat analysis or correct for time-to-transplantation. Although allo-SCT might prevent more relapses than chemotherapy, the number needed for transplantation (with allo-SCT) to prevent one relapse is in the order of 10 patients. Moreover, overall survival is similar with both methods in most recent studies, apparently because of increased salvagability of a relapse when initial therapy concerned chemotherapy only, and because of a higher treatment-related mortality with allo-SCT. Because allo-SCT also gives more severe side effects and results more often in secondary malignancies than chemotherapy, we do not recommend allo-SCT in first remission for pediatric AML in general. Further research should focus on the possibility that subgroups might benefit from allo-SCT, aiming at further improvements in the prognosis of pediatric AML.
Introduction
The treatment of children with acute myeloid leukemia (AML) has improved considerably. This was mainly achieved by more intensive chemotherapy and better supportive care. Currently, up to 90% of all patients achieve remission, and 60% to 65% will be long-term survivors.1
The treatment of AML consists of an induction period with diverse intensive chemotherapy regimens that is based on cytarabine and an anthracycline followed by consolidation. Possibilities for consolidation are allogeneic stem cell transplantation (allo-SCT), autologous stem cell transplantation (auto-SCT), and chemotherapy. For reasons of simplicity, we entitle all transplantations using different sources of hematopoietic stem cells, cord blood, bone marrow, and peripheral blood as SCT in this review.
Allo-SCT is a procedure in which a person receives stem cells from a donor. Currently, patients and donors are typed by molecular techniques for human leukocyte antigen (HLA) class I and II antigens. A well-matched donor matches for at least 9 of 10 alleles at HLA-A, -B, -C, -DRB1, and -DQB1. The donor can be a matched related donor (MRD), most often a matched sibling donor (MSD). If no MRD is available, a matched unrelated donor (MUD) may be available. MUD is sometimes used in high-risk (HR) patients in first complete remission (CR) or after relapse, and more studies suggest that MUD–allo-SCT provides similar outcome as MSD–allo-SCT. For children who lack both a MSD and a MUD, a haploidentical related donor or an unrelated cord blood donor can be used.2
In auto-SCT, the patients' own stem cells are used for rescue after myeloablative therapy. Graft-versus-host disease (GVHD) does not occur, so usually, treatment-related mortality (TRM) is low. However, the relapse rate is higher compared with allo-SCT because a potential graft-versus-leukemia effect is lacking. It is generally accepted that auto-SCT in first remission is not different from chemotherapy in terms of survival.3-7
Since 1985, allo-SCT has been widely recommended for patients with newly diagnosed AML with a MSD after induction chemotherapy. The question addressed in this review is whether results of allo-SCT are better than contemporary chemotherapy for these patients. In the absence of randomized studies that compared allo-SCT with other types of postremission therapy, Mendelian/genetic randomization with intention-to-treat analysis is the least biased method to compare outcomes.8 In an intention-to-treat analysis, the patients are analyzed in the arm to which they are allocated, irrespective of treatment actually received. Other studies use the as-treated analysis, in which the patients are analyzed according to the treatment they actually received. However, this approach has many methodologic pitfalls, as mentioned later in the discussion. The as-treated analysis should at least be corrected for time-to-transplantation to adjust for any events that may occur during the waiting time to transplantation.
It would be helpful to stratify patients into risk groups on the basis of clinical and biologic characteristics and to evaluate response to different treatments within these risk groups to identify the appropriate treatment, including SCT, for each individual patient.1,3 There is heterogeneity in risk group stratification between study groups. However, most groups agree that patients with t(8;21), inv(16), t(16;16), and t(15;17) belong to the low-risk (LR) group, and patients with monosomy 5, monosomy 7, deletions of 5q, or greater than 15% blasts in the marrow after the first course of chemotherapy belong to the HR group.9,10 Most groups agree that children who have acute promyelocytic leukemia (APL), myeloid leukemia of Down syndrome (ML-DS), t(8,21) or inv(16)/t(16,16) should not receive allo-SCT during first remission. However, indications for allo-SCT differ significantly between study groups for the remaining patients. In Europe the tendency is to avoid SCT in CR1, whereas the Children's Oncology Group (COG) recommends allo-SCT in a larger proportion of patients. An overview of the selected clinical trials is given first, followed by a discussion on the usefulness of allo-SCT in perspective of its effectiveness, side effects, and costs compared with chemotherapy only (including auto-SCT) in children and adolescents with newly diagnosed AML.
Methods
PubMed was searched in December 2008 using the search terms “acute myeloid leukemia,” “pediatric,” and “allogeneic stem cell transplantation” and was limited to human trials not started before 1985, reporting patients with AML younger than 21 years and written in English. The point at which the intention-to-treat analysis started, if used, differs per study group. This information was not consistently provided by the investigators of the reviewed studies. To summarize risk group classification for the different study groups was beyond the purpose of this review and has been reviewed previously.10 APL, myelodysplastic syndrome (MDS), secondary AML, and ML-DS were preferred to be excluded from analysis. Event-free survival (EFS) had been calculated from the date of diagnosis to last follow-up or first event (failure to achieve remission, resistant leukemia, relapse, second malignancy, or death from any cause). Overall survival (OS) had been calculated from the date of diagnosis to death of any cause or last follow-up and disease-free survival (DFS) from the date of remission to last date of follow-up or event (relapse, second malignancy, or death of any cause). To calculate the amount of patients who need to receive a transplant to prevent one relapse, the number needed for transplantation (NNT) was used. The NNT was calculated with the following formula: NNT = 1/(EFSallo-SCT − EFSchemotherapy).
Pediatric cooperative group trials that compared allo-SCT with chemotherapy according to the intention-to-treat analysis are summarized in Table 1. Pediatric cooperative group trials that did not use the intention-to-treat analysis but did correct for time-to-transplantation are summarized in Table 2. Trials that did not use one of these analyses are not presented in the tables, because these study results are too flawed.
Studies on allo-SCT versus chemotherapy in pediatric newly diagnosed AML with analysis according to the intention-to-treat principle
| Group/study/patients . | Period . | Indication for allo-SCT in CR1 . | Age, y . | Percentage of EFS/% of DFS . | OS, % . | With donor, % . | Underwent allo-SCT, % . | TRM, % . |
|---|---|---|---|---|---|---|---|---|
| AML BFM 98 + Interim9850 | July 1998 to March 2004 | HR patients n = 275 (CR) | < 18 | 5-y DFS (P = .52) | 5-y OS (P = .16) | 23 | 13 | |
| Total, n = 494 | ||||||||
| Donor, n = 63 | 47 ± 7 | 58 ± 9 | 3 | |||||
| No donor, n = 188 | 43 ± 4 | 56 ± 4 | 4 | |||||
| POG88217,26 | 1988-1993 | All patients | ≤ 21 | 3-y EFS | 3-y OS | 16* | 14† | P = .005 |
| Total, n = 321 | ||||||||
| Allo-SCT, n = 89 | 52 ± 8 | Not given | Not given | |||||
| Auto-SCT, n = 115 | 38 ± 6 (P = .01) | Lower than allo-SCT (P = .007) | 15 | |||||
| Chemo, n = 117 | 36 ± 6 (P = .06) | Similar to allo-SCT (P = .15) | 2.7 | |||||
| CCG 28916,7 | October 1989 to April 1995 | All patients | < 21 | 8-y DFS (chemo vs allo, P = .01) | 8-y OS (chemo vs allo, P = .05) | 28* | 19† | |
| Total, n = 537 | ||||||||
| Allo-SCT, n = 181 | 55 ± 9 | 60 ± 9 | 14 | |||||
| Auto-SCT, n = 177 | 42 ± 8 | 48 ± 8 | 5 | |||||
| Chemo, n = 179 | 47 ± 8 | 53 ± 8 | 4 | |||||
| CCG-251, -213, -2861, -2891, and -294116 | 1979-1996 | All patients | < 21 | 8-y DFS (P = .005) | 8-y OS (P = .026) | 25* | Not given | P < .001 |
| Total, n = 1464 | ||||||||
| Allo-SCT, n = 373 | 47 ± 6 | 54 ± 6 | 17 ± 4 | |||||
| No donor, n = 1091 | 37 ± 4 | 45 ± 4 | 7 ± 2 | |||||
| CCG 296117 | August 1996 to March 2002 | All patients | < 21 | 5-y DFS (P = .021) | 5-y OS (P = .425) | 27* | Not given | P = .007 |
| Total, n = 633 | ||||||||
| Donor, n = 170 | 60 ± 8 | 67 ± 8 | 8 | |||||
| No donor, n = 463 | 50 ± 5 | 62 ± 5 | 3 | |||||
| EORTC-CLG 5892120 | June 1993 to December 2000 | All patients | < 18 | 5-y DFS | 5-y OS | 22† | 19† | |
| Total, n = 145 | ||||||||
| Donor, n = 39 | 63 ± 8 | 78 ± 7 | 8 | |||||
| No donor, n = 106 | 57 ± 5 | 65 ± 5 | 5 | |||||
| UK-MRC AML 104,32 | May 1988 to March 1995 | All patients | < 14 | Not given | 10-y OS (P = .3) | 28† | 20† | P = .001 |
| Total, n = 315 | ||||||||
| Donor, n = 85 | 68 | 9 | ||||||
| No donor, n = 230 | 59 | 1‡ |
| Group/study/patients . | Period . | Indication for allo-SCT in CR1 . | Age, y . | Percentage of EFS/% of DFS . | OS, % . | With donor, % . | Underwent allo-SCT, % . | TRM, % . |
|---|---|---|---|---|---|---|---|---|
| AML BFM 98 + Interim9850 | July 1998 to March 2004 | HR patients n = 275 (CR) | < 18 | 5-y DFS (P = .52) | 5-y OS (P = .16) | 23 | 13 | |
| Total, n = 494 | ||||||||
| Donor, n = 63 | 47 ± 7 | 58 ± 9 | 3 | |||||
| No donor, n = 188 | 43 ± 4 | 56 ± 4 | 4 | |||||
| POG88217,26 | 1988-1993 | All patients | ≤ 21 | 3-y EFS | 3-y OS | 16* | 14† | P = .005 |
| Total, n = 321 | ||||||||
| Allo-SCT, n = 89 | 52 ± 8 | Not given | Not given | |||||
| Auto-SCT, n = 115 | 38 ± 6 (P = .01) | Lower than allo-SCT (P = .007) | 15 | |||||
| Chemo, n = 117 | 36 ± 6 (P = .06) | Similar to allo-SCT (P = .15) | 2.7 | |||||
| CCG 28916,7 | October 1989 to April 1995 | All patients | < 21 | 8-y DFS (chemo vs allo, P = .01) | 8-y OS (chemo vs allo, P = .05) | 28* | 19† | |
| Total, n = 537 | ||||||||
| Allo-SCT, n = 181 | 55 ± 9 | 60 ± 9 | 14 | |||||
| Auto-SCT, n = 177 | 42 ± 8 | 48 ± 8 | 5 | |||||
| Chemo, n = 179 | 47 ± 8 | 53 ± 8 | 4 | |||||
| CCG-251, -213, -2861, -2891, and -294116 | 1979-1996 | All patients | < 21 | 8-y DFS (P = .005) | 8-y OS (P = .026) | 25* | Not given | P < .001 |
| Total, n = 1464 | ||||||||
| Allo-SCT, n = 373 | 47 ± 6 | 54 ± 6 | 17 ± 4 | |||||
| No donor, n = 1091 | 37 ± 4 | 45 ± 4 | 7 ± 2 | |||||
| CCG 296117 | August 1996 to March 2002 | All patients | < 21 | 5-y DFS (P = .021) | 5-y OS (P = .425) | 27* | Not given | P = .007 |
| Total, n = 633 | ||||||||
| Donor, n = 170 | 60 ± 8 | 67 ± 8 | 8 | |||||
| No donor, n = 463 | 50 ± 5 | 62 ± 5 | 3 | |||||
| EORTC-CLG 5892120 | June 1993 to December 2000 | All patients | < 18 | 5-y DFS | 5-y OS | 22† | 19† | |
| Total, n = 145 | ||||||||
| Donor, n = 39 | 63 ± 8 | 78 ± 7 | 8 | |||||
| No donor, n = 106 | 57 ± 5 | 65 ± 5 | 5 | |||||
| UK-MRC AML 104,32 | May 1988 to March 1995 | All patients | < 14 | Not given | 10-y OS (P = .3) | 28† | 20† | P = .001 |
| Total, n = 315 | ||||||||
| Donor, n = 85 | 68 | 9 | ||||||
| No donor, n = 230 | 59 | 1‡ |
CLG indicates Children Leukemia Group; and UK-MRC, United Kingdom Medical Research Council.
Percentage calculated from patients who achieved CR and are eligible for random assignment.
Percentage calculated from total group of patients.
Three deaths, 1 after auto-SCT and 2 after MUD
Studies on allo-SCT versus chemotherapy in pediatric newly diagnosed AML without intention-to-treat analysis but with correction for time-to-transplantation
| Group/study/patients . | Period . | Indication for allo-SCT in CR1 . | Age, y . | Percentage of EFS/% of DFS . | OS, % . | Have a donor, % . | Underwent allo-SCT, % . | TRM, % . |
|---|---|---|---|---|---|---|---|---|
| AIEOP LAM 87-92 trials11 | March 1982 to September 2001 | All patients | < 15 | 5-y DFS | Not given | Not given | 29* (LAM92) | Not given |
| Total, n = 388 | ||||||||
| Allo-SCT, n = 78 | 64 ± 6 | |||||||
| Auto-SCT, n = 110 | 55 ± 5 | |||||||
| Chemo, n = 89 | 28 ± 5 | |||||||
| P | Not given | |||||||
| TCCSG AML M91-13 and AML M96-1430 | August 1991 to September 1998 | All patients | 5-y DFS | Not given | Not given | 20†‡ | Not given | |
| Total, n = 170 | ||||||||
| Allo-SCT, n = 34 | 81 ± 19 | |||||||
| Auto-SCT, n = 32 | ||||||||
| Chemo, n = 104 | 66 ± 10 | |||||||
| P | .22 | |||||||
| AML BFM87 and -9313 | December 1986 to June 1998 | HR patients | < 15 | Not given | 5-y OS | Not given | 11 | |
| Total, n = 356 | ||||||||
| Allo-SCT, n = 39 | 62 ± 3 | 10 | ||||||
| No allo-SCT, n = 317 | 64 ± 8 | Not given | ||||||
| P | .40 | |||||||
| LAME 89/9121 | December 1988 to December 1998 | All patients | < 20 | 5-y DFS | 5-y OS | 26* (LAME91) | 26%* (LAME91) | Not given |
| Total, n = 244 | ||||||||
| Allo-SCT, n = 74 | 57 ± 7 | 71 ± 7 | ||||||
| Chemo, n = 170 | 52 ± 4 | 55 ± 4 | ||||||
| P | .18 | .006 |
| Group/study/patients . | Period . | Indication for allo-SCT in CR1 . | Age, y . | Percentage of EFS/% of DFS . | OS, % . | Have a donor, % . | Underwent allo-SCT, % . | TRM, % . |
|---|---|---|---|---|---|---|---|---|
| AIEOP LAM 87-92 trials11 | March 1982 to September 2001 | All patients | < 15 | 5-y DFS | Not given | Not given | 29* (LAM92) | Not given |
| Total, n = 388 | ||||||||
| Allo-SCT, n = 78 | 64 ± 6 | |||||||
| Auto-SCT, n = 110 | 55 ± 5 | |||||||
| Chemo, n = 89 | 28 ± 5 | |||||||
| P | Not given | |||||||
| TCCSG AML M91-13 and AML M96-1430 | August 1991 to September 1998 | All patients | 5-y DFS | Not given | Not given | 20†‡ | Not given | |
| Total, n = 170 | ||||||||
| Allo-SCT, n = 34 | 81 ± 19 | |||||||
| Auto-SCT, n = 32 | ||||||||
| Chemo, n = 104 | 66 ± 10 | |||||||
| P | .22 | |||||||
| AML BFM87 and -9313 | December 1986 to June 1998 | HR patients | < 15 | Not given | 5-y OS | Not given | 11 | |
| Total, n = 356 | ||||||||
| Allo-SCT, n = 39 | 62 ± 3 | 10 | ||||||
| No allo-SCT, n = 317 | 64 ± 8 | Not given | ||||||
| P | .40 | |||||||
| LAME 89/9121 | December 1988 to December 1998 | All patients | < 20 | 5-y DFS | 5-y OS | 26* (LAME91) | 26%* (LAME91) | Not given |
| Total, n = 244 | ||||||||
| Allo-SCT, n = 74 | 57 ± 7 | 71 ± 7 | ||||||
| Chemo, n = 170 | 52 ± 4 | 55 ± 4 | ||||||
| P | .18 | .006 |
TCCSG indicates Tokyo Children's Cancer Study Group.
Percentage calculated from total group of patients.
Percentage calculated from patients who achieved CR and are eligible for random assignment.
Twenty-four related donors, 8 unrelated donors, and 2 unrelated cord blood donors.
Results
Associazione Italiana di Ematologia e Oncologia Pediatrica
The Associazione Italiana di Ematologia e Oncologia Pediatrica (AIEOP) group has conducted 4 consecutive studies since 1982.11 AIEOP LAM92 included patients with newly diagnosed AML, in the age range from birth to 15 years. All patients received induction and consolidation chemotherapy. This was followed by auto-SCT or allo-SCT if there was a HLA-matched family donor available or consolidation chemotherapy, irrespective of risk group. The patients were stratified into LR and HR groups, which showed different outcomes in OS, 67% ± 6% versus 47% ± 5% respectively (P = .04). When the studies AIEOP LAM87, LAM87M, and LAM92 were taken together, an advantage in 5-year DFS was found for allo-SCT (64% ± 6%) over chemotherapy (28% ± 5%) and auto-SCT (55% ± 5%) after correction for time-to-transplantation (Table 2). We note that all the data should be interpreted with caution because of the retrospective and uncontrolled nature of this analysis. In the most recent AIEOP 2002 study, LR patients are only eligible for SCT after they relapse. HR patients are eligible for allo-SCT if a family donor is available; otherwise, they will undergo auto-SCT after induction chemotherapy (Table 3). For patients in CR2 or patients with M7 AML, unrelated donors for allo-SCT are used.11
Recent and ongoing studies in pediatric AML without information on treatment outcome but with guidelines on risk-group stratified allo-SCT or not
| Group/study . | Period . | Risk groups, % . | Low risk . | Standard risk . | High risk . | Who underwent allo-SCT . |
|---|---|---|---|---|---|---|
| AIEOP LAM 2002 | December 2002 to December 2009 | LR, 18; HR, 82 | Isolated t(8;21), inv(16)/t(16;16), and CR after course 1 | — | All others | HR patients; SR patients after relapse only |
| AML-BFM 200415 | March 2004 to December 2009 | LR, 30; HR, 70 | FAB M1/M2 with Auer rods, FAB M4Eo+, t(8;21), inv(16), and blasts on day 15 < 5% and absence of FLT3/ITD | — | All patients who are not low risk | HR patients (until 2006); afterward no patients in CR1 |
| St Jude AML0830 | March 2008-2013 | LR, 31; SR, 32; HR, 37 | t(8;21) or inv(16) or t(16;16) and good response to therapy | All others | t(6;9), t(8;16), t(16;21), −7, −5, or 5q−, FLT3-ITD unless MRD negative after induction course I, FAB M0 or M6, FAB M7 without t(1;22), treatment related-AML, RAEB-2 or secondary AML after MDS, patients with poor response to therapy (MRD > 5% at day 22, and/or MRD > 0.1% after induction course II) | HR patients; also eligible for natural killer cell therapy, like SR patients |
| NOPHO-AML 200425 | January 2004- 2014 | LR, 80; HR, 20 | Less than 15% blasts after the first and CR after the second course, or t(8;21), inv(16), t(16;16), t(9;11) and CR after the second course | — | 11q23 abnormalities other than t(9;11), > 15% blasts at day 15, or lack of remission after 2 courses of chemotherapy | HR patients |
| COG AAML053118 | August 2006-2010 | LR, 25; SR, 57; HR, 18 | t(8;21), inv(16), t(16;16) | All others | −7, −5, −5q; bone marrow M3 (> 15% blasts) after course 1, except for those with low risk cytogenetics | SR and HR patients |
| ELAM0223 | March 2005-2012 | LR, 14; SR, 81; HR, 5 | t(8;21) | All others | −7, −5q, t(9;22), t(6;9) | SR and HR patients |
| JPLSG AML-05 | November 2006-2013 | LR, 40; SR, 40; HR, 20 | t(8;21), inv(16), t(16;16) | All others | −7, −5q, t(9;22), t(16;21), FLT3/ITD, no CR after course 1 | HR patients |
| MRC/DCOG AML 15 | March 2005 to February 2010 | LR, 30; SR, 55; HR, 15 | t(8;21) and inv(16)/t(16;16), irrespective of marrow status after first course or the presence of other genetic abnormalities | All others | More than 15% blasts after the first course, or adverse cytogenetics [−5, −7, del(5q), abn(3q), t(9;22), complex karyotype] | HR patients |
| Group/study . | Period . | Risk groups, % . | Low risk . | Standard risk . | High risk . | Who underwent allo-SCT . |
|---|---|---|---|---|---|---|
| AIEOP LAM 2002 | December 2002 to December 2009 | LR, 18; HR, 82 | Isolated t(8;21), inv(16)/t(16;16), and CR after course 1 | — | All others | HR patients; SR patients after relapse only |
| AML-BFM 200415 | March 2004 to December 2009 | LR, 30; HR, 70 | FAB M1/M2 with Auer rods, FAB M4Eo+, t(8;21), inv(16), and blasts on day 15 < 5% and absence of FLT3/ITD | — | All patients who are not low risk | HR patients (until 2006); afterward no patients in CR1 |
| St Jude AML0830 | March 2008-2013 | LR, 31; SR, 32; HR, 37 | t(8;21) or inv(16) or t(16;16) and good response to therapy | All others | t(6;9), t(8;16), t(16;21), −7, −5, or 5q−, FLT3-ITD unless MRD negative after induction course I, FAB M0 or M6, FAB M7 without t(1;22), treatment related-AML, RAEB-2 or secondary AML after MDS, patients with poor response to therapy (MRD > 5% at day 22, and/or MRD > 0.1% after induction course II) | HR patients; also eligible for natural killer cell therapy, like SR patients |
| NOPHO-AML 200425 | January 2004- 2014 | LR, 80; HR, 20 | Less than 15% blasts after the first and CR after the second course, or t(8;21), inv(16), t(16;16), t(9;11) and CR after the second course | — | 11q23 abnormalities other than t(9;11), > 15% blasts at day 15, or lack of remission after 2 courses of chemotherapy | HR patients |
| COG AAML053118 | August 2006-2010 | LR, 25; SR, 57; HR, 18 | t(8;21), inv(16), t(16;16) | All others | −7, −5, −5q; bone marrow M3 (> 15% blasts) after course 1, except for those with low risk cytogenetics | SR and HR patients |
| ELAM0223 | March 2005-2012 | LR, 14; SR, 81; HR, 5 | t(8;21) | All others | −7, −5q, t(9;22), t(6;9) | SR and HR patients |
| JPLSG AML-05 | November 2006-2013 | LR, 40; SR, 40; HR, 20 | t(8;21), inv(16), t(16;16) | All others | −7, −5q, t(9;22), t(16;21), FLT3/ITD, no CR after course 1 | HR patients |
| MRC/DCOG AML 15 | March 2005 to February 2010 | LR, 30; SR, 55; HR, 15 | t(8;21) and inv(16)/t(16;16), irrespective of marrow status after first course or the presence of other genetic abnormalities | All others | More than 15% blasts after the first course, or adverse cytogenetics [−5, −7, del(5q), abn(3q), t(9;22), complex karyotype] | HR patients |
FAB indicates French-American, British; RAEB, refractory anemia with excess blasts; NOPHO, Nordic Society of Pediatric Hematology and Oncology; MRC, Medical Research Council; and DCOG, Dutch Childhood Oncology Group.
Berlin-Frankfurt-Munster
The Berlin-Frankfurt-Munster (BFM) group has completed 5 consecutive AML trials since 1978; AML-BFM 78, 83, 87, 93, and 98. Only HR patients were eligible for allo-MSD-SCT. In trial AML-BFM 93, all HR patients received induction and consolidation chemotherapy, followed by allo-SCT if a sibling donor was available. Morphologic French-American-British type and day 15 marrow were used to determine a standard-risk (SR) and a HR group, which had a 5-year survival of 71% ± 4% and 50% ± 3%, respectively. The as-treated results were corrected for time-to- transplantation according the Mantel-Byar analysis.12 For AML-BFM 87 and 93 taken together, the OS of the HR patients who underwent allo-SCT was similar to the OS of HR patients who did not receive a SC transplant (62% ± 3% vs 64% ± 8%; P = .4). In trial AML-BFM 98, patients were again stratified into SR and HR groups, and only HR patients received allo-SC transplants, if they had a matched family donor. Mantel-Byar analysis was used to test the effect of SCT on survival. Five-year OS for HR patients was 54% ± 3%, and it was for SR patients 75% ± 4%. Five-year OS for HR patients with or without SCT in CR1 was similar (as treated, 69% ± 14% vs 64% ± 4%; P = .25).12,13
One aim of trial AML-BFM 98 was to evaluate prospectively the effect of MSD-SCT in HR patients of this study and the following interim phase in 2003. The intention-to-treat analysis showed no advantage of allo-MSD-SCT in children and adolescents with HR-AML (47% ± 7% for 5-year DFS donor vs 43% ± 4% no donor, P = .52; and 58% ± 9% for 5-year OS donor vs 56% ± 4% for no donor, P = .16)14 (Table 1).
The ongoing AML-BFM 2004 trial aims to optimize therapy for children and adolescents with AML. Patients are again stratified into HR and SR subgroups. Chemotherapy is intensified, and the quality of supportive therapy is optimized. During the first 2 years of the trial HR patients received allo-SC transplant in CR1. Since 2006, when the data of the intention-to-treat analysis from trial AML-BFM 98 became available, allo-SCT became restricted to patients in second CR and refractory AML (Table 3).15
Children's Cancer Group (United States)
The Children's Cancer Group (CCG) has completed 5 trials for children younger than 21 years with newly diagnosed AML (CCG-251, -213, -2861, -2891, and -2941). All comparisons were analyzed as intention-to-treat. Overall, children with a donor had a significantly better OS and DFS than did children without donor (54% vs 45%, P = .026 for OS; 47% vs 37%, P = .005 for DFS; Table 1). The relapse risk at 8 years was significantly lower for children with a donor than for children without a donor (36% vs 56%; P < .001).16
In trial CCG-2891, children with a MSD were allocated to allo-SCT, the others were randomly assigned between auto-SCT or consolidation chemotherapy. No risk group stratification was done. Allo-SCT had a significantly better 8-year DFS and OS than did chemotherapy (60% ± 9% vs 53 ± 8%, P = .05 for OS; 55% ± 9% vs 47% ± 8%, P = .01 for DFS; Table 1).6,7
In trial CCG-2961, patients were stratified into 2 risk groups on the basis of cytogenetics. The 5-year OS for LR patients (72%) differed from the OS of HR patients (39%). For consolidation treatment, patients were assigned to allo-SCT or to intensive chemotherapy. Five-year DFS for those with donor was 60% ± 8%, better than the 50% ± 5% of those without donor (P = .021), but OS at 5 years was not significantly different (67% ± 8% vs 62% ± 5%; P = .425; Table 1). In a subsequent subgroup analysis, no significant differences in either DFS or OS among LR patients with and without a donor were seen. Of the HR patients, only 14 patients were left for consolidation treatment. Of these, 57% with donor and 14% without donor survived. In CCG AML trials since 1987, patients with a MRD have had significantly better DFS and OS than patients receiving chemotherapy.17
The CCG and the Pediatric Oncology Group (POG) are now merged into the COG. Because allo-SCT did not result in a significantly better DFS or OS than chemotherapy in LR patients, the COG is no longer recommending MRD-SCT in CR1 for this group of patients (Table 3).18
The COG compared POG 8821, CCG-2891, CCG-2961, and MRC AML10 in a meta-analysis and found significant differences in outcome for SR patients who were eligible for allo-SCT in CR1 compared with consolidation chemotherapy. The 8-year DFS for patients undergoing allo-SCT compared with chemotherapy was 58% ± 7% and 39% ± 5% (P < .01), and the OS was 62% ± 7% and 51% ± 5% (P < .01), respectively. For LR and HR patients no significant differences were found. Without risk stratification, allo-SCT was significantly superior to chemotherapy for OS, DFS, and relapse.9
Dutch Childhood Oncology Group
The Dutch Childhood Oncology Group AML92/94 study included patients younger than 18 years with newly diagnosed AML, ML-DS, myelosarcoma, or AML after MDS.19 As consolidation therapy, patients were eligible for allo-SCT if a MSD was available; the other patients were randomly assigned between auto-SCT and chemotherapy. No significant difference was observed in relapse risk between patients treated with chemotherapy alone and patients who received a transplant. This study was not designed to compare results of chemotherapy with SCT as optimal consolidation, and compliance with the protocols for transplantation was often violated, resulting in strong selection bias.19 In 1998, the Dutch Childhood Oncology Group joined the MRC AML-12 and -15 studies (Table 3).
European Organization for Research and Treatment of Cancer Children Leukemia Group
The Children Leukemia Group of the European Organization for Research and Treatment of Cancer (EORTC) conducted 2 trials, of which the prospective randomized trial 58921 studied the value of early allo-SCT with an HLA-identical sibling versus chemotherapy. Maintenance chemotherapy was given to patients who did not receive a transplant. Included were children younger than 18 years with newly diagnosed AML or HR myelodysplasias. The 5-year DFSs for the donor versus no-donor groups are 63% ± 8% and 57% ± 5% respectively, and the 5-year OSs from CR for donor versus no donor are 78% ± 7% and 65% ± 5%, respectively (Table 1). Relapse occurred in 29% of the donor group and in 38% of the no donor group. No P values have been given because of the insufficient number of events.20
Leucémie Aiguë Myéloblastique Enfant
Trial LAME91 of the Leucémie Aiguë Myéloblastique Enfant (LAME) cooperative group included patients from birth to 20 years of age. After induction and 1 or 2 courses of consolidation chemotherapy, patients with a HLA-identical family donor were allocated to allo-SCT. Although auto-SCT was not scheduled, some children did receive this treatment. Maintenance chemotherapy was only given to patients who did not receive a transplant. The comparison of chemotherapy and allo-SCT was corrected for time-to-transplantation. No formal intention-to-treat analysis was performed, but all patients in CR1 with a HLA-identical family donor actually received the scheduled allograft.21 Allo-SCT resulted in a lower relapse risk (28% ± 14% for allo-SCT vs 47% ± 9% for chemotherapy; P = .02)22 and a nonsignificantly higher 5-year DFS (57% ± 7% for allo-SCT vs no 52% ± 4% for allo-SCT; P = .18).21 The OS for patients who received an allograft was significantly better compared with patients who did not receive an allograft (71% ± 7% vs 55% ± 4%; P = .006; Table 2). Allo-SCT did not significantly improve the outcome of patients with t(8;21)- or inv(16)-positive AML.21 In their subsequent ELAM02 clinical trial they do not offer allo-SCT to patients with t(8;21)23 (Table 3).
Nordic Society of Pediatric Hematology and Oncology
The Nordic Society of Pediatric Hematology and Oncology AML93 trial included patients younger than 17 years.24 Allo-SCT was recommended to all patients in CR1 with a HLA-matched family donor, the other patients mainly received chemotherapy. Auto-SCT was optional in the first part of the trial, on a nonrandomized basis, and MUD was performed in a few patients. Patients who received an auto-SC transplant have been included in the chemotherapy group. Intention-to-treat analysis was not performed, and no correction for time-to-transplantation was done; therefore, results should be interpreted with great caution, and we only refer to overall survival. The OS of patients who underwent allo-SCT or chemotherapy was similar (68%; P = .9). This was due to a significantly better prognosis for the children who relapsed after chemotherapy in CR1 and received a SC transplant in CR2 (EFS = 64% ± 8%) than for children who relapsed after SCT in CR1 (EFS = 18% ± 15%) and children who received chemotherapy only in CR1 and CR2 (EFS = 6% ± 4%).24
Pediatric Oncology Group (United States)
The POG 8821 trial included patients with previously untreated AML. Patients with an isolated myelosarcoma or secondary AML were eligible if the previous treatment was for another type of malignancy. After induction therapy, patients with a MRD were eligible for allo-SCT, others for auto-SCT or consolidation chemotherapy.5 With the use of an intention-to-treat analysis, the 3-year EFS after randomization was better for children who underwent allo-SCT (52% ± 8%) than for those given chemotherapy (36% ± 6%; P = .06) and significantly better than for children who underwent auto-SCT (38% ± 6%; P = .01). OS after allo-SCT was not significantly different to that after chemotherapy (P = .15) but superior to that after auto-SCT (P = .007). No OS rates were given (Table 1).26
In the next POG 9421 study, children younger than 21 years were eligible for allo-SCT if they had a HLA-identical sibling donor; otherwise, they were allocated to consolidation chemotherapy. The 3-year OS for patients who underwent consolidation chemotherapy or allo-SCT were 37% and 67%, respectively (P < .001). These are as-treated data without correction for time-to-transplantation and thus are probably biased.27
St Jude Children's Hospital
Trial AML91 at the St Jude Children's Research Hospital applied allo-SCT if patients had a HLA-matched sibling donor and auto-SCT or chemotherapy in the other patients. After adjustment for time-to-transplantation, patients receiving chemotherapy alone did not have a significantly greater risk of an event than patients receiving allo-SCT or auto-SCT. The use of SCT during first remission did not improve survival, although no percentages were given.28
St Jude trial AML-97 excluded ML-DS in the analysis. Only patients with HR AML were candidates for allo-SCT. Others were eligible for auto-SCT, but in 1998 auto-SCT was substituted with 2 courses of chemotherapy. The final results have not yet been reported.28 Their most recent trial AML-02 also stratified the patients into risk groups (Table 3).29
Japan
The Tokyo Children's Cancer Study Group conducted 2 trials, AML M91-13 and AML M96-14.30 Allo-SCT was allocated in CR1, if a HLA-matched donor, either related or unrelated, was available. Children that did not have a donor were allocated to chemotherapy or auto-SCT (selected by physician's choice). To adjust for time-to-transplantation, the landmark analysis was used. The 5-year DFS of the chemotherapy group was not significantly lower compared with the allo-SCT group (66% ± 10% vs 81% ± 19%, respectively; P = .22; Table 2).30
After these 2 trials, the Japanese Childhood AML Cooperative Study Group conducted trial AML99. In this trial, allo-SCT (related and unrelated) was given in CR1 to all HR patients and was recommended to SR patients with a MRD. The remaining SR patients were randomly assigned between chemotherapy plus auto-SCT or chemotherapy only. However, auto-SCT was eliminated in June 2002. All LR patients received chemotherapy only. For the SR patients, the 5-year DFS of the matched donor group was significantly higher than that of the no donor group (as-treated: 82% [67%-100%] vs 53% [42%-67%]; P = .029), respectively. Five-year OS was not statistically different for matched donor versus no donor (as-treated: 82% [67%-100%] vs 69% [58%-82%]; P = .38). The 5-year OS of 16 HR patients who underwent allo-SCT (6 related SCT, 6 unrelated SCT, and 4 cord blood SCT) was 69%.31
United Kingdom Medical Research Council
The United Kingdom Medical Research Council trials AML10 and AML12 only included patients with de novo AML in their analysis. In MRC AML10 the pediatric part concerned patients younger than 14 years and allocated patients to allo-SCT if they had a matched sibling donor. Patients without a MSD were randomly assigned to receive auto-SCT after 4 courses of consolidation chemotherapy or no further treatment. Comparisons were based on an intention-to-treat analysis.4 Patients with a donor had a significantly reduced relapse risk than did the patients without a donor (26% vs 42%; P = .02), although fewer relapses were counterbalanced by more procedure-related deaths (P = .001). No statistically significant difference was observed in survival between children with or without a donor at 10 years (68% vs 59%; P = .34 ; Table 1). The investigators concluded that allo-SCT or auto-SCT after 4 courses of intensive chemotherapy showed no survival advantage.32
Risk group stratification on the basis of cytogenetics and response to the first course of chemotherapy was done in MRC AML12, for the pediatric part in children younger than 16 years. In this trial, allo-SCT was limited to SR and HR patients. LR patients received chemotherapy only, as well as the SR and HR patients without a donor. No auto-SCT was scheduled. When the results of AML10 and AML12 were combined, no significant improvement of DFS (2 P = .06) or OS (2 P = .1) was seen in any risk group with allo-SCT, analyzed according to the intention-to-treat principle.4 In addition, the 4-year survival with chemotherapy only was 62%.33
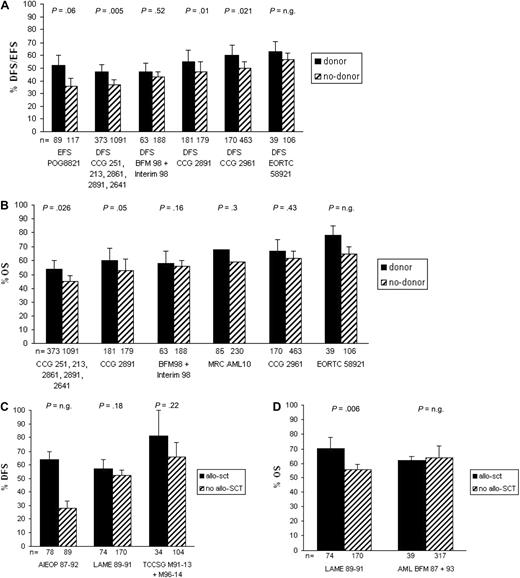
In trial MRC AML15, allo-SCT in CR1 was restricted to HR patients (results not yet published; Table 3; Figure 1).
Survival of studies on allo-SCT versus chemotherapy in pediatric newly diagnosed AML. (A) EFS/DFS for studies analyzed according to the intention-to-treat principle, (B) OS for studies analyzed according to the intention-to-treat principle, and (C) DFS and (D) OS for studies analyzed according to the as-treated principle corrected for time-to-transplantation; n.g. indicates not given. For the BFM98 + Interim98 study, only HR patients were included. The studies are ordered from lowest to highest survival with chemotherapy only.
Survival of studies on allo-SCT versus chemotherapy in pediatric newly diagnosed AML. (A) EFS/DFS for studies analyzed according to the intention-to-treat principle, (B) OS for studies analyzed according to the intention-to-treat principle, and (C) DFS and (D) OS for studies analyzed according to the as-treated principle corrected for time-to-transplantation; n.g. indicates not given. For the BFM98 + Interim98 study, only HR patients were included. The studies are ordered from lowest to highest survival with chemotherapy only.
Number needed to transplant to avoid relapse
To further assess the effectiveness of allo-SCT in comparison with chemotherapy, the number needed for transplantation was calculated. The number needed for transplantation to prevent one relapse varies from 6.3 to 14.3 (6.3, 10, 10, 11.1, 12.5, and 14.3), with a median of 10 patients in the 6 studies, which showed a significant advantage of allo-SCT.6,16,17,21
Toxicity of SCT
Little is known about the incidence of and the risk factors for the late effects of treatment of childhood AML because there have been few real long-term survivors. However, because the number of survivors is increasing, it is important to evaluate the toxicities of the different treatment methods. The survival advantage given by allo-SCT needs to be considered in the context of the risks of the procedure. These include acute and late toxicities and quality of life (QOL), but also financial costs.
The advantages of SCT are often counterbalanced by mortality, related mainly to the complications of infection and GVHD, and the long-term toxicity of the treatment. The TRM rate after allo-SCT of our reviewed studies ranged from 7% to 17% and for chemotherapy from 1% to 8%.7,16,17,20,32,34 Because little information is available on the toxicity of SCT exclusively in children, also information about toxicity in adults is included. Overall, the acute toxicities of SCT include infectious complications, hepatic veno-occlusive disease, interstitial pneumonia, and acute GVHD. The late toxicities of SCT include endocrinologic deficiencies that lead to growth failure and infertility, cataract, neurocognitive abnormalities, chronic GVHD, and cardiac dysfunction. Secondary malignancies occur after SCT in a higher percentage than after chemotherapy only.35,36 Obviously, chemotherapy has side effects as well. The acute toxicities observed after chemotherapy include severe myelosuppression with infections in neutropenia, mucositis, and acute cardiotoxicity. The late toxicities of chemotherapy include late cardiotoxicity, including cardiac failure and dysrhythmia, neurocognitive dysfunction, and secondary malignancies.
The effect on QOL of intensive consolidation therapy and SCT was determined in patients older than 18 years, 1 year after treatment in the MRC AML10 trial. Patients who received SCT had significantly more side effects than patients who received chemotherapy. The side effects after allo-SCT were also worse than after auto-SCT, both of which were worse than after chemotherapy.35 Similar outcomes were reported by Zittoun et al36 They studied 98 patients from 15 to 60 years old after inclusion of the EORTC-GIMEMA AML8A trial and found that QOL, physical condition, and sexual functioning were significantly worse after allo-SCT than after auto-SCT or chemotherapy. Leahey et al37 compared the late effects in 26 survivors of AML and MDS younger than 18 years treated with chemotherapy with or without cranial irradiation and 26 patients treated with chemotherapy followed by either auto-SCT, MSD-SCT, or MRD-SCT. Chemotherapy and SCT had similar adverse effects on growth, renal, and cardiac function. Significantly more patients after SCT needed estrogen supplementation for ovarian failure than did patients who received chemotherapy. Leung et al38 evaluated the late consequences of treatment in 77 patients who survived pediatric AML for more than 10 years. Patients treated with chemotherapy plus total body irradiation as part of allo-SCT had significantly more side effects than patients who received chemotherapy only. Parsons et al39 did a quality-adjusted survival analysis on pediatric trial POG8821, using the quality-adjusted time without symptoms or toxicity (q-TWiST) method. Patients who received allo-SCT spent significantly more time in TOX (the period of treatment-related symptomatic toxicities with grade ≥ 3), more time in TWiST, the period representing the best possible quality of life, during which patients experience no toxicities of treatment or symptoms of disease, had more relapse-free time (P = .03) and time alive (P = .07) than chemotherapy-only patients according to the intention-to-treat principle. The time after relapse was not significantly different, 6.2 months for chemotherapy and 4.5 months for allo-SCT. Finally, Goemans et al40 reported that significantly more children with relapsed AML had late effects if treated with allo-SCT than if treated with chemotherapy only.
Cost-effectiveness of SCT
In the review by Redaelli et al41 it appeared that transplantation is the most expensive procedure in the treatment of AML and is mainly because of the costs of hospitalization. The costs of auto-SCT are 30% to 50% less than the costs of allo-SCT when the costs of relapse are excluded. With the costs of relapse included, allo-SCT is significantly less expensive than auto-SCT because of more relapse after auto-SCT. Three studies have evaluated cost-effectiveness of chemotherapy compared with allo-SCT.42-44 In general, those studies have found that SCT was cost-effective because, despite the higher overall costs of SCT, allo-SCT resulted in longer quality-adjusted survival.41 However, such comparisons may change in case of more effective chemotherapy as used now, at least in pediatric AML.
Uyl-de Groot et al45 performed an analysis of the costs of patients with AML younger than 65 years in 2 different hospitals in the Netherlands, applying either the Hemato-Oncologie voor Volwassenen Nederland 29 protocol (n = 37) or the EORTC AML10 protocol (n = 47). The costs of allo-SCT are the highest in both studies, followed by auto-SCT. Allo-SCT and auto-SCT with the use of marrow-derived SCs appear to be a lot more expensive than for peripheral blood SCT (PBSCT). Therefore, a reduction in costs could be realized when PBSCT would replace marrow transplantation.45 However, most studies in adults that compared PBSCs and SCs from bone marrow as source show increased chronic GVHD after PBSCT. In children, mortality rates were higher after using PBSCs from a MSD for transplantation. However, when using unrelated donors in children, no difference between PBSCs and SCs from bone marrow was observed.46
Hematopoietic SCs from an unrelated cord blood (UCB) transplant are possibly a good option for children that lack a MSD. With an UCB transplant, grafts are readily available, thereby limiting time pressure on transplantation timing as dictated by donor availability, especially in the unrelated setting. However, the antileukemic efficacy of UCB in comparison to other SC sources is currently not completely clear.2
Discussion
The question addressed in this review is whether allo-SCT is a better treatment for children with newly diagnosed AML than chemotherapy alone. A similar analysis was done in 2002. At that time, allo-SCT in CR1 was recommended in Europe for most patients excluding LR patients. However, there was already a trend to reduce the use of SCT in CR1.47
In general, allo-SCT results in a significantly lower relapse risk than chemotherapy alone as consolidation therapy for AML.4,9,16,21,22 In the 7 studies that showed a significant advantage of allo-SCT, the median NNT to prevent one relapse was 10 patients (range, 6-14 patients). Therefore, when all studies would be included, this number will most probably be higher. However, fewer relapses are often counterbalanced by a higher TRM and more toxicity caused by allo-SCT. Moreover, the salvage rate from relapse after allo-SCT in CR1 is often lower than if children had chemotherapy only. Thus, overall survival with allo-SCT is comparable with intensive chemotherapy in most recent studies. An exception is seen in the studies reported by Perel et al21 and Entz-Werle et al,20 who report improved OS with allo-SCT, with larger differences in favor of allo-SCT between OS and DFS/EFS, suggesting increased salvage rate for relapse after allo-SCT in CR1. However, a confounder in both studies was the maintenance chemotherapy given only to patients who did not receive a transplant in CR1. This maintenance chemotherapy results in inferior outcome,21,48 and thus has a negative effect on long-term outcome of the chemotherapy only group.
Despite lack of convincing evidence in favor of allo-SCT in newly diagnosed pediatric AML, it cannot be excluded that subgroups benefit. There is consensus that the LR group has an OS with chemotherapy only, which is so good that allo-SCT is not applied in these patients. In SR patients, however, a benefit of allo-SCT was found in some studies, and the meta-analysis on CCG, POG, and MRC AML 10 studies by Horan et al9 reported a significant benefit in DFS and OS for SR patients treated with allo-SCT. Tsukimoto et al31 found this benefit only for DFS but not for OS in the Japanese study, whereas in the United Kingdom Medical Research Council AML trials no benefit was found at all. It remains an open question whether SR patients benefit from allo-SCT in this time of effective chemotherapy, and this requires further clinical research, especially to determine which patients, if any, within this group will actually benefit from allo-SCT.
The studies that reported subgroup analyses did not show a benefit of allo-SCT for HR patients.4,9,14 Only study CCG-296117 suggested a benefit, but patient numbers were small, and no P values were provided. Thus, allo-SCT cannot be considered standard of care in HR patients, and, if done, it should be performed in the setting of a clinical study.
Another key question in general is the timing of allo-SCT in the context of the chemotherapy protocol. Allo-SCT given after only 1 or 2 courses of chemotherapy might have inferior results because of relatively high minimal residual disease levels in such a situation. However, allo-SCT given after 5 courses of intensive chemotherapy may not add much antileukemic activity any more. This issue was beyond the limits of the present review.
Despite limited information about the cost-effectiveness of allo-SCT compared with chemotherapy, it is clear that allo-SCT is more expensive than chemotherapy. Most studies that reported about the cost-effectiveness (done in adults) conclude that allo-SCT is more cost-effective than chemotherapy.41-44 However, the survival rates with chemotherapy only are increasing, so a more recent analysis on this important subject is warranted, including pediatric studies.
Because chemotherapy has become significantly more intensive and effective in the past decades, the actual survival benefit of allo-SCT has become less and statistically nonsignificant for the total group of patients. Comparison of outcome for patients treated with chemotherapy alone and patients who also underwent allo-SCT is difficult for several reasons. (1) There is considerable heterogeneity of the type and intensity of pretransplantation chemotherapy and also for the chemotherapy for patients who did not receive a transplant. The amount and type of preceding chemotherapy may have an effect on the efficacy of allo-SCT. (2) Only patients in remission normally undergo SCT (refractory disease, early relapse, and early treatment-related death in CR excluded), which wrongly suggests allo-SCT to be more effective. (3) Patients with severe side effects can often not undergo SCT, also leading to a selection bias. (4) Social and cultural circumstances could also influence the actual compliance to treatment. In some studies only 60% to 70% of patients with a donor actually received a transplant. (5) However, not all the eligible patients have a compatible sibling, and the availability of a donor might be correlated with outcome. It might be that patients in families with a large number of siblings have a different prognosis with treatment than patients with a low number of siblings. This could be explained by socioeconomic or ethnic differences.
In lack of actual randomized studies, the best approach is, therefore, to compare patient groups by the availability of a donor by intention-to-treat analysis. The intention-to-treat analysis is important to avoid selection bias as explained earlier. However, the intention-to-treat analysis also has some limitations. For example, caution should be taken in the estimation of the effect of treatment because of dilution due to noncompliance, and interpretation becomes difficult if a large proportion of participants cross over to opposite treatment arms.8 Subsequently, in some studies the rules of the intention-to-treat analysis were not followed correctly.48,49
Conclusions
The value of allo-SCT in pediatric AML remains controversial and depends on the results obtained with chemotherapy alone. During the past 10 to 20 years not only the efficacy of intensive chemotherapy and salvage therapy after relapse has improved, but also methodical problems comparing treatment groups are better taken in account. This has reinforced the trend to reduce the indication for allo-SCT in CR1.47 Most groups agree that allo-SCT is not to be recommended to LR patients in CR1 as defined by APL, ML-DS, t(8;21), and inv(16). For remaining patients, most recent studies with good results with chemotherapy only do not show a benefit for allo-SCT in terms of OS or even DFS. Possible exceptions are the studies LAME 89/9121 and EORTC-Children Leukemia Group 58921,20 which showed an OS benefit with allo-SCT. However, those studies have maintenance therapy as the main confounder as was discussed earlier.
On the basis of the data reviewed, allo-SCT is not recommended for good-risk pediatric patients with newly diagnosed AML, has unknown efficacy in high-risk patients, and is used by some groups, but not others, for standard-risk patients.
Authorship
Contribution: D.N. wrote the paper; and U.C., M.B.B., and G.J.L.K. discussed the format and content of the article and contributed to the writing, review, and editing of the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Gertjan J. L. Kaspers, VU University Medical Center, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands; e-mail: gjl.kaspers@vumc.nl.