Abstract
T-cell ubiquitin ligand-2 (TULA-2) is a recently discovered histidine tyrosine phosphatase thought to be ubiquitously expressed. In this work, we have investigated whether TULA-2 has a key role in platelet glycoprotein VI (GPVI) signaling. This study indicates that TULA-2 is expressed in human and murine platelets and is able to associate with Syk and dephosphorylate it. Ablation of TULA-2 resulted in hyperphosphorylation of Syk and its downstream effector phospholipase C-γ2 as well as enhanced GPVI-mediated platelet functional responses. In addition, shorter bleeding times and a prothrombotic phenotype were observed in mice lacking TULA-2. We therefore propose that TULA-2 is the primary tyrosine phosphatase mediating the dephosphorylation of Syk and thus functions as a negative regulator of GPVI signaling in platelets.
Introduction
The platelet is the primary hemostatic cell that circulates in the bloodstream in a quiescent, discoid state. After damage to the vascular wall, collagen, as part of the extracellular matrix, becomes exposed. Platelets interact with the exposed collagen. This serves as a trigger that causes platelet activation and the development of a hemostatic plug to arrest bleeding.1 Of all the receptors expressed on the platelet surface, it is thought that the glycoprotein VI (GPVI)/Fc receptor-γ chain (FcR-γ chain) complex is the primary collagen receptor to elicit the activation of platelets.2 After the interaction with collagen, a signaling cascade is initiated downstream of the GPVI receptor involving numerous proteins.2 On binding of collagen to GPVI, it is thought that clustering of the receptor occurs and phosphorylation of the immunoreceptor tyrosine-based activation motif (ITAM) of the FcR-γ chain follows.3 This event is thought to be mediated by the Src-family tyrosine kinases Fyn and Lyn, which are constitutively associated with GPVI.4 After phosphorylation of the ITAM, the tyrosine kinase, spleen tyrosine kinase (Syk), is recruited to the phosphorylated ITAM.5 Once bound, Syk undergoes tyrosine phosphorylation at multiple sites, including Y525 and Y526 (Y519/20 in murine Syk) to increase its kinase activity.6 The activation of Syk leads to the formation of a protein complex containing several adapter proteins, such as linker-for-activation of T cells, and kinases, such as phosphatidylinositol-3-kinase that leads to the eventual phosphorylation and activation of phospholipase C-γ2 (PLC-γ2) and release of Ca2+ from intracellular stores.7 As a result, the secretion of several autocoids occurs that in turn activate nearby circulating platelets to recruit them to the site of injury to form a stable platelet plug. The central role of Syk in GPVI signaling is undisputed, as Syk-deficient platelets fail to aggregate, secrete stored granules, form arachidonic acid, and exhibit no PLC-γ2 phosphorylation in response to GPVI agonists.8
Although a lot of work has been undertaken to reveal the molecular mechanisms of GPVI-mediated platelet activation, less is known about negative regulation of the signaling cascade. In T cells, such negative regulation is mediated by T-cell ubiquitin ligand-2 (TULA-2), a histidine phosphatase. TULA-2, also known as either suppressor of T-cell receptor signaling-1 or ubiquitin-associated domain (UBA) and SH3 domain-containing protein B (UBASH3B), and TULA (suppressor of T-cell receptor signaling-2; UBASH3A) belong to the TULA family of proteins.9 Both family members have been shown to be important negative regulators of T-cell signaling, with T cells deficient in both TULA and TULA-2 displaying a hyper-responsiveness to T-cell receptor engagement as well as hyperphosphorylation of the Syk homolog ζ-chain-associated protein kinase 70.10 More recently, it has been shown that the TULA family of proteins possesses tyrosine phosphatase activity, with TULA-2 exhibiting manyfold greater activity than TULA and that the phosphatase activity of TULA-2 is partly responsible for the hyperactivity of T cells deficient in both TULA and TULA-2.11-13 In addition, it has been shown that TULA-2 can associate with Syk in HEK293T cells overexpressing Syk and TULA-2 and TULA-2 exerts a specificity toward Syk, in terms of dephosphorylation, over other tyrosine kinases.11 Given the critical importance of Syk in the GPVI signaling cascade and the similarities between T-cell receptor and GPVI signaling, we investigated the possible role of TULA family proteins in the negative regulation of Syk activity in GPVI-mediated platelet activation.
Methods
Materials
All reagents were from Sigma-Aldrich unless stated. Anti-Syk (4D10), anti-Syk (01), anti-Syk (N-19), anti–PLC-γ2 (B10), anti–α tubulin (B7), anti–β tubulin (D10), anti–horseradish peroxidase-conjugated goat anti–mouse, anti–rat, or anti–rabbit immunoglobulin G (IgG) antibodies, and protein A/G PLUS agarose were purchased from Santa Cruz Biotechnology. Anti–phosphospecific-Syk (Tyr525/6), PLC-γ2 (Tyr759), and anti-Erk 1/2 (3A7) antibodies were purchased from Cell Signaling Technology. Anti–TULA-2 antibody was purchased from Rockland Immunochemicals. Antiphosphotyrosine (4G10) was purchased from Millipore. Anti-GPVI (JAQ1) was purchased from Emfret. Infrared dye-labeled goat anti–mouse and anti–rabbit IgG antibodies were purchased from Licor. Type I collagen was purchased from Chronolog. Convulxin was purified as described by Polgar et al.14 Collagen-related peptide (CRP) was purchased from Dr Richard Farndale. Anti-TULA antibody was produced as previously described.15 Glutathione S transferase (GST)–Syk and FURA2-AM were purchased from Invitrogen.
Synthesis and purification of GST-TULA-2 and GST-TULA-2 H380A
TULA-2 and TULA-2 H380A were amplified by polymerase chain reaction from a pAlterMax plasmid containing the TULA-2 or TULA-2 H380A gene. The polymerase chain reaction products were then purified, cut with KpnI and XhoI, ligated into pAcGHLT-B, and transformed into XL-10 Gold competent cells (Agilent Technologies). Purified TULA-2 or TULA-2 H380A containing pAcGHLT-B was then used to express and purify TULA-2 and TULA-2 H380A protein from Sf9 cells according to the manufacturer's instructions (Baculogold Transfection kit, catalog no. 560129, BD Biosciences).
Human platelet isolation and preparation
Blood was drawn from informed healthy volunteers according to a protocol approved by the Institutional Review Board of Temple University in accordance with the Declaration of Helsinki into one-sixth volume acid citrate dextrose (85mM sodium citrate, 111mM glucose, 71.4mM citric acid). Platelet-rich plasma (PRP) was isolated by centrifugation at 200g for 15 minutes and incubated with 1mM aspirin for 30 minutes at 37°C. Platelets were obtained by centrifugation for 10 minutes at 800g and resuspended in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid–buffered Tyrode solution (10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4, 137mM NaCl, 2.7mM KCl, 2mM MgCl2, 0.42mM NaH2PO4, 5mM glucose), 0.2% bovine serum albumin, and 0.2 U/mL apyrase.
Murine platelet isolation and preparation
All mice were maintained and housed in a specific pathogen-free facility, and animal procedures were carried out in accordance with the institutional guidelines after the Temple University Animal Care and Use Committee approved the study protocol. Blood was drawn via cardiac puncture into one-tenth volume of 3.8% sodium citrate. Blood was then spun at 100g for 10 minutes and the PRP removed. Red blood cells were mixed with 400 μL 3.8% sodium citrate and spun for a further 10 minutes at 100g. Resulting PRPs were combined, 100nM carbacyclin added and centrifuged for 10 minutes at 400g. Platelet-poor plasma was removed, and the platelet pellet resuspended in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid-buffered Tyrode solution (pH 7.4) containing 0.2 U/mL apyrase.
Murine platelet preparation for Ca2+ mobilization
Murine blood was drawn as described in “Murine platelet isolation and preparation” and mixed with 4 volumes of piperazine-1,4-bis-2-ethanesulfonic acid-buffered Tyrode solution (pH 6.5) containing 500μM ethyleneglycoltetraacetic acid, 10μM indomethacin, and 100nM carbacyclin. The mixture was centrifuged at 100g for 15 minutes at room temperature. The PRP was removed, diluted to 10 mL with the aforementioned buffer, and centrifuged at 800g for 15 minutes at room temperature. The platelet pellet was resuspended in the aforementioned buffer (1 mL per mouse) and incubated with 5μM FURA-2 AM for 45 minutes at room temperature. The platelets were centrifuged at 800g for 10 minutes at room temperature and finally resuspended in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid–buffered Tyrode solution. The platelets were allowed to recover for 15 minutes at room temperature before starting the experiment. The details of Ca2+ measurement have been previously documented.16 The data in the graph represent the maximal Ca2+ concentration minus basal Ca2+ concentration.
Platelet activation
Samples (300 or 500 μL) of washed platelets were placed into a lumi-dual aggregometer (Chronolog) set at a temperature of 37°C and stirring rate of 1200 rpm. The samples were then treated as described in “Results” for each experiment.
Platelet-dense granule secretion measurements
Adenosine triphosphate secretion was monitored using a luciferin-luciferase reagent (DuPont) at 1.6 mg/mL and was performed simultaneously with the platelet activation.
Lysate preparation
After activation, platelets were either treated with one-tenth volume 6.6M HClO4 to precipitate the protein, centrifuged for 10 minutes at 12 000g at 4°C, washed with deionized H2O, and resuspended in 1 × sample buffer (62.5mM Tris, pH 6.8, 2% sodium dodecyl sulfate [SDS], 10% glycerol, 100mM dithiothreitol, 0.01% bromophenol blue) or lysed with 500 μL 2 × RIPA (100mM Tris, pH 7.4, 300mM NaCl, 2% Nonidet P-40, 0.2% SDS, 1% deoxycholate) or 2 × NP-40 (100mM Tris, pH 7.4, 300mM NaCl, 2% Nonidet P40) lysis buffer or 2 × NP-40 supplemented with 0.2% SDS), incubated on ice for 10 minutes, and centrifuged for 10 minutes at 12 000g at 4°C or 30 minutes at 100 000g at 4°C.
GST pulldown assay
Cleared lysates were incubated with 2 μg of agarose-coupled GST-TULA-2 H380A for 1 hour at 4°C while rocking, or equimolar agarose-coupled GST as a negative control. After 1 hour, the beads were pelleted and washed 3 times with 1 × NP-40 lysis buffer; 2× sample buffer was then added to the samples, boiled for 5 minutes, and the samples subjected to SDS–polyacrylamide gel electrophoresis and immunoblotting.
Coimmunoprecipitation assay
After lysis with 2 × NP-40 supplemented with 0.2% SDS and ultracentrifugation, platelet lysates were incubated with 2 μg/mL of anti-TULA-2 antibody or rabbit IgG at 4°C while rocking. After 1 hour, 20 μL/mL protein A/G agarose was added and incubated overnight at 4°C with rocking. The next day, agarose beads were washed 5 times for 5 minutes with 4 mL of 1 × NP-40 lysis buffer with 0.1% SDS; 2 × sample buffer was then added to the samples, boiled for 5 minutes, and the samples subjected to SDS-polyacrylamide gel electrophoresis and immunoblotting.
Immunoblotting
Prepared proteins were resolved on SDS-polyacrylamide gels and transferred to either Immobilon-P (Millipore) or nitrocellulose membranes (Whatman). Immobilon-P membranes were blocked with 5% nonfat dry milk in Tris-buffered saline with 0.05% Tween-20 (TBS-T), and nitrocellulose membranes were blocked with Odyssey blocking buffer (Licor). Membranes were probed overnight at 4°C with the desired primary antibody and then washed 4 times with TBS-T. Immobilon-P membranes were incubated with horseradish peroxidase-conjugated secondary antibody and nitrocellulose membranes incubated with infrared dye-labeled secondary antibodies for 60 minutes at room temperature and washed 4 times with TBS-T. Immobilon-P membranes were developed using chemiluminescent substrate (Millipore) and visualized using the Fujifilm LAS-1000 (Fujifilm). Nitrocellulose membranes were developed using the Odyssey imaging system (Licor)
In vitro kinase assay
Kinase assays were performed as previously described17 with the following modifications: Syk was immunoprecipitated from wild-type and TULA-dKO mice using anti-Syk clone N-19 polyclonal antibody. Blots were probed for tubulin as a loading control.
FeCl3-induced in vivo thrombosis injury model
The left common carotid artery from mice was exposed, and a miniature Doppler flow probe placed around the artery. To injure, the arterial blood flow was stopped and a 1 × 2-mm 7.5% FeCl3-soaked piece of filter paper was placed on the artery for 2 minutes followed by rinsing with saline. Blood flow was then resumed and monitored for 30 minutes, and the time to occlusion and thrombus stability was noted. A stable thrombus was defined as one that fully occluded the vessel for at least 5 minutes.
Tail-bleeding assay
Bleeding time assay was performed as previously described.18
Statistical analysis
All statistics were calculated using GraphPad Prism software (version 5.0c). Time courses were analyzed by 2-way analysis of variance. Dose-response curves were fit to a simple hyperbolic equation, and differences between the fits of wild-type and mutant cells were determined using comparisons built into GraphPad Prism software (version 5.0c). All other data were analyzed using a Student t test.
Results
TULA-2 is expressed in platelets
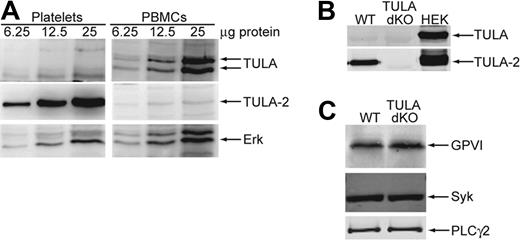
To evaluate the role of the TULA family proteins in platelet GPVI signaling, we investigated whether these proteins are expressed in human and murine platelets using Western blot analysis. Previous reports indicate that TULA-2 is ubiquitously expressed; however, TULA expression is confined to lymphoid cells.9,10,15,19,20 Figure 1A shows a Western blot of increasing amounts of human platelet protein and peripheral blood mononucleocytes (PBMCs) probed with antibodies against TULA and TULA-2. The results indicate a high expression of TULA-2 in human platelets compared with PMBCs and no expression of TULA in human platelets. The doublet for TULA seen in the PBMCs represents both the long- and short-form splice variants of TULA, which are both found in PBMCs.9 We further analyzed platelet lysates from wild-type mice and mice deficient in both TULA family members (TULA-dKO) as well as HEK293T cells overexpressing each family member. As shown in Figure 1B, TULA-2 is clearly present in the wild-type platelets and not in TULA-dKO platelets, whereas no TULA is detectable in either set of murine platelets. These data suggest that TULA-2 is the only TULA family member expressed in human and murine platelets.
Expression of TULA family members in human and murine platelets and expression of GPVI signaling proteins in murine platelets. (A) Increasing amounts of human platelet and PBMC protein were blotted and probed for TULA family members or the loading control Erk. (B) TULA family protein expression is compared in wild-type and TULA-dKO murine platelets with HEK293T cells overexpressing each member as a positive control. (C) Relative expression of GPVI, Syk, and PLC-γ2 is compared between wild-type and TULA-dKO murine platelets.
Expression of TULA family members in human and murine platelets and expression of GPVI signaling proteins in murine platelets. (A) Increasing amounts of human platelet and PBMC protein were blotted and probed for TULA family members or the loading control Erk. (B) TULA family protein expression is compared in wild-type and TULA-dKO murine platelets with HEK293T cells overexpressing each member as a positive control. (C) Relative expression of GPVI, Syk, and PLC-γ2 is compared between wild-type and TULA-dKO murine platelets.
TULA-2 can dephosphorylate Syk
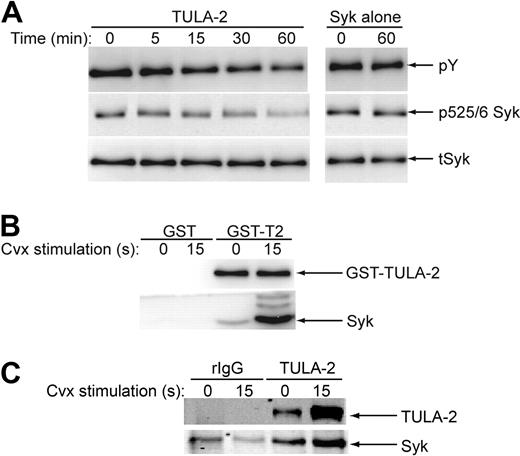
As TULA-2 dephosphorylates tyrosine kinases in T cells and Syk is a key tyrosine kinase downstream of GPVI signaling in platelets, we sought to ascertain whether full-length TULA-2 could dephosphorylate Syk. Full-length GST-TULA-2 was heterologously expressed and purified from Sf9 insect cells and incubated with phosphorylated Syk. As shown in Figure 2A, full-length TULA-2 caused a reduction in the total tyrosine phosphorylation of Syk as well as dephosphorylation at tyrosines 525 and 526 in the activation loop of Syk. These data further confirm that Syk is a substrate for TULA-2.
TULA-2 dephosphorylation of Syk and association with Syk. (A) Active TULA-2 was purified from Sf9 cells, incubated with phosphorylated Syk for the indicated times, analyzed by Western blotting, and probed for phosphotyrosine, pY525/6 Syk, and total Syk. (B) NP-40 lysed lysates from convulxin-treated and untreated human platelets were precleared and incubated with GST-TULA-2 (H380A) or GST. Pulldowns were analyzed by Western blotting and probed for Syk and TULA-2. (C) NP-40 with 0.1% SDS-lysed lysates from convulxin-treated and untreated human platelets were precleared and incubated with rabbit IgG or anti–TULA-2 antibody. Immunoprecipitates were analyzed by Western blotting and probed for Syk and TULA-2. All blots are representative of 3 experiments.
TULA-2 dephosphorylation of Syk and association with Syk. (A) Active TULA-2 was purified from Sf9 cells, incubated with phosphorylated Syk for the indicated times, analyzed by Western blotting, and probed for phosphotyrosine, pY525/6 Syk, and total Syk. (B) NP-40 lysed lysates from convulxin-treated and untreated human platelets were precleared and incubated with GST-TULA-2 (H380A) or GST. Pulldowns were analyzed by Western blotting and probed for Syk and TULA-2. (C) NP-40 with 0.1% SDS-lysed lysates from convulxin-treated and untreated human platelets were precleared and incubated with rabbit IgG or anti–TULA-2 antibody. Immunoprecipitates were analyzed by Western blotting and probed for Syk and TULA-2. All blots are representative of 3 experiments.
TULA-2 can associate with Syk
Previous work has shown that TULA-2 can associate with Syk in HEK293T cells overexpressing Syk and TULA-2.11 Therefore, we investigated whether Syk and TULA-2 can form a complex in platelets. Unstimulated and convulxin-stimulated precleared platelet lysates were incubated with agarose-coupled full-length enzymatically inactive mutant GST-TULA-2 (GST-TULA H380A). The resulting pulldown proteins were then probed for Syk to evaluate whether a complex is formed between Syk and TULA-2. Figure 2B shows the Western blots from the GST pulldown and shows an agonist-dependent interaction between Syk and TULA-2. In addition to GST pulldowns, coimmunoprecipitation studies were performed. Precleared lysates from unstimulated and convulxin-stimulated platelet lysates were incubated with anti-TULA-2 antibodyand the immunoprecipitates probed for Syk via Western blotting. Figure 2C shows the resulting Western blots and confirms that Syk and TULA-2 associate in platelets. These data suggest that a complex forms between TULA-2 and Syk after GPVI activation, which may allow TULA-2 to negatively regulate Syk phosphorylation downstream of GPVI signaling.
Lack of TULA-2 causes aberrant Syk phosphorylation
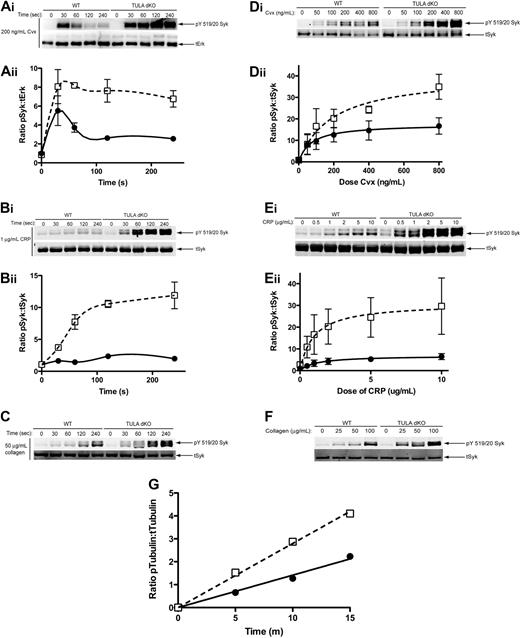
Having established that TULA-2, but not TULA, is expressed in platelets and can interact with Syk and dephosphorylate it, we investigated the role of TULA-2 in regulating Syk phosphorylation in platelets. These studies were performed in TULA-dKO murine platelets; and as TULA is not expressed in platelets (Figure 1A-B), the effects observed are the result of lack of TULA-2 in these mice. Figure 3Ai shows a time course of Syk phosphorylation from wild-type and TULA-dKO platelets using convulxin. In the wild-type platelets, an increase in Syk phosphorylation was observed after activation, peaking at 30 seconds, followed by a dephosphorylation of Syk. However, in the mutant platelets, a hyperphosphorylation of Syk compared with wild-type platelets was seen at all time points. In addition, the TULA-dKO platelets lacked an apparent dephosphorylation seen at later time points in the wild-type platelets. CRP, a peptide more directly related to collagen, produced slower phosphorylation of Syk. However, the difference between Syk phosphorylation in TULA-dKO and wild-type platelets was greater after stimulation with CRP than it was after convulxin treatment (Figure 3Bi). Plotting of the mean Syk phosphorylation from 3 independent experiments for convulxin and CRP time courses and subsequent statistical analysis indicated a significant potentiation of Syk phosphorylation in TULA-dKO platelets (Figure 3Aii,Bii). Figure 3C shows that the physiologic agonist collagen produces an increase in Syk phosphorylation in both wild-type and TULA-dKO platelets up to 4 minutes. Again, a hyperphosphorylation of Syk was seen in the TULA-dKO platelets.
Syk phosphorylation in wild-type and TULA-dKO murine platelets. (Ai) Isolated wild-type and TULA-dKO platelets were stimulated with 200 ng/mL convulxin for 0, 30, 60 120, and 240 seconds, the protein precipitated, and pY519/20 Syk and total Erk were probed. (Aii) Mean relative Syk phosphorylation of 3 experiments for covulxin in wild-type (■) and TULA-dKO (□) platelets was plotted against time and a 2-way analysis of variance performed (P < .001, WT vs dKO). (Bi) Same as subpanel Ai, except that platelets were stimulated with 1 μg/mL CRP and total Syk was used as a loading control. (Bii) Same as subpanel Aii, except that CRP was used as agonist (P < .001, WT vs dKO). (Ci) Same as subpanel Bi, except that platelets were stimulated with 50 μg/mL collagen. (Di) Isolated wild-type and TULA-dKO platelets were stimulated for 2 minutes with 50, 100, 200, 400, or 800 ng/mL convulxin, processed for Western blotting, and probed for pY519/20 Syk and total Syk. (Dii) Same as subpanel Aii, except that Syk phosphorylation was plotted as a function of convulxin and curves were fitted using a nonlinear regression (P < .001, WT vs dKO). (Ei) Same as subpanel Di, except that platelets were stimulated with 0.5, 1, 2, 5, or 10 μg/mL CRP. (Eii) Same as subpanel Dii, except that CRP was used as agonist (P < .001, WT vs dKO). (F) Same as subpanel Di, except that platelets were stimulated for 1 minute with 25, 50, or 100 μg/mL collagen. (G) Isolated wild-type and TULA dKO platelets were left untreated or treated with 200 ng/mL convulxin for 4 minutes. Kinase activity of the resulting immunoprecipitates was then measured and plotted as function of time. Shown is a representative plot from 5 independent experiments.
Syk phosphorylation in wild-type and TULA-dKO murine platelets. (Ai) Isolated wild-type and TULA-dKO platelets were stimulated with 200 ng/mL convulxin for 0, 30, 60 120, and 240 seconds, the protein precipitated, and pY519/20 Syk and total Erk were probed. (Aii) Mean relative Syk phosphorylation of 3 experiments for covulxin in wild-type (■) and TULA-dKO (□) platelets was plotted against time and a 2-way analysis of variance performed (P < .001, WT vs dKO). (Bi) Same as subpanel Ai, except that platelets were stimulated with 1 μg/mL CRP and total Syk was used as a loading control. (Bii) Same as subpanel Aii, except that CRP was used as agonist (P < .001, WT vs dKO). (Ci) Same as subpanel Bi, except that platelets were stimulated with 50 μg/mL collagen. (Di) Isolated wild-type and TULA-dKO platelets were stimulated for 2 minutes with 50, 100, 200, 400, or 800 ng/mL convulxin, processed for Western blotting, and probed for pY519/20 Syk and total Syk. (Dii) Same as subpanel Aii, except that Syk phosphorylation was plotted as a function of convulxin and curves were fitted using a nonlinear regression (P < .001, WT vs dKO). (Ei) Same as subpanel Di, except that platelets were stimulated with 0.5, 1, 2, 5, or 10 μg/mL CRP. (Eii) Same as subpanel Dii, except that CRP was used as agonist (P < .001, WT vs dKO). (F) Same as subpanel Di, except that platelets were stimulated for 1 minute with 25, 50, or 100 μg/mL collagen. (G) Isolated wild-type and TULA dKO platelets were left untreated or treated with 200 ng/mL convulxin for 4 minutes. Kinase activity of the resulting immunoprecipitates was then measured and plotted as function of time. Shown is a representative plot from 5 independent experiments.
In addition to the time course of Syk phosphorylation, we measured dose-responses for convulxin-, CRP-, and collagen-mediated Syk phosphorylation in wild-type and TULA-dKO murine platelets. Figures 3Di, Ei, and F are representative blots of convulxin, CRP, and collagen dose-responses, respectively, from wild-type and TULA-dKO murine platelets. These blots show that a potentiation of Syk phosphorylation occurs in the TULA-dKO platelets, across all doses of agonist with all 3 GPVI agonists. Plotting mean Syk phosphorylation from 3 independent experiments against dose for convulxin and CRP, fitting using a nonlinear regression, and comparing the curves indicated a significant potentiation of Syk phosphorylation in TULA-dKO platelets (Figure 3Dii,Eii).
Although an increase in Syk phosphorylation at tyrosines 519 and 520 is usually taken as evidence of increased kinase activity, we took this a step further by performing in vitro kinase assays using Syk immunoprecipitated from wild-type and TULA-dKO platelets to test whether Syk immunoprecipitated from TULA-dKO platelets exhibited increased kinase activity compared with Syk from wild-type platelets. Platelets were treated with convulxin for 4 minutes before lysis and immunoprecipitation. The activity of Syk was assessed by the phosphorylation of a known substrate tubulin.17 Figure 3G shows a representative time course from 5 independent experiments in which tubulin phosphorylation by Syk was monitored. An increased rate of tubulin phosphorylation by Syk immunoprecipitated from TULA-dKO platelets compared with wild-type platelet is evident. This suggests that TULA-2 negatively regulates Syk kinase activity in GPVI-stimulated platelets and confirms that phosphorylation of Tyr519/520 reflects Syk activity in our system.
It is possible that hyperphosphorylation of Syk could be a result in part of the hyperphosphorylation of the FcR-γ chain ITAM motif. Thus, we compared FcR-γ chain phosphorylation in wild-type and TULA-deficient platelets. Supplemental Figure 1A (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) shows that there is no significant increase in FcR-γ chain phosphorylation in TULA-dKO platelets. In the same experiment, Syk was hyperphosphorylated at Y519/20. Quantitation of phosphorylation for 3 independent experiments indicated that there was no statistically significant difference in FcR-γ chain phosphorylation (supplemental Figure 1B) between wild-type and TULA-dKO platelets, although Syk was significantly hyperphosphorylated in the TULA-dKO platelets. These data suggest that TULA-2 does not regulate FcR-γ chain phosphorylation and confirms that TULA-2 directly regulates Syk phosphorylation.
Lack of TULA-2 causes aberrant PLC-γ2 phosphorylation
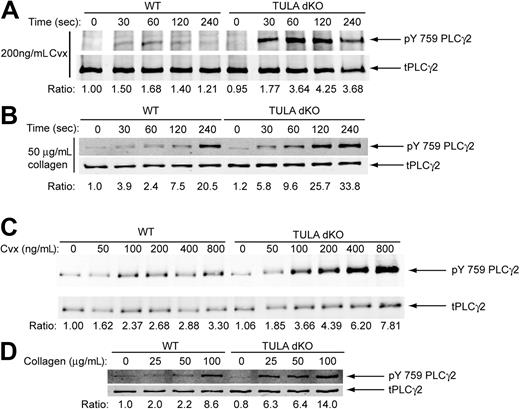
PLC-γ2 is the phospholipase C isoform downstream of Syk activation and responsible for mobilization of calcium and platelet activation downstream of GPVI.21-24 Thus, we assessed the phosphorylation of PLC-γ2 tyrosine 759, an important residue for its activation,22,25 in wild-type and TULA-dKO platelets to determine whether increased Syk phosphorylation caused a concurrent increase in PLC-γ2 phosphorylation. A time course of PLC-γ2 phosphorylation after GPVI stimulation with convulxin and collagen is shown in Figure 4A and Figure 4B, respectively. Similar to the Syk phosphorylation time course (Figures 3Ai,C), PLC-γ2 exhibits enhanced and sustained phosphorylation in the TULA-dKO platelets. In addition, convulxin and collagen-induced PLC-γ2 phosphorylation was potentiated in a dose-dependent manner in TULA-dKO platelets (Figure 4C and Figure 4D, respectively), mirroring the phosphorylation of Syk (Figure 3Di,F). PLC-γ2 was also hyperphosphorylated in TULA-dKO platelets in response to CRP (data not shown).
PLC-γ2 phosphorylation in wild-type and TULA-dKO murine platelets. (A) Isolated wild-type and TULA-dKO platelets were stimulated with 200 ng/mL convulxin for 0, 30, 60, 120, and 240 seconds, the protein precipitated, analyzed by Western blotting, and pY759 PLC-γ2 and total PLC-γ2 probed. (B) Same as panel A, except that platelets were stimulated with 50 μg/mL collagen. (C) Isolated wild-type and TULA-dKO platelets were stimulated for 2 minutes with 50, 100, 200, 400, or 800 ng/mL convulxin, processed for Western blotting, and probed for pY759 PLC-γ2 and total PLC-γ2 probed. (D) Same as panel C, except that platelets were stimulated with 25, 50, or 100 μg/mL collagen. Ratios were derived by dividing pY759 PLC-γ2 band quantitation by total PLC-γ2 band quantitation. Convulxin blots are representative of 3 experiments.
PLC-γ2 phosphorylation in wild-type and TULA-dKO murine platelets. (A) Isolated wild-type and TULA-dKO platelets were stimulated with 200 ng/mL convulxin for 0, 30, 60, 120, and 240 seconds, the protein precipitated, analyzed by Western blotting, and pY759 PLC-γ2 and total PLC-γ2 probed. (B) Same as panel A, except that platelets were stimulated with 50 μg/mL collagen. (C) Isolated wild-type and TULA-dKO platelets were stimulated for 2 minutes with 50, 100, 200, 400, or 800 ng/mL convulxin, processed for Western blotting, and probed for pY759 PLC-γ2 and total PLC-γ2 probed. (D) Same as panel C, except that platelets were stimulated with 25, 50, or 100 μg/mL collagen. Ratios were derived by dividing pY759 PLC-γ2 band quantitation by total PLC-γ2 band quantitation. Convulxin blots are representative of 3 experiments.
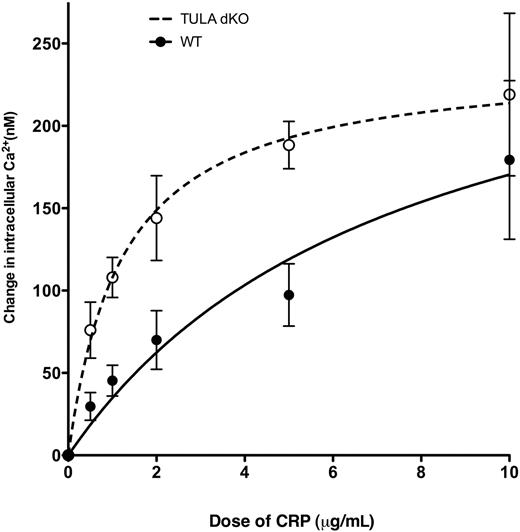
Ca2+ mobilization is potentiated in platelets deficient in TULA-2
Hyperphosphorylation of PLC-γ2 (Figure 4) is a reflection of its hyperactivity leading to increased generation of IP3 and mobilization of intracellular calcium. Thus, Ca2+ mobilization after GPVI activation was monitored in wild-type and TULA-dKO murine platelets to assess whether the apparent lack of Syk regulation in TULA-dKO platelets led to a potentiation of Ca2+ release. Figure 5 shows a graph of change in Ca2+ versus CRP concentration and shows a potentiation of Ca2+ mobilization in TULA-dKO murine platelets compared with wild-type. Statistical analysis of the curve fits showed a statistically significant difference between the groups (P = .001).
Ca2+ mobilization in wild-type and TULA-dKO platelets. FURA-2-AM-loaded murine platelets were stimulated with various doses of CRP in the presence of 100μM 2MeSAMP, 10μM MRS2719, 150nM echistatin, 10μM indomethacin, and 2mM probenecid, and the Ca2+ mobilization was measured using a spectrofluorometer. The change in intracellular calcium was then measured, plotted against dose of CRP, and fitted with a hyperbolic curve. Data points are constructed from 3 independent experiments.
Ca2+ mobilization in wild-type and TULA-dKO platelets. FURA-2-AM-loaded murine platelets were stimulated with various doses of CRP in the presence of 100μM 2MeSAMP, 10μM MRS2719, 150nM echistatin, 10μM indomethacin, and 2mM probenecid, and the Ca2+ mobilization was measured using a spectrofluorometer. The change in intracellular calcium was then measured, plotted against dose of CRP, and fitted with a hyperbolic curve. Data points are constructed from 3 independent experiments.
Platelet functional responses are potentiated in platelets deficient in TULA-2
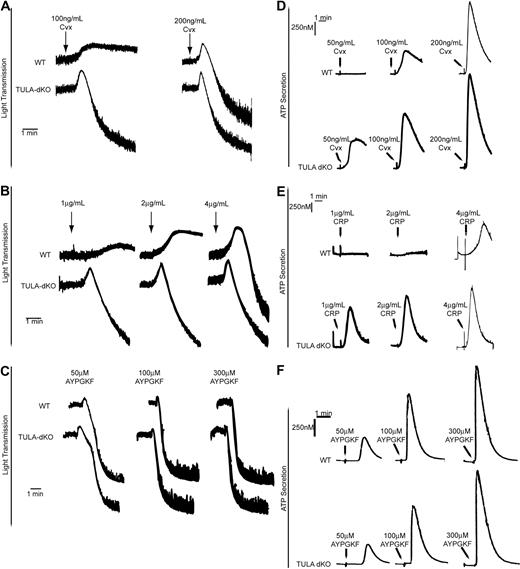
To test whether the increased GPVI signaling in TULA-dKO platelets resulted in enhanced platelet responses, we evaluated the platelet functional responses in TULA-dKO platelets. We first studied platelet aggregation; and as shown in Figure 6, GPVI-mediated platelet aggregation in TULA-dKO murine platelets was potentiated with the GPVI agonists convulxin (Figure 6A) and CRP (Figure 6B), respectively. No difference in platelet aggregation was observed with the protease-activated receptor 4 (PAR4) agonist AYPGKF (Figure 6C).
Platelet aggregation and dense granule secretion in wild-type and TULA-dKO platelets. (A) Isolated wild-type and TULA-dKO platelets were stimulated with 100 ng/mL or 200 ng/mL convulxin, and the change in light transmittance was observed. (B) Same as panel A, except that platelets were stimulated with 1, 2, or 4 μg/mL CRP. (C) Same as panel A, except that platelets were stimulated with 50, 100, or 300μM AYPGKF. (D) Simultaneous to platelet aggregation studies, dense granule secretion was measured by monitoring adenosine triphosphate release using a luciferin-luciferase reagent; 50, 100, and 200 ng/mL convulxin were used. (E) Same as panel D, except that platelets were stimulated with 1, 2, or 4 μg/mL CRP. (F) Same as panel D, except that platelets were stimulated with 50, 100, or 300μM AYPGKF. All traces are representative of 3 experiments.
Platelet aggregation and dense granule secretion in wild-type and TULA-dKO platelets. (A) Isolated wild-type and TULA-dKO platelets were stimulated with 100 ng/mL or 200 ng/mL convulxin, and the change in light transmittance was observed. (B) Same as panel A, except that platelets were stimulated with 1, 2, or 4 μg/mL CRP. (C) Same as panel A, except that platelets were stimulated with 50, 100, or 300μM AYPGKF. (D) Simultaneous to platelet aggregation studies, dense granule secretion was measured by monitoring adenosine triphosphate release using a luciferin-luciferase reagent; 50, 100, and 200 ng/mL convulxin were used. (E) Same as panel D, except that platelets were stimulated with 1, 2, or 4 μg/mL CRP. (F) Same as panel D, except that platelets were stimulated with 50, 100, or 300μM AYPGKF. All traces are representative of 3 experiments.
To further study the functional role of TULA-2 in GPVI signaling, dense granule secretion was compared between wild-type and TULA-dKO murine platelets in response to convulxin and CRP. A potentiation of dense granule secretion was observed in TULA-dKO platelets, relative to wild-type murine platelets, in response to convulxin (Figure 6D) and CRP (Figure 6E), respectively. Dense granule secretion downstream of the PAR4 receptor was not significantly different in the TULA-dKO platelets compared with wild-type platelets (Figure 6F). These data suggest that TULA-2's major functional role is in the negative regulation of GPVI signaling.
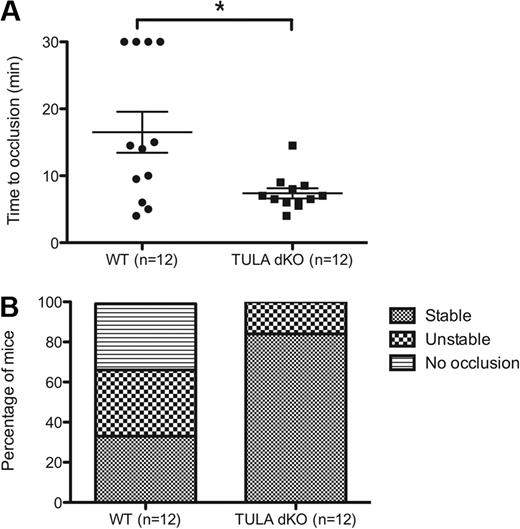
TULA-2-deficient mice are prothrombotic and have shorter bleeding times
As functional responses in the platelets isolated from TULA-dKO are enhanced in response to GPVI agonists, we evaluated the in vivo physiologic implications of these enhanced responses. To evaluate this, the well-established FeCl3-induced thrombosis injury model was used.26 Damage to the endothelial layer by FeCl3 leads to the exposure of subendothelial collagen, so it is an appropriate model to use to test enhanced GPVI signaling.27 Isolated carotid arteries from wild-type and TULA-dKO mice were injured with 7.5% FeCl3 for 2 minutes, and the time to occlusion and the thrombus stability was measured. The time to occlusion for wild-type and TULA-dKO mice is shown in Figure 7A, and the results clearly show a statistically significant shorter time to occlusion in the TULA-dKO mice (P < .05). Figure 7B is a plot of thrombus stability and shows an enhanced thrombus stability in TULA-dKO mice compared with wild-type, with 87% of mice forming stable thrombi in the TULA-dKO mice and only 33% in the wild-type.
FeCl3 thrombosis injury model in wild-type and TULA-dKO murine platelets. Wild-type and TULA-dKO mice were subjected to injury of the left carotid artery by 7.5% FeCl3 for 2 minutes, and the time to occlusion and thrombus stability was noted. (A) Plot of time to occlusion for each group of mice; n = 12 for each group. Calculations were 16.5 ± 3 minutes for wild-type and 7.37 ± 0.76 minutes for TULA-dKO (mean ± SD). Statistical analysis was performed using an unpaired t test. (B) Plot of thrombus stability, defined as a complete occlusion for at least 5 minutes.
FeCl3 thrombosis injury model in wild-type and TULA-dKO murine platelets. Wild-type and TULA-dKO mice were subjected to injury of the left carotid artery by 7.5% FeCl3 for 2 minutes, and the time to occlusion and thrombus stability was noted. (A) Plot of time to occlusion for each group of mice; n = 12 for each group. Calculations were 16.5 ± 3 minutes for wild-type and 7.37 ± 0.76 minutes for TULA-dKO (mean ± SD). Statistical analysis was performed using an unpaired t test. (B) Plot of thrombus stability, defined as a complete occlusion for at least 5 minutes.
To assess the role of TULA-2 in hemostatic function, tail-bleed assays were performed in wild-type and TULA-dKO mice. The mean time to cessation of bleeding the TULA-dKO mice was significantly shorter compared with wild-type mice (P > .047). Wild-type mice had a mean bleeding time of 188 plus or minus 47 seconds (n = 12) and TULA-dKO mice had a mean bleeding time of 98 plus or minus 17 seconds (n = 11). These data, taken together with the thrombosis injury model, indicate that TULA-2 has a physiologically relevant function in vivo to negatively regulate hemostasis and thrombus formation.
Discussion
The platelet GPVI receptor is important for the initiation of platelet activation and hemostasis after damage to the vascular endothelium. Therefore, its negative regulation is of critical importance to prevent unnecessary platelet activation and potentially harmful thrombus formation. In the studies presented here, we have shown that the histidine phosphatase TULA-2 is a negative regulator of GPVI signaling by virtue of its association with Syk and its subsequent dephosphorylation and has an important functional role in vivo to prevent uncontrolled thrombus formation.
Both members of the TULA family of proteins have been proposed to be negative regulators of signaling; indeed, in T cells, depletion of both TULA and TULA-2 is required to see a hyper-responsive phenotype.10 Reexpresssion of TULA-2 in TULA double-deficient T cells leads to substantial reversal of the phenotype, indicating that TULA-2 is responsible, in part, for the phenotype observed.13 In our studies, we show that only TULA-2 is expressed in platelets to a measurable extent. This finding is not surprising, as TULA is thought to be a protein expressed primarily in lymphoid cells.9 Therefore, any effects seen in platelets by the depletion of TULA proteins are the result of a TULA-2 deficiency.
In these studies, we show an increase in Syk phosphorylation in TULA-dKO murine platelets (Figure 3). Furthermore, we show that TULA-2 does not regulate the dephosphorylation of the FcR-γ chain ITAM motif (supplemental Figure 1). These data are consistent with the proposed phosphatase function of TULA-2 and are in agreement with Agrawal et al11 who have shown that TULA-2 can associate with Syk and preferentially dephosphorylate Syk over other tyrosine kinases. The hyperphosphorylation of Syk seen in our study, and the association and dephosphorylation of Syk (Figure 2) suggest a similar mechanism of negative regulation of Syk by TULA-2 is occurring in platelets. However, in T cells, hyperphosphorylation of the Syk homolog ZAP-70 is only observed in cells deficient in both TULA family members and not TULA-2(−/−) cells.10,13 This suggests that the negative regulation of Zap-70 after T-cell receptor engagement relies on both TULA and TULA-2. Thus, the TULA-2-mediated negative regulation of signaling may differ slightly between platelets and T cells.
In this study, we have also presented data that shows hyperphosphorylation of PLC-γ2 after GPVI activation in platelets deficient in TULA-2. These data could be interpreted in 2 ways. First, PLC-γ2 could be a substrate for TULA-2; thus, a lack of TULA-2 leads to PLC-γ2 hyperphosphorylation and enhanced platelet function. Second, hyperphosphorylation of Syk in TULA-2-deficient platelets leads to increased kinase activity of Syk and therefore increased GPVI signaling, leading to enhanced PLC-γ2 phosphorylation and platelet activation. We think that the second interpretation is most probably based on the dephosphorylation of Syk by TULA-2 and the association between Syk and TULA-2 seen in Figure 2. In addition, data published by Agrawal et al,11 which indicate that Syk is a substrate for TULA-2, and data published by Mikhailik et al,13 demonstrating that TULA-2 can dephosphorylate the Syk homolog Zap-70, are consistent with this interpretation.
Calcium mobilization is a critical event in the activation of platelets. Thus, we tested whether the lack of Syk regulation by TULA-2 in TULA-2-deficient platelets had a concomitant effect on calcium mobilization. The potentiation of calcium mobilization seen in TULA-2-deficient platelets is consistent with the negative regulatory function of TULA-2 in GPVI signaling and correlates with the hyperphosphorylation of Syk and PLC-γ2 presented here. The enhanced Ca2+ mobilization leads to the increased platelet functionality seen in aggregation, secretion, and possibly the in vivo thrombosis model.
In addition to potentiated Syk and PLC-γ2 phosphorylation and calcium mobilization, our data also show an enhancement of platelet functional responses in TULA-dKO murine platelets (Figure 6). These data are consistent with data published in T cells after T-cell receptor engagement where TULA double-deficient T cells exhibit a hyper-responsive phenotype to T-cell receptor engagement.10 Our data are also in agreement with data published by Raguz et al,28 indicating that epidermal growth factor receptor signaling is also negatively regulated by TULA-2.
The enhanced platelet function when TULA-2 is deleted appears to be specific to GPVI signaling because no such enhanced function was found downstream of the G-protein coupled receptor PAR-4. In terms of the PAR-4 receptor, we observed no difference in platelet aggregation or dense granule secretion in TULA-dKO platelets.
To assess the role of TULA-2 in thrombus formation in vivo, the ferric chloride thrombosis model was used. Before performing these experiments, the relative expression of the critical proteins in the GPVI signaling cascade was measured to ensure up-regulated protein expression of these proteins because deletion of TULA-2 would not affect the in vivo studies. Expression of GPVI, Syk, and PLC-γ2 in wild-type and TULA-dKO murine platelets was comparable (Figure 1C). Our data show that there is an enhanced time to occlusion in TULA-dKO mice, and the thrombi formed by 7.5% FeCl3 injury are more stable in TULA-dKO mice compared with wild-type. This is consistent with the hypothesis that TULA-2 is a negative regulator of GPVI signaling and correlates with the enhanced phosphorylation of GPVI signaling proteins, Ca2+ mobilization, and enhanced GPVI-mediated platelet functional responses shown here. These data are also consistent with in vivo thrombosis model data published for other reported negative regulators of platelet function. Falati et al29 reported that platelet endothelial cell adhesion molecule-1-deficient mice are prothrombotic, indicated by a shorter time to occlusion of the carotid artery in the FeCl3 thrombosis model. In addition, carcinoembryonic antigen cell adhesion molecule-1-deficient mice also exhibit a prothrombotic phenotype, when mesenteric arterioles are subject to FeCl3 injury.30
In addition to the FeCl3-induced thrombosis injury model tail-bleeding times were performed in wild-type and TULA-dKO mice. Bleeding times were significantly shorter in TULA-dKO mice. These data are consistent with the proposed role of TULA-2 in the negative regulation of GPVI signaling.
The data presented here suggest that the histidine phosphatase TULA-2 has an important negative regulatory role in GPVI signaling. However, little is known about the mechanisms regulating the activation of TULA-2.9 Previous work has shown that TULA-2 can undergo phosphorylation at tyrosine 19 after CD3 ligation; however, no function has been attributed to this phosphorylation.31 Another mechanism proposed to regulate the function of TULA-2 is its modification with a ubiquitin residue, which blocks the ability of TULA-2 to interact with ubiquitinated proteins by forming an intramolecular interaction with its UBA.32 However, our results provide no indication of either ubiquitination or tyrosine phosphorylation of TULA-2 in platelets (data not shown). Therefore, the mechanisms by which TULA-2 is regulated in platelets remain to be investigated.
In conclusion, the data presented here suggest that the phosphatase TULA-2 is an important negative regulator of GPVI-mediated platelet functional responses and does so by interacting with Syk and dephosphorylating it. This may serve to prevent unnecessary and uncontrolled platelet activation and the onset of thrombosis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Monica Dupon for her assistance with the tail-bleed assays.
This work was supported by the Pennsylvania Department of Health (research grants) (J.L.D.), the Southeastern Pennsylvania Affiliate of the American Heart Association (J.L.D.), and the National Institutes of Health (grants HL60683, HL80444, and HL81322 to S.P.K. and CA 78499 to A.Y.T.).
National Institutes of Health
Authorship
Contribution: D.H.T. designed and performed experiments and wrote the manuscript; T.M.G., T.N.N., and C.A.D. performed experiments; N.C. contributed vital reagents and animals; S.P.K. designed experiments and analyzed data; A.Y.T. provided direction, designed experiments, and analyzed data; and J.L.D. provided overall direction, designed experiments, and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James L. Daniel, Department of Pharmacology, Temple University Medical School, 3420 N. Broad St, Philadelphia, PA 19140; e-mail: jdaniel@temple.edu; or Alexander Y. Tsygankov, Department of Microbiology, Temple University Medical School, 3420 N. Broad St, Philadelphia, PA 19140; e-mail: tsygan@temple.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal