Abstract
Osteoporosis is a frequent problem in disorders characterized by iron overload, such as the thalassemias and hereditary hemochromatosis. The exact role of iron in the development of osteoporosis in these disorders is not established. To define the effect of iron excess in bone, we generated an iron-overloaded mouse by injecting iron dextran at 2 doses into C57/BL6 mice for 2 months. Compared with the placebo group, iron-overloaded mice exhibited dose-dependent increased tissue iron content, changes in bone composition, and trabecular and cortical thinning of bone accompanied by increased bone resorption. Iron-overloaded mice had increased reactive oxygen species and elevated serum tumor necrosis factor-α and interleukin-6 concentrations that correlated with severity of iron overload. Treatment of iron-overloaded mice with the antioxidant N-acetyl-L-cysteine prevented the development of trabecular but not cortical bone abnormalities. This is the first study to demonstrate that iron overload in mice results in increased bone resorption and oxidative stress, leading to changes in bone microarchitecture and material properties and thus bone loss.
Introduction
Transfusion of red blood cells can be a life-saving therapy for patients with congenital or acquired anemias, such as the thalassemias, aplastic anemias, and myelodysplastic syndromes. Patients with sickle cell disease and severe vaso-occlusive complications may also be placed on regular transfusions. Each unit of transfused blood contains 220 to 250 mg of iron bound to hemoglobin, an amount much larger than the 1 to 2 mg of iron that is absorbed daily to maintain normal human iron homeostasis.1,2 Because there is no way of excreting iron in humans, chronic transfusions lead to progressive and pathologic iron accumulation. Nontransfusional iron overload can occur in conditions in which ongoing erythroid destruction leads to inappropriately high intestinal iron absorption. For example, iron overload is a feature in β-thalassemia intermedia, a condition for which most affected persons are not transfused. Other examples include congenital dyserythropoietic anemia and sideroblastic anemia. Finally, hereditary hemochromatosis, which is a genetic disorder characterized by high intestinal iron absorption, also results in increased tissue iron accumulation.1,3
Osteoporosis and fractures occur frequently in disorders associated with iron overload, such as the thalassemias4,5 and hereditary hemochromatosis.6 Whether this is caused by a direct effect of iron on bone or is the result of concomitant endocrine deficiencies, such as hypogonadism, ineffective erythropoiesis, other comorbidities that may affect bone metabolism, or the effect of chronic illness itself remains unclear.5-8 Recent limited animal and in vitro data support the idea that iron excess directly controls bone formation and/or remodeling.9-11
In this report, we developed a mouse model of iron overload in which C57/BL6 mice were injected with iron dextran. This approach resulted in increased iron content in various organs, including liver, spleen, and heart, similar to that seen in transfused patients with iron excess.12 We found that BL6 iron-overloaded mice have trabecular and cortical bone abnormalities that are related to the severity of tissue iron overload. In addition, our results ascertain the adverse role of iron in the development of low bone mass in states of iron excess through a process that involves increased oxidative stress (reactive oxygen species [ROS]), with accompanying increases in serum levels of the pro-osteoclastogenic cytokines tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6).
Methods
Animals
Groups (n = 8) of 2-month-old C57/BL6 male mice were treated intraperitoneally once a week for 2 months with low (0.1 g/kg) or high (1.0 g/kg) iron dextran [a complex of ferric hydroxide, Fe(OH)3 and low molecular weight (5000) dextran] or placebo (phosphate-buffered saline). To evaluate the role of ROS, additional groups were given high-dose iron as above plus N-acetyl-L-cysteine (NAC; Sigma-Aldrich; 100 mg/kg per day) given subcutaneously 5 days/week.
At the time of death, blood was collected for measurement of serum cytokines, assayed by commercially available enzyme-linked immunosorbent assay (BioLegend). After death, one femur was stored in 90% ethanol and scanned by micro-computed tomography (micro-CT). The other femur was embedded in methyl methacrylate, and longitudinal 2-μm sections were mounted on barium fluoride (BaF2) infrared windows (Spectral Systems) for Fourier-transformed infrared spectroscopic imaging (FTIRI) analyses. Additional sections (5 μm) were used for histomorphometry. One tibia was fixed in 10% buffered formalin at 4°C, decalcified, and embedded in paraffin; 5-μm-thick deparaffinized sections were used for histology, whereas additional sections were stained for iron using the Pearl Prussian blue reaction. Liver, spleen, and cleaned vertebrae were collected for measurement of iron content. Liver, spleen, pancreas, and heart were also embedded in paraffin, sectioned, and stained with Pearl reaction for iron.
Tissue and bone iron content
Spleen, liver, and vertebral iron content was determined by atomic absorption spectroscopy. Tissue weight was determined and representative samples placed in tarred porcelain crucibles. Samples were dried at 110°C overnight, dry weight was determined, after which they were ashed in a Muffle furnace at 550°C. The ash was dissolved in 3N HCl, and its total Fe content was determined by atomic absorption. Mineral content was calculated as the ratio of ash weight to dry weight.
Micro-CT
Densitometric and morphometric micro-CT analysis was performed on cortical bone from mid-diaphyses of femurs and trabecular bone from the distal third of femurs. Bones were analyzed on a Scanco mCT 35 (Scanco Medical) system using a 6-μm voxel size, 55 KVp, and 0.36 degrees rotation step. The Scanco mCT software (HP, DEC windows Motif, Version 1.6) was used for three-dimensional reconstruction and data processing. A global threshold of 0.4 g/cm3 was used.
Cortical and trabecular regions of interest (ROIs) were first defined on all volumes using a utility of the processing system. In the femurs, the ROIs extended from 50 μm proximally to the end of the distal growth plate more than 1.7 mm toward the diaphysis. Trabecular ROIs were free-hand drawn on sequential slices to include the endosteal envelope, conforming to the endosteal contour on each slice. Cortical ROIs were defined as 1.7-mm segments of the mid-diaphysis of the femurs. Cortical ROIs resulted from digital subtraction of the respective trabecular ROIs from the whole bone volume. Bone mineral density measurements were performed on cortical and trabecular volumes after segmentation of bone voxels using the global threshold, to only include bone tissue. Cortical and trabecular morphometry was performed after binarization by the threshold assigned to each system dataset. Cortical bone morphometry included the cortical periosteal or outer perimeter, the cortical endosteal or inner perimeter, cortical thickness and area, and marrow area. The morphometric parameters defining trabecular bone mass and microarchitecture included bone volume fraction, trabecular thickness, trabecular number, and trabecular spacing.
FTIRI
Spectral images of proximal femur sections were obtained by FTIRI using the PerkinElmer Spotlight Imaging system (PerkinElmer Life and Analytical Sciences). Cortical and trabecular bone were examined separately. The spectral resolution was either 4 or 8 cm, whereas the spatial resolution was approximately 7 μm. Background spectra were collected under identical conditions from the same BaF2 windows. After correcting for background and the presence of polymethyl methacrylate, spectra were transformed to yield images corresponding to infrared band areas, peak height ratios, and integrated area ratios by a combination of the spectrometer software and ISYS Chemical Imaging Software (Version 2.1; Spectral Dimensions).
Five spectroscopic parameters were calculated: mineral-to-matrix ratio, carbonate-to-mineral ratio, carbonate-to-matrix ratio, collagen cross-link ratio, and crystallinity. The mineral-to-matrix is the integrated areas ratio of the PO4 band (900-1200 cm) to amide I band (1590-1720 cm), a measure that corresponds to ash weight.13 Carbonate-to-mineral ratio (carbonate band [840-890 cm]/PO4 band [900-1200 cm]) indicates carbonate substitutions in hydroxyapatite.14 Carbonate-to-amide I ratio15 is calculated to eliminate the effect of changes in phosphate on the assessment of carbonate incorporation into mineral. The collagen cross-link ratio is a parameter reflecting the relative ratio of nonreducible and reducible collagen cross-links, expressed as the absorbance ratio at 1660 and 1690 cm.16 Mineral crystallinity was calculated from the intensity ratio of the phosphate sub-bands 1030/1020 cm.17 In the spectral images, pixels devoid of bone (no mineral and/or matrix spectral signature) were set to zero and masked so as to be excluded from the calculations. The spectroscopic results were expressed as histograms describing the pixel distribution of the measured spectroscopic parameters. Means and SD of the pixel distributions and corresponding color-coded images were generated at the same time using ISYS software. Means were averaged for multiple sites in each animal and among the 6 different animals for each age and genotype using Microsoft Excel 2007.
X-ray diffraction
Vertebrae from iron-overloaded and control animals were ground to a fine powder in a liquid nitrogen cooled mill (Spex Industries) and subjected to wide-angle X-ray diffraction on a Bruker AXS diffractometer with Ni-filtered CuK α radiation. Scans were run from 4° 2 θ to 50° 2 θ. The particle size in the c-axis direction was estimated by line-broadening analysis of the 002 peak (25.85° 2 θ) using DIFFRACplus software where the full width at half-maximum is linearly related to the crystallite size and perfection (t) based on the Debye Scherrer equation.
Bone histomorphometry
Longitudinal femur sections were stained using the von Kossa method and Goldner trichrome as previously described.18 Measurements were performed in cancellous tissue of the proximal metaphysis beginning 1 mm from the central region of the growth plate to exclude the primary spongiosa and 500 μm from the endocortical margins to exclude modeling bone. The Bioquant program (Bioquant-True Color Windows, Advanced Image Analysis Software, sVGA Frame Grabber Image Processing Board and Optronics DEI-470 Video Camera) was used to collect the raw data. Trabecular bone volume (BV/TV), osteoclast surface, and osteoid surface for bone surface (BS) were measured. Osteoclast number per BS (no/mm) and BS covered by osteoblasts corrected for total BS (percentage) were measured using the Bioquant program. Osteoclasts and osteoblasts were identified in paraffin-embedded longitudinal tibia sections by staining for tartrate-resistant acid phosphatase (TRAP) using a commercially available kit (Sigma-Aldrich) and by immunostaining with the procollagen type I antibody clone SP1.D8 (Developmental Studies Hybridoma Bank, University of Iowa), respectively. Active osteoclasts and osteoblasts were identified as TRAP or procollagen type I positive cells on the BS, respectively.
For dynamic histomorphometry, mice were injected with calcein (100 mg/kg) and xylenol orange (90 mg/kg), respectively, at 7 and 3 days before death. Fluorochrome-based indices of bone formation were measured in unstained 5-μm longitudinal sections of distal femur. Double- and single-labeled perimeters were identified. Interlabel width of double-labeled regions was measured using the Bioquant program. The mineral apposition rate (MAR, μm/day) and bone formation rate (BFR/BS, μm2/μm/day) were then determined.
Quantitative reverse-transcription polymerase chain reaction
RNA from crushed femurs of iron-overloaded animals and their controls was extracted after bone marrow was flushed out. Total RNA was prepared using TRIzol reagent (Sigma-Aldrich) according to the manufacturer's instructions. First-strand cDNA was synthesized with a SuperScript First-Strand Synthesis System for reverse-transcription polymerase chain reaction using SuperScript II reverse transcriptase enzyme (Invitrogen). All reactions were performed in a Chromo4 thermocycler (MJ Research) using FastStart SYBR Green Master Mix (Roche Diagnostics). The primers used for expression analysis, cathepsin K (CTK; forward, 5′-CAGCAGGATGTGGGTGTTCA-3′ and reverse, 5′-ACACTGGCCCTGGTTCTTGA-3′), receptor activator of nuclear factor-κB ligand (RANKL; forward, 5′-GGCCACAGCGCTTCTCAG-3′ and reverse, 5′-GAGTGACTTTATGGGAACCCGAT-3′), osteoprotegerin (OPG; forward, 5′-AGCTGCTGAAGCTGTGGAA-3′ and reverse, 5′-TGTTCGAGTGGCCGAGAT-3′), osteopontin (OPN; forward, 5′-GAAACTCTTCCAAGCAATTC-3′ and reverse, 5′-GGACTAGCTTGTCCTTGTGG-3′), osteocalcin (OC; forward, 5′-CAGACAAGTCCCACACAGCA-3′ and reverse, 5′-CTTTATTTTGGAGCTGCTGT-3′), and mouse HPRT housekeeping gene (forward 5′-AGTGTTGGATACAGGCCAGAC-3′ and reverse, 5′- CGTGATTCAAATCCCTGAAGT-3′) were purchased from Invitrogen. All primer pairs amplified a single band with the expected molecular weight as analyzed by agarose gel electrophoresis. The quantities of all samples were normalized to the mouse HPRT housekeeping gene.
Determination of oxidative stress (ROS)
Bone marrow was flushed from femurs of placebo and high-dose iron dextran-treated mice, and total protein was extracted. The carbonyl content of total protein, which reflects oxidized protein,19 was determined by Western blot using the OxiBlot Detection Kit (Chemicon International), normalized to β-actin.
Statistical analysis
Results are presented as mean plus or minus SD, each group including 8 animals. The differences between 2 groups (ie, high-dose iron overload vs placebo, placebo vs high-dose iron + NAC) were evaluated by Student t test. The differences among 3 groups (ie, placebo vs low-dose iron vs high-dose iron) were evaluated by one-way analysis of variance. Statistical analysis was performed using the STATA/IC software. Differences with P values less than .05 were considered significant.
Results
Bone abnormalities associated with iron overload
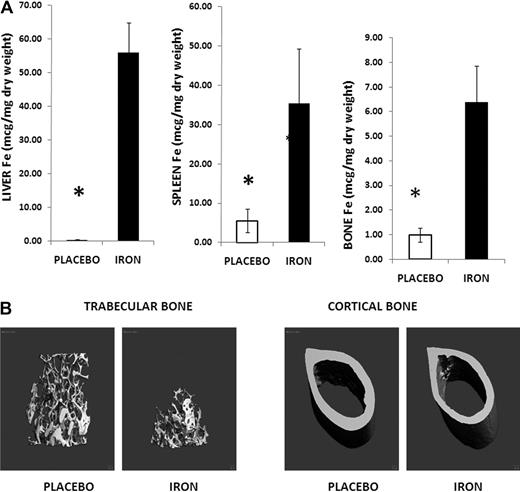
Administration of iron dextran (1 g/kg/wk) intraperitoneally for 2 months resulted in severe tissue iron overload. We first measured liver and spleen iron content because iron is cleared from plasma by spleen and liver macrophages.1 As expected, liver and spleen iron content were markedly increased (Figure 1A). In addition, we documented iron deposition in other tissues, such as pancreas and heart (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), similar to that observed in iron-overloaded humans.12 Although bone iron content was also increased in iron-treated mice compared with placebo (Figure 1A), the increase was not as pronounced as in liver and spleen. Pearl staining for iron of bone sections indicated that most of the metal was accumulated in the bone marrow, and not on the BS, and was present intracellularly in macrophage-like cells (supplemental Figure 1B).
Iron-overloaded mice exhibit osteoporosis. (A) Liver, spleen, and femur iron contents increased in iron dextran-treated animals (▬) compared with controls (▭). Note the change in scale. Data are mean ± SD. *P < .01. (B) Micro-CT images of iron-overloaded animals and their placebo controls: femoral sections showing thinning of trabecular bone, with mid-diaphysis femur demonstrating thinning of the cortical bone and increased marrow area.
Iron-overloaded mice exhibit osteoporosis. (A) Liver, spleen, and femur iron contents increased in iron dextran-treated animals (▬) compared with controls (▭). Note the change in scale. Data are mean ± SD. *P < .01. (B) Micro-CT images of iron-overloaded animals and their placebo controls: femoral sections showing thinning of trabecular bone, with mid-diaphysis femur demonstrating thinning of the cortical bone and increased marrow area.
To establish whether iron excess induces bone abnormalities, we performed micro-CT studies of the iron-overloaded mouse (1 g/kg/wk for 2 months) and its control. Micro-CT analysis of trabecular bone (distal femur) revealed a decreased bone volume fraction, decreased number of trabeculae and trabecular thickness, and increased trabecular spacing in iron-overloaded mice compared with placebo (Table 1; Figure 1B). Analysis of cortical bone (mid-diaphysis femur) in the iron-overloaded animals showed thinner cortices and decreased cortical area compared with controls (Table 1; Figure 1B).
Micro-CT analysis of mice treated with placebo, high-dose iron dextran (1 g/kg per week), and low-dose iron dextran (0.1 g/kg per week)
| . | Placebo . | High-dose iron . | Low-dose iron . |
|---|---|---|---|
| Trabecular bone | |||
| BMD, mg/mm3 | 841.63 ± 11.42 | 832.38 ± 19 | 822 ± 8.05 |
| BVF, percentage | 19.79 ± 3.6 | 11.21 ± 2.9* | 13.7 ± 1.9‡ |
| Trabecular number, N/mm | 4.93 ± 0.334 | 3.94 ± 0.568* | 4.46 ± 0.16‡ |
| Trabecular thickness, μm | 48.836 ± 4.9 | 43.566 ± 4.8† | 45.37 ± 4.48‡ |
| Trabecular spacing, μm | 190.04 ± 14.15 | 254.62 ± 41* | 215.825 ± 8.3‡ |
| Cortical bone | |||
| Cortical thickness, mm | 0.192 ± 0.0064 | 0.151 ± 0.0002* | 0.17 ± 0.00738‡ |
| Inner perimeter, mm | 3.44 ± 0.38 | 4.02 ± 0.045 | 3.84 ± 0.3 |
| Outer perimeter, mm | 5.10 ± 0.36 | 5.21 ± 0.764 | 5.14 ± 0.328 |
| Cortical area, mm2 | 0.9117 ± 0.1 | 0.769 ± 0.0592* | 0.864 ± 0.04437‡ |
| Marrow area, mm2 | 1.06 ± 0.1774 | 1.1885 ± 0.08† | 1.14 ± 0.159‡ |
| . | Placebo . | High-dose iron . | Low-dose iron . |
|---|---|---|---|
| Trabecular bone | |||
| BMD, mg/mm3 | 841.63 ± 11.42 | 832.38 ± 19 | 822 ± 8.05 |
| BVF, percentage | 19.79 ± 3.6 | 11.21 ± 2.9* | 13.7 ± 1.9‡ |
| Trabecular number, N/mm | 4.93 ± 0.334 | 3.94 ± 0.568* | 4.46 ± 0.16‡ |
| Trabecular thickness, μm | 48.836 ± 4.9 | 43.566 ± 4.8† | 45.37 ± 4.48‡ |
| Trabecular spacing, μm | 190.04 ± 14.15 | 254.62 ± 41* | 215.825 ± 8.3‡ |
| Cortical bone | |||
| Cortical thickness, mm | 0.192 ± 0.0064 | 0.151 ± 0.0002* | 0.17 ± 0.00738‡ |
| Inner perimeter, mm | 3.44 ± 0.38 | 4.02 ± 0.045 | 3.84 ± 0.3 |
| Outer perimeter, mm | 5.10 ± 0.36 | 5.21 ± 0.764 | 5.14 ± 0.328 |
| Cortical area, mm2 | 0.9117 ± 0.1 | 0.769 ± 0.0592* | 0.864 ± 0.04437‡ |
| Marrow area, mm2 | 1.06 ± 0.1774 | 1.1885 ± 0.08† | 1.14 ± 0.159‡ |
BMD indicates bone mineral density; and BVF, bone volume fraction.
P < .001, high-dose vs placebo.
P < .05, high-dose vs placebo.
P < .05, high-dose vs low-dose vs placebo.
FTIRI analysis was undertaken to determine whether iron overload induced changes in bone composition, and the results are summarized in Table 2. Mineral-to-matrix and carbonate-to-amide I ratios were decreased in both cortical and trabecular bone of iron-overloaded animals, indicating changes in mineralization and an increase of the nonmineralized matrix. The carbonate-to-mineral ratio was decreased, reflecting increased bone turnover. No changes in collagen or hydroxyapatite structure were found.
Changes in mineral parameters from iron overload
| FTIRI parameter . | Category . | Placebo . | Iron dextran . | P . |
|---|---|---|---|---|
| Cortical bone | Mineral-to-matrix | 7.7 ± 0.4 | 6.3 ± 0.7 | .002 |
| Carbonate-to-mineral | 0.0074 ± 0.0003 | 0.006 ± 0.001 | .01 | |
| Carbonate-to-matrix | 0.057 ± 0.004 | 0.038 ± 0.009 | .001 | |
| Cross-link | 3.4 ± 0.36 | 3.3 ± 0.36 | NS | |
| Crystallinity | 1.12 ± 0.015 | 1.10 ± 0.03 | .01 | |
| Trabecular bone | Mineral-to-matrix | 4.0 ± 0.5 | 3.5 ± 0.2 | .06 |
| Carbonate-to-mineral | 0.0046 ± 0.001 | 0.0030 ± 0.001 | .01 | |
| Carbonate-to-matrix | 0.019 ± 0.006 | 0.010 ± 0.0001 | .01 | |
| Cross-link | 3.1 ± 0.37 | 3.4 ± 0.4 | NS | |
| Crystallinity | 1.054 ± 0.014 | 1.050 ± 0.01 | NS | |
| X-ray diffraction | Crystal size, nm | 13.5 ± 4.6 | 18 ± 5.4 | NS |
| FTIRI parameter . | Category . | Placebo . | Iron dextran . | P . |
|---|---|---|---|---|
| Cortical bone | Mineral-to-matrix | 7.7 ± 0.4 | 6.3 ± 0.7 | .002 |
| Carbonate-to-mineral | 0.0074 ± 0.0003 | 0.006 ± 0.001 | .01 | |
| Carbonate-to-matrix | 0.057 ± 0.004 | 0.038 ± 0.009 | .001 | |
| Cross-link | 3.4 ± 0.36 | 3.3 ± 0.36 | NS | |
| Crystallinity | 1.12 ± 0.015 | 1.10 ± 0.03 | .01 | |
| Trabecular bone | Mineral-to-matrix | 4.0 ± 0.5 | 3.5 ± 0.2 | .06 |
| Carbonate-to-mineral | 0.0046 ± 0.001 | 0.0030 ± 0.001 | .01 | |
| Carbonate-to-matrix | 0.019 ± 0.006 | 0.010 ± 0.0001 | .01 | |
| Cross-link | 3.1 ± 0.37 | 3.4 ± 0.4 | NS | |
| Crystallinity | 1.054 ± 0.014 | 1.050 ± 0.01 | NS | |
| X-ray diffraction | Crystal size, nm | 13.5 ± 4.6 | 18 ± 5.4 | NS |
Values are mean ± SD.
NS indicates not significant.
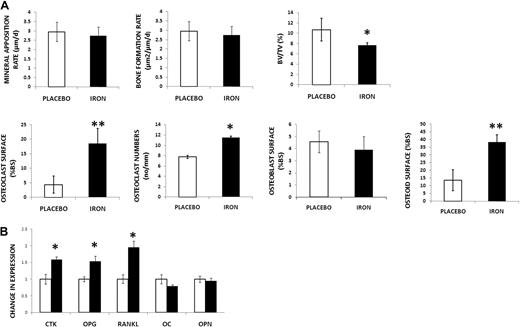
Dynamic histomorphometry was then performed to determine changes in bone formation with iron. Based on fluorochrome-derived indices, MAR and BFR were calculated (Figure 2A; supplemental Figure 2) and were found unchanged with iron treatment, suggesting that osteoblast function was unaltered. Thus, we turned to static histomorphometry to obtain additional information on bone remodeling. Using the von Kossa method to stain the mineralized matrix, we observed decreased BV/TV in iron-overloaded animals (Figure 2A; supplemental Figure 2), a finding consistent with our micro-CT results. Bone resorptive surfaces were increased with iron (Figure 2A). In addition, osteoclasts were identified as TRAP-positive cells on the BS, and their numbers were increased with iron dextran (Figure 2A; supplemental Figure 2: TRAP stain). Taken together, these results indicate increased resorption with iron excess. BS covered by active osteoblasts was identified using the procollagen I stain (Figure 2A; supplemental Figure 2). Consistent with the results of dynamic histomorphometry, no differences were found between treatment groups, indicating no changes in osteoblast number. Despite these last findings, we observed a significant increase in osteoid (Figure 2A; supplemental Figure 2), suggesting a mineralization defect.
Iron-overloaded mice exhibit decreased bone mass, increased resorption, and increased deposition of osteoid. (A) First row: No changes in MAR and BFR by double-label technique in the iron-overloaded mice compared with placebo. Decreased BV/TV (von Kossa stain) with iron excess. Second row: Increased osteoclast surface (Goldner trichrome stain) and increased osteoclast numbers (no/mm) by TRAP in iron-treated mice. No changes were found in osteoblast numbers between placebo and iron-treated mice using stain for procollagen type I. Increased osteoid was present in the iron-treated animals. Data are mean ± SD. *P < .05. **P < .01. (B) Increased expression of genes associated with bone resorption (CTK, OPG, and RANKL) in iron-overloaded mice. No changes in expression of OC and OPN. Data are mean ± SD. *P < .01.
Iron-overloaded mice exhibit decreased bone mass, increased resorption, and increased deposition of osteoid. (A) First row: No changes in MAR and BFR by double-label technique in the iron-overloaded mice compared with placebo. Decreased BV/TV (von Kossa stain) with iron excess. Second row: Increased osteoclast surface (Goldner trichrome stain) and increased osteoclast numbers (no/mm) by TRAP in iron-treated mice. No changes were found in osteoblast numbers between placebo and iron-treated mice using stain for procollagen type I. Increased osteoid was present in the iron-treated animals. Data are mean ± SD. *P < .05. **P < .01. (B) Increased expression of genes associated with bone resorption (CTK, OPG, and RANKL) in iron-overloaded mice. No changes in expression of OC and OPN. Data are mean ± SD. *P < .01.
To further evaluate bone turnover, mRNA from the femurs of iron-overloaded and placebo-treated animals was extracted after bone marrow was flushed. We observed increased expression of genes involved in bone resorption (CTK, OPG, and RANKL), again in agreement with our histologic findings. In contrast, there was no difference in expression of genes reflecting bone formation (OC and OPN; Figure 2B)
Iron-induced bone abnormalities are dose-dependent
To evaluate the possible dose dependency of iron overload bone loss, we repeated our studies using mice with placebo (phosphate-buffered saline) or high- or low-dose iron dextran (1 and 0.1 g/kg/wk) intraperitoneally, respectively) for 2 months. As expected, the low-dose iron dextran resulted in liver and spleen iron concentrations intermediate between those of the placebo and high-dose groups (liver iron content: 0.35 ± 0.14 vs 16.7 ± 2 vs 55.0 ± 8.9 μg/mg dry weight, placebo vs low-dose vs high-dose, P < .001; spleen iron content: 5.54 ± 2.9 vs 19.5 ± 8.5 vs 35 ± 1.08 μg/mg dry weight, placebo vs low-dose vs high-dose, P < .01). Micro-CT analysis of cortical bone at mid-diaphysis femur and trabecular bone at distal femur from animals that received the low-dose iron showed abnormalities similar in nature, although less severe than those seen in the animals that were treated with high-dose iron dextran (Table 1). Otherwise, their micro-CT values plotted in an intermediate range between those of the placebo and high-dose iron dextran-treated animals.
Bone abnormalities induced by iron overload correlate with an inflammatory process
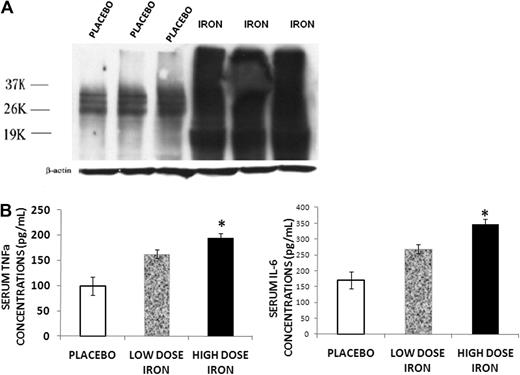
Because iron was found to be abundant in the bone marrow of the iron-overloaded animals, and iron overload has been implicated in the development of increased ROS in other systems,3,12 we determined the levels of ROS in bone marrow protein extracted from placebo and iron dextran-treated mice using the OxiBlot Detection Kit. The method detects the carbonyl content of proteins, the concentration of which correlates with ROS.19 An overlapping series of bands were present in the specimens from iron-overloaded animals, indicating increased carbonyl content in a number of bone marrow proteins consistent with increased ROS in the bone marrow of these mice (Figure 3A). We also measured TNF-α and IL-6 concentrations in the serum of low- and high-dose iron treated and control animals, as these cytokines are elevated in other conditions associated with increased ROS and increased resorption.20,21 Both serum TNF-α and IL-6 concentrations were increased with iron overload (Figure 3B). Furthermore, the increase in serum cytokines occurred in a dose-dependent fashion related to the severity of iron overload.
Iron overload induces inflammatory changes. (A) Western blot for protein carbonyl content from bone marrow of placebo and iron dextran-treated mice. An overlapping series of bands were present in the iron-overloaded animals, indicating increased carbonyl content in a number of bone marrow proteins consistent with increased ROS in the bone marrow of these mice. (B) Serum TNF-α and IL-6 concentrations in mice treated for 2 months with high-dose (▬) or low-dose iron dextran ( ) and placebo (▭). Serum cytokines increase in a dose-dependent fashion. Data are mean ± SD. *P < .01.
) and placebo (▭). Serum cytokines increase in a dose-dependent fashion. Data are mean ± SD. *P < .01.
Iron overload induces inflammatory changes. (A) Western blot for protein carbonyl content from bone marrow of placebo and iron dextran-treated mice. An overlapping series of bands were present in the iron-overloaded animals, indicating increased carbonyl content in a number of bone marrow proteins consistent with increased ROS in the bone marrow of these mice. (B) Serum TNF-α and IL-6 concentrations in mice treated for 2 months with high-dose (▬) or low-dose iron dextran ( ) and placebo (▭). Serum cytokines increase in a dose-dependent fashion. Data are mean ± SD. *P < .01.
) and placebo (▭). Serum cytokines increase in a dose-dependent fashion. Data are mean ± SD. *P < .01.
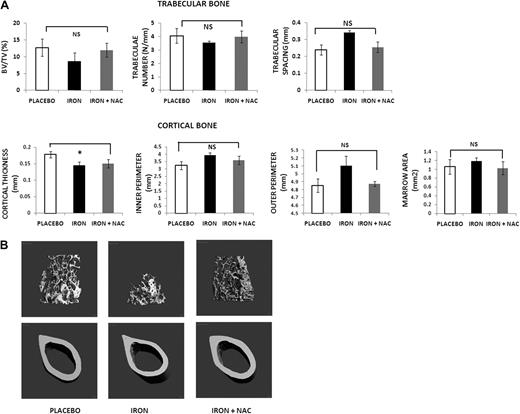
Because our data indicate that iron overload in the mouse is associated with increased ROS, we sought to determine whether ROS are prime mediators of the bone abnormalities that we observed. Thus, we treated mice with placebo versus iron dextran (1 g/kg/wk given intraperitoneally) versus iron dextran (1 g/kg/wk given intraperitoneally) plus the antioxidant NAC (100 mg/kg/d, subcutaneously, 5 d/wk) for 2 months. The micro-CT analysis and pictures (shown in Figure 4) reveal that NAC administration largely prevented the bone abnormalities of iron overload, confirming that these changes are mediated at least in part by ROS. In agreement with these observations, serum TNF-α and IL-6 in animals given the antioxidant were similar to those in controls (TNF-α: 98 ± 15 vs 193 ± 8 vs 112 ± 8 pg/mL, placebo vs iron vs iron + NAC; IL-6: 165 ± 12 vs 337 ± 12 vs 182 ± 11 pg/mL, placebo vs iron vs iron + NAC). As expected, liver and spleen iron content from animals treated with iron dextran + NAC was increased compared with placebo, with levels similar or even higher than the iron dextran mice (liver iron content 0.38 ± 0.15 vs 59.0 ± 7.5 vs 58.5 ± 8.2 μg/mg dry weight, placebo vs iron vs iron + NAC, P < .001; spleen iron content: 6.1 ± 3.5 vs 35 ± 9.08 vs 48 ± 12.3 μg/mg dry weight, placebo vs iron vs iron + NAC, P < .001).
Treatment with an antioxidant prevents the bone loss induced by iron overload. (A) Micro-CT analysis of mice treated with placebo, iron dextran alone, or iron dextran plus NAC for 2 months: placebo (▭), iron dextran (▬), and iron + NAC ( ). Data are mean ± SD. NS indicates not significant. *P < .01. (B) Micro-CT images of placebo, iron dextran, and iron dextran + NAC animals. Trabecular bone at distal femur and cortical bone at mid-diaphysis femur.
). Data are mean ± SD. NS indicates not significant. *P < .01. (B) Micro-CT images of placebo, iron dextran, and iron dextran + NAC animals. Trabecular bone at distal femur and cortical bone at mid-diaphysis femur.
Treatment with an antioxidant prevents the bone loss induced by iron overload. (A) Micro-CT analysis of mice treated with placebo, iron dextran alone, or iron dextran plus NAC for 2 months: placebo (▭), iron dextran (▬), and iron + NAC ( ). Data are mean ± SD. NS indicates not significant. *P < .01. (B) Micro-CT images of placebo, iron dextran, and iron dextran + NAC animals. Trabecular bone at distal femur and cortical bone at mid-diaphysis femur.
). Data are mean ± SD. NS indicates not significant. *P < .01. (B) Micro-CT images of placebo, iron dextran, and iron dextran + NAC animals. Trabecular bone at distal femur and cortical bone at mid-diaphysis femur.
Discussion
The mouse model of iron overload described in this study had a pronounced bone phenotype, with abnormalities occurring in a dose-dependent fashion. Changes included trabecular and cortical thinning and alterations in the material properties of the bone. They were associated with increased resorption and oxidative stress. The bone loss was largely prevented by treatment with the antioxidant NAC, which replenishes intracellular glutathione levels, a central component in the cellular defense against oxidative stress.22 The prevention of bone abnormalities with NAC supports the hypothesis that oxidative stress is involved in the pathogenesis of bone loss in iron excess.
Iron has been implicated in the development of osteoporosis in diseases characterized by iron overload, such as the thalassemias and hemochromatosis, although the evidence is not consistent.5-7,23,24 Studies in patients with such disorders have been complicated by sample size limitations and multiple disease covariants that may also have an effect on the bone. Iron status has been so far assessed by serum ferritin, which is subject to variability and may not accurately reflect the severity of iron overload,25-29 explaining some of the inconsistencies in clinical studies. Certainly, the use of recent magnetic resonance imaging technology to assess liver iron stores is expected to shed light on the role of iron on bone remodeling.
The study of osteoporosis in thalassemia, in particular, is quite complex. The exact role of ineffective erythropoiesis in regularly transfused patients is unclear. Low ascorbate concentrations have been frequently described in regularly transfused patients and may contribute to bone loss.30-32 Alternatively, our findings of unaltered collagen synthesis suggest that levels of ascorbate have not decreased to the point of inhibiting hydroxylation of key proline residues in collagen, a rate-limiting step in collagen synthesis.33 Finally, the iron chelator desferrioxamine has been associated with bone lesions that resemble those of rickets.34 These facts demonstrate the complexities of the clinical studies, some of which can be addressed by animal experiments, which allow direct manipulation of individual factors. The iron overload achieved in the mice used in this study resulted from parental administration of iron and mimics transfusional siderosis. Similar to what is seen in humans, the iron-overloaded mouse has iron accumulation in multiple tissues, including liver, spleen, and heart.12 Hepatic iron concentrations reflect total body iron stores, as liver is the main site of iron storage.1-3 As expected, we observed higher iron content in the liver compared with other tissues. However, hepatic iron concentrations were much higher in the iron-overloaded mouse than are measured in transfused patients. Species differences and length of exposure must be taken into account when considering the relationship between severity of iron overload and development of osteoporosis.
The concept that iron overload leads to increased oxidative stress and inflammation is not new.35-38 In iron-overloaded states, the binding capacity of transferrin, the major iron transport protein, is surpassed.1-3,12 The resulting increase in non–transferrin-bound iron has been associated with increased ROS. In addition, the intracellular storage capacity of ferritin is exceeded and the labile pool of intracellular iron is increased. This metabolically active iron catalyzes the formation of free radicals, which can lead to cell damage and eventually death.35 Indeed, a number of in vitro and in vivo studies link iron to the generation of oxidative species. With regards to the situation in humans, a growing body of literature comes from studies of iron-overloaded patients with thalassemia, in whom increased biomarkers of oxidative stress, increased serum cytokines, and alteration in lymphocyte subsets have been described.36-39 Although the ineffective erythropoiesis of thalassemia itself has been associated with increased oxidative stress in these studies, there is also evidence that the excess iron burden is an additional culprit. Immune alterations have also been described in hereditary hemochromatosis.40 Two studies have described an association between increased bone resorption and serum cytokine in states of iron excess.9,38 To our knowledge, however, ours is the first study directly linking iron-induced oxidative stress to low bone mass. We also observed increased serum inflammatory cytokines and provided evidence that their production is also regulated by oxidative stress. Further studies are needed to determine whether proinflammatory cytokines mediate bone loss in this model.
The bone loss that was observed in our model was associated with increased resorption, which is most probably related to the presence of increased ROS. The proinflammatory cytokines TNF-α and IL-6, which were increased in the sera of iron-treated mice, increase osteoclast formation. Increased resorption is responsible for the bone loss seen in a wide range of conditions, including joint inflammation, estrogen withdrawal, and aging.20,21,41,42 ROS are known to activate NF-κB, a key factor involved in osteoclastogenesis.20,21 Increased resorption has also been described in patients with thalassemia5,43 and in iron-overloaded rats.9 In contrast, the literature on the effect of iron on osteoblastogenesis is limited and conflicting. A negative effect of iron on mineralization and bone formation has been described in vitro11 and in iron-overloaded pigs,44 whereas the opposite has been reported in rats.9 In our study, we did not observe a significant decrease in bone formation by dynamic histomorphometry.
Although our data provide evidence that increased oxidative stress leads to the described bone phenotype, it is feasible that iron, as a heavy metal, may also act directly on bone. Aluminum toxicity, for example, has been reported to cause mineralization defects similar to rickets.45 Indeed, we found that the bone iron content was increased in the iron-overloaded animals compared with controls. Both our histology, showing a large increase of deposited nonmineralized osteoid, and FTIRI data indicate a defect in mineralization that supports the idea of a direct effect of iron on specific aspects of osteoblast function, an area in which we provide no insights. Our studies, using FTIRI and x-ray powder diffraction to better understand changes in bone chemical properties with iron, failed to detect changes in hydroxyapatite crystal size and perfection.
There are some limitations to our study that must be indicated. First, the interactions between the immune system and bone constitute the focus a new rapidly growing field of osteoimmunology.42,46 Results must be considered in light of the expanse of information being developed. It is already known, however, that cytokines, such as TNF-α and IL-6, mediate bone loss primarily by increasing bone resorption.46-48 There is additional evidence that TNF-α mediates bone loss in cases of increased oxidative stress.20,21 The second limitation is that our controls did not receive dextran because the iron dextran that was used in our experiments contained dextran of low molecular weight (∼ 5000) a nonimmunogenic form that was not available for injection into the controls. This is in contrast to the high molecular weight dextrans, which are immunogenic and are part of certain iron dextran preparations.49,50 Finally, in terms of the antioxidant studies, treatment of iron-overloaded mice with NAC showed a different response of the trabecular versus cortical bone. The alterations of the trabecular bone were completely rescued with NAC, whereas the cortical bone continued to demonstrate abnormalities. Cortical and trabecular bone are known to have differential turnover, which may explain these results. Higher doses of NAC or its daily administration instead of 5 days per week, as done in other animal studies,21 may have yielded comparable results. Regardless, this is the first experiment to suggest an alternative or an adjunctive therapy to iron chelation in iron-overloaded states and carries the promise of significant translational applications.
In conclusion, taking the literature and our results into account, we propose a model where iron excess leads to increased oxidative stress. Oxidative stress then induces inflammatory changes, again in a dose-dependent fashion, which then mediate bone loss through changes in bone remodeling. Further studies are needed to confirm this model in animals as well its relevance in humans. Consistent with the proposed model of systemic inflammation driving the bone abnormalities in the iron-overloaded mouse, iron was primarily localized intracellularly in the bone marrow cavity instead of on the BS or within the bone.
An Inside Blood analysis of this article appears at the front of this issue.
Presented in abstract form at the 31st annual meeting of the American Society of Bone and Mineral Research, Denver, CO, September 11, 2009.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health (grant KO8 HL 088231) and the Weill Cornell Clinical and Translational Science Center (pilot award). The study used the Core Center facilities for Musculoskeletal Integrity at the Hospital for Special Surgery (AR046121).
National Institutes of Health
Authorship
Contribution: J.T. performed research in the areas of micro-CT and FTIRI analysis, analyzed data, and contributed to the writing of the paper; Z.Y., R.C., and H.L. performed research in the areas inflammation; F.P.R., P.M.-K., P.J.G., and A.L.B. participated in the study design; S.C.-R. contributed to the study design in the area of inflammation; S.B.D. performed the bone histology and contributed to the study design; R.W.G. measured tissue iron measurements and contributed to the study design; and M.G.V. designed and performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Maria G. Vogiatzi, Weill Cornell Medical College, Pediatric Endocrinology, Box 103, 525 East 68th St, New York, NY 10065; e-mail: mvogiatz@med.cornell.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal