Abstract
The prevailing idea regarding the mechanism(s) by which therapeutic immunosuppressive dendritic cells (DCs) restrain alloimmunity is based on the concept that they interact directly with antidonor T cells, inducing anergy, deletion, and/or regulation. However, this idea has not been tested in vivo. Using prototypic in vitro–generated maturation-resistant (MR) DCs, we demonstrate that once MR-DCs carrying donor antigen (Ag) are administered intravenously, they decrease the direct and indirect pathway T-cell responses and prolong heart allograft survival but fail to directly regulate T cells in vivo. Rather, injected MR-DCs are short-lived and reprocessed by recipient DCs for presentation to indirect pathway CD4+ T cells, resulting in abortive activation and deletion without detrimental effect on the number of indirect CD4+ FoxP3+ T cells, thus increasing the regulatory to effector T cell relative percentage. The effect on the antidonor response was independent of the method used to generate therapeutic DCs or their viability; and in accordance with the idea that recipient Ag-presenting cells mediate the effects of therapeutic DCs in transplantation, prolongation of allograft survival was achieved using donor apoptotic MR-DCs or those lacking surface major histocompatibility complex molecules. We therefore conclude that therapeutic DCs function as Ag-transporting cells rather than Ag-presenting cells to prolong allograft survival.
Introduction
Historically, randomly selected, haplotype-shared, donor-specific transfusion (DST) of whole blood or leukocytes before transplantation, alone or in combination with immunosuppressive agents, was one of the first cell-based therapies used to restrain the antidonor response.1-7 The beneficial effect of DST depends on the presence of leukocytes and donor antigen (Ag),8-10 the load and immunogenicity of the allo-Ag transferred,4,9 and the time of administration before transplantation.6,10 Early studies suggested that DST-mediated immunosuppression requires that T cells recognize directly donor-Ag expressed by the transfused leukocytes.7 However, it was later demonstrated that presentation of donor-Ag in the context of self–major histocompatibility complex (MHC) molecules by recipient Ag-presenting cells (APCs), through the indirect pathway of allorecognition, is critical for the DST effect.10-12 The finding that DST sensitizes a percentage of recipients and the introduction of new immunosuppressive agents discontinued the clinical use of DST in the 1980s.7
During the past 15 years, a new generation of cell therapies based on intravenous administration of donor- or recipient-derived dendritic cells (DCs) expanded in vitro and rendered immunosuppressive by pharmacologic or genetic methods has been used to down-regulate the host-versus-graft13-25 and graft-versus-host26 responses. These in vitro–generated immature, maturation-resistant (MR), or alternatively activated DCs have been used with variable success to prevent/delay allograft rejection and graft-versus-host disease in murine models.27 However, the mechanisms of action of therapeutic DCs in vivo in transplantation have not been elucidated because previous studies have analyzed the function of the therapeutic DCs in vitro, or their impact on the antidonor response ex vivo.13-25 As originally assumed for DST, the prevailing dogma states that therapeutic DCs down-regulate the antidonor response by interacting directly with donor-reactive T cells, promoting anergy, deletion, and/or regulation. However, to our knowledge, this simple proposition has not been tested. Alternatively, the injected DCs could function, as shown in DST, by simply providing donor-Ag to recipient APCs and through the indirect pathway (donor-Ag presented by recipient MHC). Considering the cost, time, and potential risks of future DC-based therapies in transplantation: what would be the benefit of introducing new therapies based on in vitro–generated immunosuppressive DCs if they simply function through the DST effect?
In this study, we used MR-DCs as prototypic therapeutic immunosuppressive DCs to investigate the mechanisms by which DC-based therapies regulate alloimmunity in vivo. Our findings indicate that systemically injected MR-DCs do not directly present donor-Ag but rather serve as a source of donor-Ag for recipient DCs for presentation to indirect pathway T cells, down-regulation of the antidonor response, and prolongation of allograft survival, similar to DST.11,12 Our findings suggest a shared mechanism of action between these therapies and call into question the potential clinical superiority of current DC-based therapies in transplantation.
Methods
Mice and reagents
C57BL/6 (B6), BALB/c, C3H, B6.129-H2dlAb1-Eα/J (MHC II−/−), B6.FVB-Tg (Itagx-DTR/eGFP)57Lan/J (CD11c-eGFP), B6.129P2-B2mtm1Unc/J (MHC I−/−), B6.SJL-PtprcaPepcb/BoyJ (CD45.1), and RAG1−/− B6 mice were from The Jackson Laboratory, and B6.129-H2-Ab1tm1GruB2mtm1JaeN17 (MHC I/IIdouble KO) from Taconic Farms. 1H3.1 and 2C RAG1−/− B6 mice were bred in our animal facility. Studies were approved by the Institutional Animal Care and Use Committee. Granulocyte-macrophage colony-stimulating factor and interleukin-4 (IL-4) were from PeproTech; PKH26, 1α,25-(OH)2 vitamin D3 (VD3), and polyriboinosinic acid/polyribocytidylic acid from Sigma-Aldrich; and CD40 (FGK45.5) and NK1.1 (PK136) antibody (Ab) from BioXCell.
Generation of MR-DCs
Bone marrow–derived DC precursors were purified as described28 and cultured in RPMI 1640 with 10% fetal calf serum, glutamine, nonessential amino acids, sodium pyruvate, N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 2-mercaptoethanol, antibiotics, and 1000 U/mL granulocyte-macrophage colony-stimulating factor and 500 U/mL IL-4, either with addition of 10nM VD3 beginning on day 2 of culture (MR-DCs) or not (control-DCs). Medium, cytokines, and VD3 were renewed every other day. MR-DCs were purified from CD86+ DCs by negative depletion (Dynabeads). To generate lipopolysaccharide (LPS)-matured DCs, control DCs (day 6) were cultured with LPS (200 ng/mL) overnight. For in vitro challenge, control DCs and MR-DCs (day 6) were treated for 48 hours with a DC1-maturation cocktail containing interferon-γ IFN-γ (20 ng/mL), IL-1β (20 ng/mL), tumor necrosis factor-α (50 ng/mL), CpG (1μM), and polyriboinosinic acid/polyribocytidylic acid (1μM); or with LPS (50 ng/mL); or agonistic CD40 Ab (10 μg/mL, HM40-3). Early apoptotic (annexin-V+ propidium iodide−) MR-DCs and splenocytes were generated by 3-minute ultraviolet B (UVB) irradiation as previously described.28
Isolation and adoptive transfer of TCRtg T cells
CD4+ 1H3.1 and CD8+ 2C T cells were purified from spleens and lymph nodes of transgenic T-cell receptor (TCRtg) mice with either CD4+ or CD8+ Dynabeads negative isolation kits, stained with 7.5μM Vybrant CFDA SE Cell Tracer (Invitrogen) and administered intravenously at 3 × 106 T cells per B6 mouse.
Heart transplantation
Heterotopic (abdomen) vascularized cardiac transplantation was performed according to the method of Corry et al.29
Microscopic analysis and immunostaining
Paraffin-embedded allograft sections were processed for hematoxylin and eosin. Graft fragments were snap-frozen, cryosectioned and fixed in 95% ethanol, and then treated with 5% normal goat serum followed by avidin/biotin blocking kit (Vector Laboratories). Sections were stained with Alexa-488-CD4 Ab and biotin-FoxP3 Ab or biotin-CD8α Ab plus Cy3-streptavidin. For MR-DC trafficking studies, sections were labeled with CD11c Ab plus Cy2-antihamster IgG, biotin-CD45.2 Ab plus Cy3-streptavidin, and Alexa-647-CD3 Ab.
DC-enriched suspensions were generated from CD45.1+ B6 hosts injected intravenously with CD45.2+ MR-DCs. Briefly, spleens were digested with 400 U/mL collagenase (30 minutes, 37°C), followed by rinsing in ice-cold Ca-free 0.01M ethylenediaminetetraacetic acid-phosphate-buffered saline and centrifugation on a 16% Histodenz gradient (700g, 20 minutes, 4°C). DC-enriched suspensions (20%-25% DCs) were centrifuged with a Shandon cytocentrifuge (35g, 5 minutes), and cytospins were fixed in 4% formaldehyde and incubated with biotin-CD45.2 Ab plus Cy3-streptavidin, CD11c Ab plus Cy5 anti–hamster IgG, and fluorescein isothiocyanate TdT-mediated dUTP nick end labeling (Roche Diagnostics). To detect 1H3.1 CD4+ T cells in peripheral tissues, heart, liver, and kidney cryosections were labeled with Alexa-647-CD4 Ab and biotin-Thy1.1 Ab plus Cy2-streptavidin. Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI; Invitrogen). Slides were examined on a Nikon Eclipse E800 microscope at magnifications of ×200 and ×400 with Nikon 20×/0.17 and 40×0.95 numeric aperture objectives. Images were acquired with a Spot RT Slider CCD camera (Diagnostic Instruments) using Spot Advanced Version 4.6 software. Digital images were processed with Adobe Photoshop CS3 Extended 10.0 software.
For confocal microscopy, splenic DC-enriched suspensions from CD11c-eGFP B6 mice injected with PKH26-labeled BALB/c MR-DCs were attached to poly-L-lysine–treated slides, fixed with paraformaldehyde, and imaged with an Olympus 1×81 microscope.
MLCs and ELISPOT assays
The allostimulatory ability of γ-irradiated BALB/c control- and MR-DCs was tested in 3-day mixed leukocyte cultures (MLCs) using B6 splenic T cells purified by Dynabeads negative depletion. Cell proliferation was evaluated by assessment of [3H]thymidine incorporation.
To analyze the direct pathway response, purified splenic T cells (enrichment columns, R&D Systems) from B6 mice transplanted 7 days before with BALB/c hearts were incubated with CD3-depleted, γ-irradiated, splenic B6, BALB/c, or C3H APCs (3 × 104 T cells + 2.5 × 105 APCs/well) in 96-well ELISPOT plates coated with IFN-γ Ab. To analyze the indirect pathway, purified recipient splenic T cells were incubated with CD3-depleted, γ-irradiated, splenic B6 APCs (3 × 105 T cells + 2.5 × 105 APCs/well) and sonicates (50μL/well) prepared from BALB/c, B6, or C3H splenocytes (from 2 × 107 cells/mL). ELISPOT plates were developed 36 hours later (BD Biosciences).
Quantification of donor-derived MR-DCs by PCR analysis
DNA (DNeasy Tissue Kit, QIAGEN) from spleen of B6 mice injected with BALB/c MR-DCs 24 hours after treatment with (or not) NK1.1 Ab (200 μg, intraperitoneally) was used as template for polymerase chain reaction (PCR) using primers for IgG2aa (BALB/c and B6 mice encode for the IgG2aa and IgG2ab alleles, respectively)30 and the ribosomal S15 housekeeping gene. The primer sequences were: IgG2aa: forward, 5′ ACAAAGTCCCTGGTTTGGTGC, and reverse, 5′ GGCATTTGCATGGAGGACAG (111-bp product); S15: forward, 5′ TTCCGCAAGTTCACCTACC, and reverse, 5′ CGGGCCGGCCATGCTTTACG (1231-bp product). For PCR, 750 ng DNA was added to Illustra PuReTaq Ready-To-Go PCR beads (GE Healthcare) and run at 94°C for 3 minutes (94°C for 30 seconds, 67.7°C for 30 seconds, 72°C for 50 seconds) for 38 cycles, and 72°C for 10 minutes.
Assay for Ag presentation
Splenic DC-enriched suspensions were labeled with fluorescein isothiocyanate-H2-Kb, APC-CD11c, APC-Cy7-CD8α, and phycoerythrin (PE)–CD45RA Ab and sorted with a FACSAria flow cytometer (BD Biosciences). Fluorescence-activated cell sorter (FACS)–sorted APCs were γ-irradiated and used as stimulators of carboxyfluorescein succinimidyl ester (CFSE)–labeled 1H3.1 CD4+ T cells (50 000 APCs: 400 000 T cells/well) in 96-well round-bottom plates. After 5 days, T cells were FACS-assayed for CFSE dilution. FACS-sorted splenic B6 CD11chiCD8− DCs pulsed with BALB/c IEα52-68 peptide were used as positive controls.
Flow cytometric analysis
Cells were blocked with 10% normal goat serum and incubated (30 minutes, 4°C) with Ab. BALB/c control- and MR-DCs were stained with PE-Cy5-CD11c and one of the following PE-Abs: anti-IAd, -H2Kd, -CD40, -CD80, or -CD86 Ab, or with PE–annexin V (BD Biosciences).
Splenocytes and blood cells were depleted of erythrocytes and incubated with PE-Cy5-CD4 or -CD8α, APC-Thy1.1, and PE-CD62L or PE-CD69 Ab, or PE–annexin V. For Treg staining, cells were surface-labeled, treated with cytofix/cytoperm solution, and stained with PE-FoxP3 Ab (eBioscience). Appropriate irrelevant Abs were used as controls. Cells were fixed in paraformaldehyde and analyzed with a FACSCalibur flow cytometer (BD Biosciences).
ELISA
Detection of IL-2, IL-4, IL-10, IL-12p70, and IFN-γ was performed by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (BD Biosciences).
Statistical analysis
GraphPad Prism Version 4 was used for statistical analyses. Results are expressed as mean plus or minus SD if one representative experiment is shown or as mean plus or minus SEM if data are averaged from more than one experiment. Comparison between 2 groups was performed by Student t test. Graft survivals were compared by Kaplan-Meier analysis and the log-rank test. A P value less than .05 was considered significant.
Results
MR-DCs, as prototypic therapeutic DCs, modulate alloimmunity in vivo
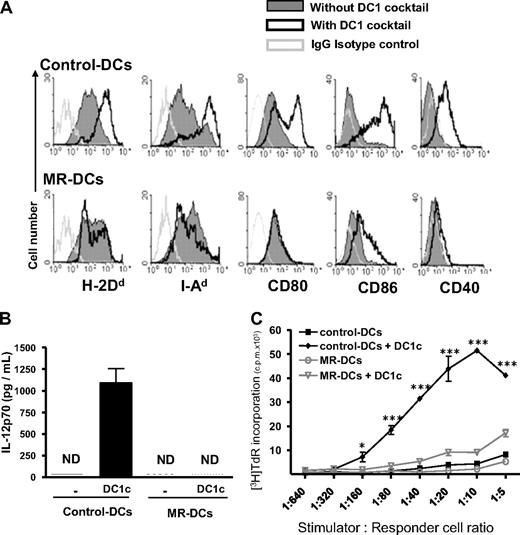
To investigate the mechanisms by which therapeutic immunosuppressive DCs restrain the antidonor response in vivo, we selected as prototype, MR-DCs generated with 1α,25(OH)2VD3, the active form of VD3, which inhibits DC maturation.31-33 These MR-DCs were MHC I/IIlo/int CD40− CD80/86−/lo and, unlike control DCs, failed to up-regulate MHC I/II, CD40, and CD80/86, secrete IL-12p70, or allostimulate T cells after challenge with a DC1-maturation cocktail (Figure 1), LPS, or agonistic CD40 Ab (supplemental Figure 1A-C, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
VD3-treated MR-DCs represent prototypic immunosuppressive DCs in vitro. Bone marrow–derived MR-DCs generated in vitro in the presence of VD3, or not (control-DCs), were challenged for 48 hours with a DC1-maturation cocktail (DC1c). (A) FACS analysis of the surface phenotype of control- and MR-DCs, with or without 48-hour stimulation with DC1c. (B) Detection by ELISA of IL-12p70 in culture supernatants of control- and MR-DCs after 48-hour stimulation with (or without) DC1c (mean ± SD shown). (C) Assessment by 3-day MLCs of the T-cell allostimulatory ability of control- and MR-DCs, untreated or after 48-hour stimulation with DC1c. (A-C) Representative data from 2 or more independent experiments. ND indicates not detected. *P < .05. ***P < .001.
VD3-treated MR-DCs represent prototypic immunosuppressive DCs in vitro. Bone marrow–derived MR-DCs generated in vitro in the presence of VD3, or not (control-DCs), were challenged for 48 hours with a DC1-maturation cocktail (DC1c). (A) FACS analysis of the surface phenotype of control- and MR-DCs, with or without 48-hour stimulation with DC1c. (B) Detection by ELISA of IL-12p70 in culture supernatants of control- and MR-DCs after 48-hour stimulation with (or without) DC1c (mean ± SD shown). (C) Assessment by 3-day MLCs of the T-cell allostimulatory ability of control- and MR-DCs, untreated or after 48-hour stimulation with DC1c. (A-C) Representative data from 2 or more independent experiments. ND indicates not detected. *P < .05. ***P < .001.
We tested the effect of administration of MR-DCs on survival of cardiac allografts in mice, a model that allowed us to compare the effect of our MR-DCs with that of previously reported immunosuppressive DCs used alone in the same model, with mean graft survival times (MSTs) ranging from 19 to 71 days13,14,16-19,21-23 and more than 100 days in one report.15 Administration (intravenous) of 5 × 106 donor-derived (BALB/c) MR-DCs 7 days before transplantation prolonged survival of BALB/c hearts in B6 mice with a MST (52.2 ± 33.4 days) within the range previously reported,13,14,16-19,21-23 and significantly compared with recipients nontreated (MST = 11.1 ± 2.2 days, P < .0001), injected with syngeneic (B6) MR-DCs (MST = 14.5 ± 0.5 days, P < .0001) or with third-party (C3H) MR-DCs (MST = 21.2 ± 8.0 days, P = .0317) (Figure 2A). We did not find statistically significant MST differences between B6 recipients treated with BALB/c MR-DCs or BALB/c immature (not MR) DCs (Im-DCs) (MST = 53.6 ± 15.1 days) (Figure 2A). A higher dose of donor-derived MR-DCs (15 × 106 DCs) was less effective at prolonging survival of BALB/c cardiac allografts in B6 mice (MST = 32.2 ± 6.3 days, N = 5, data not shown).
Therapy with donor-derived MR-DCs regulates the antidonor response and prolongs allograft survival in mice. (A) Survival of BALB/c hearts transplanted into B6 mice nontreated or injected intravenously 7 days before transplantation with (1) 5 × 106 donor (BALB/c)-derived MR-DCs, (2) 5 × 106 donor (BALB/c)-derived immature (not MR) DCs, (3) 5 × 106 recipient (B6)-derived MR-DCs, or (4) 5 × 106 third-party (C3H)-derived MR-DCs. (B) Representative images of sections of BALB/c heart grafts, 7 days after transplantation in B6 recipients nontreated or injected intravenously (7 days before transplantation) with donor-derived MR-DCs. The arrow indicates an area with leukocyte infiltration and edema. Hematoxylin and eosin (original magnification ×200). (C) Microscopic analysis and quantification of graft-infiltrating CD4+ and CD8+ T lymphocytes (as average number of cells per 10 low-powered fields [LPF]) on sections of BALB/c hearts collected 7 days after transplantation in B6 recipients. *Tissue areas detailed in insets. Fluorescence microscopy (original magnifications ×200 and ×400). (D) Assessment of the direct and indirect pathway T-cell responses by IFN-γ ELISPOT assay, 7 days after transplantation, using as responders purified T cells from spleens of naive B6 mice or B6 recipients of BALB/c hearts pretreated (or not) with BALB/c MR-DCs. Data represent 2 or more independent experiments with 3 or more animals per group (mean ± SD shown; values shown for direct pathway).
Therapy with donor-derived MR-DCs regulates the antidonor response and prolongs allograft survival in mice. (A) Survival of BALB/c hearts transplanted into B6 mice nontreated or injected intravenously 7 days before transplantation with (1) 5 × 106 donor (BALB/c)-derived MR-DCs, (2) 5 × 106 donor (BALB/c)-derived immature (not MR) DCs, (3) 5 × 106 recipient (B6)-derived MR-DCs, or (4) 5 × 106 third-party (C3H)-derived MR-DCs. (B) Representative images of sections of BALB/c heart grafts, 7 days after transplantation in B6 recipients nontreated or injected intravenously (7 days before transplantation) with donor-derived MR-DCs. The arrow indicates an area with leukocyte infiltration and edema. Hematoxylin and eosin (original magnification ×200). (C) Microscopic analysis and quantification of graft-infiltrating CD4+ and CD8+ T lymphocytes (as average number of cells per 10 low-powered fields [LPF]) on sections of BALB/c hearts collected 7 days after transplantation in B6 recipients. *Tissue areas detailed in insets. Fluorescence microscopy (original magnifications ×200 and ×400). (D) Assessment of the direct and indirect pathway T-cell responses by IFN-γ ELISPOT assay, 7 days after transplantation, using as responders purified T cells from spleens of naive B6 mice or B6 recipients of BALB/c hearts pretreated (or not) with BALB/c MR-DCs. Data represent 2 or more independent experiments with 3 or more animals per group (mean ± SD shown; values shown for direct pathway).
Seven days after transplantation, allografts from nontreated mice exhibited intense cellular infiltrate, hemorrhage, edema, and myocardial damage, whereas transplants from B6 recipients pretreated with 5 × 106 BALB/c MR-DCs showed minimum infiltrate (Figure 2B), with fewer CD8+ T cells (P = .0111) and similar numbers of CD4+ T cells compared with nontreated controls (Figure 2C). The effect of donor MR-DC therapy on the systemic antidonor T-cell response was quantified 7 days after transplantation. Donor-derived MR-DC administration significantly reduced the frequency of direct (P = .0135) and indirect (P = .0059) pathway IFN-γ-secreting T cells in the spleen, compared with that of nontreated recipients (Figure 2D). Thus, therapy with donor-derived MR-DCs generated with VD3 is associated with significant reduction of intragraft inflammation and the systemic antidonor T-cell response, and prolongation of cardiac allograft survival. We therefore conclude that VD3 MR-DCs represent a typical example of therapeutic immunosuppressive DCs to test the proposed hypothesis.
Therapeutic MR-DCs fail to regulate directly donor-reactive T cells in vivo
The current dogma assumes that therapeutic immunosuppressive DCs injected intravenously interact directly with antidonor T cells in vivo. We tested this idea in TCRtg models where the tg T cells recognize donor-Ag on the surface of injected MR-DCs. Based on previous studies of in situ targeting of quiescent DCs, the injected MR-DCs should promote abortive/defective activation and partial proliferation of allospecific T cells (1-3 days after Ag delivery) followed by deletional tolerance and, in some models, generation of Treg.34-39
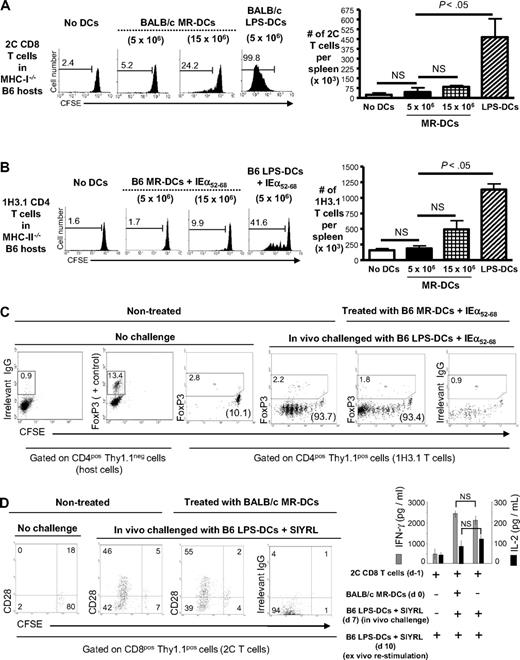
To test whether donor-derived MR-DCs interact directly with donor-reactive CD8+ T cells in vivo, we injected intravenous 5 × 106 BALB/c MR-DCs (the DC dose that optimally prolongs allograft survival in our model) into MHC I−/− B6 mice (Thy1.2) reconstituted with CFSE-labeled 2C TCRtg CD8+ T cells (Thy1.1), which are specific for BALB/c H-2Ld. Because host APCs lack surface MHC I, abortive priming of 2C T cells depends exclusively on contact with the donor-derived MR-DCs. Surprisingly, no proliferation of splenic 2C cells was detected in vivo by FACS analysis 3 days after MR-DC administration (Figure 3A). When the number of MR-DCs was increased 3-fold, a slight proliferation of 2C cells was detected (P = .0131). The fact that administration of 5 × 106 BALB/c LPS-matured DCs triggered robust proliferation of 2C T cells in MHC I−/− B6 mice (P < .0001; Figure 3A) indicates that the lack of MHC I by the host did not affect the viabilty and proliferation of 2C cells, at least during the 3-day span of the experiment.
Therapeutic MR-DCs carrying donor-Ag fail to directly modulate antidonor T cells in vivo. (A) On the left, FACS analysis of CFSE-labeled 2C CD8+ T-cell proliferation when transferred into host MHC class I−/− B6 mice injected (or not, control) the next day with 5 × 106 or 15 × 106 BALB/c MR-DCs, or 5 × 106 BALB/c LPS-matured DCs. Analysis was performed 3 days after DC administration. Numbers in histograms are percentages of dividing 2C CD8+ T cells. On the right, bar diagram shows absolute numbers of 2C CD8+ T cells in the spleen. (B) On the left, FACS analysis of CFSE-labeled 1H3.1 CD4+ T-cell proliferation when transferred into host MHC class II−/− B6 mice injected (or not, control) the next day with 5 × 106 or 15 × 106 B6 MR-DCs pulsed with the BALB/c IEα52-68 allopeptide, or 5 × 106 B6 LPS-matured DCs pulsed with IEα52-68. On the right, bar diagram shows absolute numbers of splenic 1H3.1 CD4+ T cells. (C) In vivo analysis of donor-reactive CD4+ T-cell anergy. MHC II−/− B6 mice reconstituted with CFSE-labeled 1H3.1 CD4+ T cells were injected (or not, control) with B6 MR-DCs pulsed with BALB/c IEα52-68. After 7 days, mice were challenged in vivo, or not, with B6 LPS-matured DCs pulsed with IEα52-68. Three days later, proliferation of splenic 1H3.1 CD4+ T cells (in parentheses) and FoxP3 expression (in gated regions) were analyzed by FACS. (D) In vivo analysis of antidonor CD8+ T-cell anergy. MHC I−/− B6 mice reconstituted with CFSE-labeled 2C CD8+ T cells were injected intravenously (or not, control) with BALB/c MR-DCs. After 7 days, mice were challenged in vivo, or not, with B6 LPS-matured DCs pulsed with the 2C TCR agonistic peptide SIYRYYGL (SIYRL). Three days later, proliferation and CD28 expression of splenic 2C CD8+ T cells were analyzed by FACS, and IFN-γ and IL-2 secretion by splenic 2C T cells was assessed by ELISA after ex vivo restimulation (24 hours) with B6 LPS-matured DCs pulsed with SIYRL. Numbers in dot plots indicate percentages of cells. (A-D) Results represent 2 or more independent experiments with at least 3 animals per group. NS indicates not significant.
Therapeutic MR-DCs carrying donor-Ag fail to directly modulate antidonor T cells in vivo. (A) On the left, FACS analysis of CFSE-labeled 2C CD8+ T-cell proliferation when transferred into host MHC class I−/− B6 mice injected (or not, control) the next day with 5 × 106 or 15 × 106 BALB/c MR-DCs, or 5 × 106 BALB/c LPS-matured DCs. Analysis was performed 3 days after DC administration. Numbers in histograms are percentages of dividing 2C CD8+ T cells. On the right, bar diagram shows absolute numbers of 2C CD8+ T cells in the spleen. (B) On the left, FACS analysis of CFSE-labeled 1H3.1 CD4+ T-cell proliferation when transferred into host MHC class II−/− B6 mice injected (or not, control) the next day with 5 × 106 or 15 × 106 B6 MR-DCs pulsed with the BALB/c IEα52-68 allopeptide, or 5 × 106 B6 LPS-matured DCs pulsed with IEα52-68. On the right, bar diagram shows absolute numbers of splenic 1H3.1 CD4+ T cells. (C) In vivo analysis of donor-reactive CD4+ T-cell anergy. MHC II−/− B6 mice reconstituted with CFSE-labeled 1H3.1 CD4+ T cells were injected (or not, control) with B6 MR-DCs pulsed with BALB/c IEα52-68. After 7 days, mice were challenged in vivo, or not, with B6 LPS-matured DCs pulsed with IEα52-68. Three days later, proliferation of splenic 1H3.1 CD4+ T cells (in parentheses) and FoxP3 expression (in gated regions) were analyzed by FACS. (D) In vivo analysis of antidonor CD8+ T-cell anergy. MHC I−/− B6 mice reconstituted with CFSE-labeled 2C CD8+ T cells were injected intravenously (or not, control) with BALB/c MR-DCs. After 7 days, mice were challenged in vivo, or not, with B6 LPS-matured DCs pulsed with the 2C TCR agonistic peptide SIYRYYGL (SIYRL). Three days later, proliferation and CD28 expression of splenic 2C CD8+ T cells were analyzed by FACS, and IFN-γ and IL-2 secretion by splenic 2C T cells was assessed by ELISA after ex vivo restimulation (24 hours) with B6 LPS-matured DCs pulsed with SIYRL. Numbers in dot plots indicate percentages of cells. (A-D) Results represent 2 or more independent experiments with at least 3 animals per group. NS indicates not significant.
To address the same question with donor-reactive CD4+ T cells, B6 MR-DCs pulsed with the BALB/c IEα52-68 peptide, were injected intravenously into MHC II−/− B6 mice (Thy1.2) reconstituted with CFSE-labeled 1H3.1 CD4+ T cells (Thy1.1) specific for the BALB/c IEα52-68 peptide presented by B6 IAb molecules. Similar to the 2C system, MR-DCs pulsed with IEα52-68 and administered intravenously (5 × 106 DCs) failed to trigger abortive proliferation of 1H3.1 T cells (Figure 3B), assessed by FACS 3 days later. Similar results were obtained when the number of injected MR-DCs pulsed with IEα52-68 was increased 3-fold (Figure 3B). By contrast, administration of LPS-matured DCs pulsed with IEα52-68 induced 1H3.1 T-cell proliferation (P = .0003), indicating that 1H3.1 cells remain viable and proliferate in an MHC II−/− environment during the time course of the experiment (Figure 3B).
Although our findings indicate that the injected MR-DCs do not trigger abortive priming of alloreactive T cells in vivo, they do not exclude induction of anergy through direct contact between the MR-DCs and T cells. To test this, MHC II−/− B6 mice reconstituted with CFSE-labeled 1H3.1 CD4+ T cells were injected intravenously (or not, control) the next day with B6 MR-DCs pulsed with IEα52-68. If the 1H3.1 cells, which do not proliferate under such conditions (as shown in Figure 3B), become anergic after contact with the MR-DCs, they should remain unresponsive to later challenge with stimulatory DCs presenting the same donor-Ag. The finding that splenic 1H3.1 CD4+ T cells from MHC II−/− B6 mice pretreated with B6 MR-DCs plus IEα52-68 proliferated in vivo in response to challenge with B6 LPS-matured-DCs pulsed with IEα52-68 (injected intravenously 7 days after the MR-DCs) as vigorously as 1H3.1 T cells from controls that did not receive MR-DCs, rules out induction of CD4+ T-cell anergy (Figure 3C). The absence of up-regulation of FoxP3 by 1H3.1 T cells indicates that the lack of proliferation of 1H3.1 T cells 7 days after MR-DC injection was not caused by 1H3.1 Treg generation via direct contact with the MR-DCs (Figure 3C).
A similar approach was used to test whether intravenously injected MR-DCs induce CD8+ T-cell anergy in vivo. MHC I−/− B6 mice transferred with CFSE-labeled 2C CD8+ T cells were injected intravenously (or not, control) the next day with BALB/c MR-DCs. If the 2C T cells become anergic after contact with the MR-DCs, they should not proliferate on later challenge with stimulatory DCs. However, splenic 2C CD8+ T cells from MHC I−/− B6 mice pretreated with BALB/c MR-DCs or not (control) exhibited similar levels of proliferation, CD28 expression, and ex vivo release of IFN-γ and IL-2 after challenge (7 days after MR-DC administration) with intravenous injection of B6 LPS-matured-DCs pulsed with SIYRYYGL, a potent agonistic peptide for the 2C TCR (Figure 3D). Together, these results indicate that MR-DCs carrying donor-Ag injected intravenously at the dose that optimally prolongs allograft survival are unable to effectively down-regulate the antidonor response via direct contact with T cells.
Recipient APCs regulate the antidonor T-cell response that follows DC-based therapy
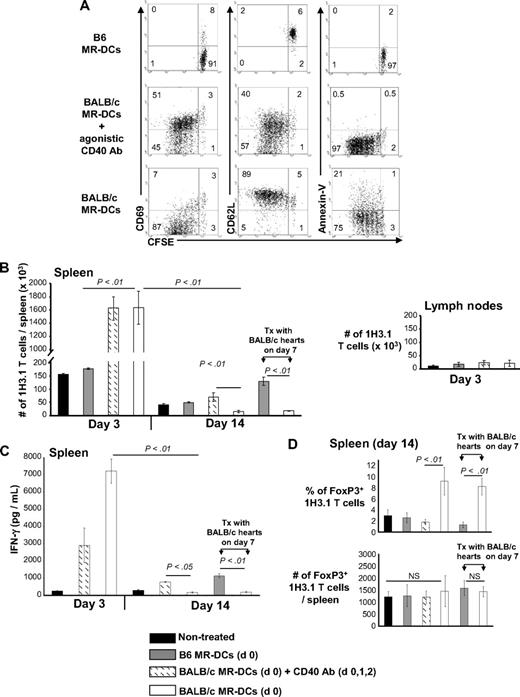
The finding that intravenously injected MR-DCs fail to directly modulate antidonor T cells prompted us to investigate whether their beneficial effect is mediated through indirect pathway T cells. Thy1.2 B6 mice received CFSE-labeled 1H3.1 CD4+ T cells (Thy1.1+) and 1 day later were injected intravenously with BALB/c MR-DCs, alone or in combination with agonistic CD40 Ab, the latter as control to trigger full 1H3.1 cell activation. Because 1H3.1 cells recognize BALB/c IEα52-68 presented by B6 IAb, the injected BALB/c MR-DCs have to be reprocessed and presented by host (B6) APCs to 1H3.1 T cells. Three days after administration of BALB/c MR-DCs, splenic 1H3.1 T cells proliferated but expressed CD69lo CD62Lhi and contained a higher percentage of apoptotic cells, all signs of abortive activation (Figure 4A). By contrast, 1H3.1 T cells from controls treated with BALB/c MR-DCs plus CD40 Ab proliferated and became fully activated (Figure 4A).
Effect of donor-derived MR-DC administration on the indirect CD4+ T-cell response. Host B6 mice received CFSE-labeled 1H3.1 Thy1.1+ CD4+ T cells and were injected intravenously (or not) the next day with control syngeneic (B6) MR-DCs, or BALB/c MR-DCs alone or plus agonistic CD40 Ab (intraperitoneally), the latter used as a positive control to promote full 1H3.1 T-cell activation. (A) Dot plots show proliferation, surface phenotype, and percentages of apoptotic cell death of splenic 1H3.1 CD4+ T cells (gated on Thy1.1 cells) analyzed by FACS, 3 days after MR-DC administration. (B) Absolute numbers of 1H3.1 Thy1.1+ CD4+ T cells in the spleen, 3 and 14 days after B6 (control) or BALB/c MR-DC (with and without CD40 Ab, intraperitoneally) intravenous administration in host B6 mice, in the absence or presence of BALB/c cardiac allografts transplanted 7 days after MR-DC administration. Absolute numbers of 1H3.1 cells did not change significantly in lymph nodes (cervical, axilar, mesenteric, inguinal), assessed 3 days after MR-DC infusion. (C) Amounts of IFN-γ (by ELISA) secreted by splenocytes of each group of recipient B6 mice restimulated ex vivo (24 hours) with the BALB/c IEα52-68 allopeptide. (D) Percentages and absolute numbers of splenic 1H3.1 FoxP3+ CD4+ T cells 14 days after MR-DC injection in each group of recipient B6 mice. NS indicates not significant. (A-D) Results represent 2 independent experiments with 3 or more animals per group (mean ± SD).
Effect of donor-derived MR-DC administration on the indirect CD4+ T-cell response. Host B6 mice received CFSE-labeled 1H3.1 Thy1.1+ CD4+ T cells and were injected intravenously (or not) the next day with control syngeneic (B6) MR-DCs, or BALB/c MR-DCs alone or plus agonistic CD40 Ab (intraperitoneally), the latter used as a positive control to promote full 1H3.1 T-cell activation. (A) Dot plots show proliferation, surface phenotype, and percentages of apoptotic cell death of splenic 1H3.1 CD4+ T cells (gated on Thy1.1 cells) analyzed by FACS, 3 days after MR-DC administration. (B) Absolute numbers of 1H3.1 Thy1.1+ CD4+ T cells in the spleen, 3 and 14 days after B6 (control) or BALB/c MR-DC (with and without CD40 Ab, intraperitoneally) intravenous administration in host B6 mice, in the absence or presence of BALB/c cardiac allografts transplanted 7 days after MR-DC administration. Absolute numbers of 1H3.1 cells did not change significantly in lymph nodes (cervical, axilar, mesenteric, inguinal), assessed 3 days after MR-DC infusion. (C) Amounts of IFN-γ (by ELISA) secreted by splenocytes of each group of recipient B6 mice restimulated ex vivo (24 hours) with the BALB/c IEα52-68 allopeptide. (D) Percentages and absolute numbers of splenic 1H3.1 FoxP3+ CD4+ T cells 14 days after MR-DC injection in each group of recipient B6 mice. NS indicates not significant. (A-D) Results represent 2 independent experiments with 3 or more animals per group (mean ± SD).
The initial expansion of 1H3.1 CD4+ T cells after BALB/c MR-DC administration was followed at day 14 by a significant reduction in (1) the number of 1H3.1 T cells in the spleen (Figure 4B) and (2) IFN-γ secretion by 1H3.1 T cells in response to ex vivo restimulation with IEα52-68 (Figure 4C), compared with mice nontreated or injected with BALB/c MR-DCs plus agonistic CD40 Ab.
The reduction in numbers of 1H3.1 T cells in spleen of B6 mice 14 days after administration of BALB/c MR-DCs was caused by deletion instead of migration to peripheral tissues because 1H3.1 T cells were not detected in heart, kidney, liver, or blood (not shown). Administration of BALB/c MR-DCs was accompanied at day 14 by a significant increase in the percentage of CD4+ FoxP3+ 1H3.1 T cells in the spleen. However, their absolute numbers did not change significantly, suggesting that CD4+ FoxP3+ 1H3.1 T cells have a survival advantage compared with effector T cells (Figure 4D). Administration of BALB/c MR-DCs in B6 hosts did not induce immune deviation in 1H3.1 cells, as neither IL-4 nor IL-10 was detected after ex vivo stimulation with IEα52-68, 3 or 14 days after MR-DC treatment (not shown). Similar down-regulatory effects on adoptively transferred 1H3.1 T cells were detected on day 14 in B6 mice transplanted with BALB/c heart allografts 7 days after intravenous administration (on day 0) of BALB/c MR-DCs, mimicking the therapeutic approach used by us and most DC-based therapies reported in transplantation models (Figure 4B-D). The effects of BALB/c MR-DCs on 1H3.1 T cells in vivo were not the result of the VD3 because similar results were achieved after administration of BALB/c Im-DCs (not MR) generated without exogenous VD3 (supplemental Figure 2).
Interestingly, intravenous administration of apoptotic (UVB-irradiated) BALB/c MR-DCs also promoted 1H3.1 T-cell deletion and increased the relative percentage of CD4+ FoxP3+ 1H3.1 T cells in spleen (supplemental Figure 3), indicating that therapeutic DCs do not to have to be alive to down-regulate the antidonor T-cell response. Interestingly, all mice treated with apoptotic MR-DCs secreted very low levels of IFN-γ (supplemental Figure 3C), probably because of the additional anti-inflammatory effects of apoptotic cells.
Donor-derived MR-DCs are short-lived and internalized by recipient DCs
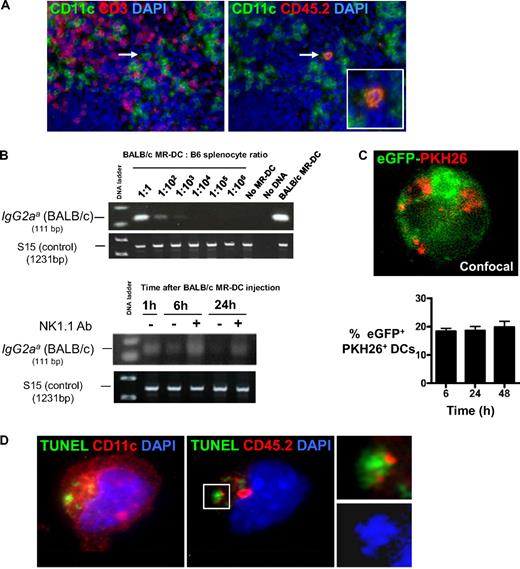
We hypothesized that, once injected intravenously, donor-derived MR-DCs alive or dead (because of natural turnover or targeting by NK cells40 ) serve as a source of donor-Ag for recipient quiescent APCs in lymphoid organs.28,37,38 To test this idea, we first analyzed trafficking of (CD45.2+) BALB/c MR-DCs injected intravenously into (CD45.1+) B6 mice. Six hours after injection (107 DCs), very few MR-DCs were detected by microscopy in the marginal zone, and between 12 and 48 hours, in the T-cell areas of the spleen (Figure 5A). Because of their low numbers, we estimated semiquantitatively the amount of MR-DCs in the spleen by PCR analysis for the IgG2aa allele, which is encoded in the BALB/c, but not B6, genome.30 Using serially diluted BALB/c MR-DCs, we determined the sensitivity of the assay to be approximately 1 BALB/c MR-DC in 10 000 B6 splenocytes (Figure 5B). The content of BALB/c DNA decreased steadily 1 hour after MR-DC inoculation and was barely detectable 24 hours later, approximately indicative of less than or equal to 10 000 BALB/c MR-DCs per spleen (Figure 5B). Treatment with the NK cell-depleting Ab NK1.1 increased the amount of BALB/c DNA detected 6 and 24 hours after MR-DC injection, indicating that host NK cells contribute to elimination of donor-derived MR-DCs. Additional experiments confirmed that BALB/c MR-DCs are killed by B6 NK cells in vitro (supplemental Figure 4) and in vivo (supplemental Figure 5). Importantly, the use of VD3 did not affect the viability of MR-DCs in vitro or their viability or capacity to migrate to secondary lymphoid tissues in vivo, compared with control DCs (supplemental Figure 5).
Donor-derived MR-DCs are processed by recipient DCs in vivo. (A) Migration of intravenously injected BALB/c MR-DCs (CD45.2+) into B6 mouse (CD45.1+) spleen, analyzed by fluorescence microscopy on tissue sections, 24 hours after MR-DC injection. Arrow points to a BALB/c MR-DC homed in the T-cell area of a splenic follicle. Nuclei were stained blue with DAPI (original magnification × 200). (B) Detection by PCR of the BALB/c IgG2aa allele after BALB/c MR-DC injection intravenously into B6 mice. (Top) Assay sensitivity. (Bottom) Detection by PCR of BALB/c MR-DCs mobilized to spleens of B6 mice 1, 6, or 24 hours after MR-DC injection. Host B6 mice were treated (+) or not (−) with the NK cell-depleting Ab NK1.1. The housekeeping gene S15 is shown to confirm equal DNA loading between samples. (C) Confocal image of a cytospin showing one recipient (eGFP+) splenic DC with fragments (in red) derived from PKH26-labeled BALB/c MR-DCs injected intravenously 12 hours earlier (original magnification × 400). Percentages of recipient splenic eGFP+ DCs with material acquired from PKH26-labeled BALB/c MR-DCs, assessed on cytospins by microscopy at different times after MR-DC injection. (D) Cytospin showing one recipient (B6) splenic DC (CD11c+, far red) containing phagocytosed apoptotic cell fragments (TUNEL+, green) derived from CD45.2+ (red) BALB/c MR-DCs injected intravenously. Nuclei were stained with DAPI. The area marked in the figure is shown in detail in the inset. Confocal, fluorescence microscopy (original magnification ×400).
Donor-derived MR-DCs are processed by recipient DCs in vivo. (A) Migration of intravenously injected BALB/c MR-DCs (CD45.2+) into B6 mouse (CD45.1+) spleen, analyzed by fluorescence microscopy on tissue sections, 24 hours after MR-DC injection. Arrow points to a BALB/c MR-DC homed in the T-cell area of a splenic follicle. Nuclei were stained blue with DAPI (original magnification × 200). (B) Detection by PCR of the BALB/c IgG2aa allele after BALB/c MR-DC injection intravenously into B6 mice. (Top) Assay sensitivity. (Bottom) Detection by PCR of BALB/c MR-DCs mobilized to spleens of B6 mice 1, 6, or 24 hours after MR-DC injection. Host B6 mice were treated (+) or not (−) with the NK cell-depleting Ab NK1.1. The housekeeping gene S15 is shown to confirm equal DNA loading between samples. (C) Confocal image of a cytospin showing one recipient (eGFP+) splenic DC with fragments (in red) derived from PKH26-labeled BALB/c MR-DCs injected intravenously 12 hours earlier (original magnification × 400). Percentages of recipient splenic eGFP+ DCs with material acquired from PKH26-labeled BALB/c MR-DCs, assessed on cytospins by microscopy at different times after MR-DC injection. (D) Cytospin showing one recipient (B6) splenic DC (CD11c+, far red) containing phagocytosed apoptotic cell fragments (TUNEL+, green) derived from CD45.2+ (red) BALB/c MR-DCs injected intravenously. Nuclei were stained with DAPI. The area marked in the figure is shown in detail in the inset. Confocal, fluorescence microscopy (original magnification ×400).
Given this rapid loss of the injected DCs in vivo, we explored whether BALB/c MR-DCs are internalized as apoptotic cells by splenic APCs. PKH26-labeled (red) BALB/c MR-DCs were injected intravenously in CD11c-eGFP B6 mice. Six, 24, and 48 hours after injection, approximately 20% of splenic eGFP+ DCs contained PKH26+ fragments (Figure 5C). To exclude potential passive transfer of PKH26 between donor and host DCs and to identify apoptotic bodies, (CD45.2+) BALB/c MR-DCs were injected intravenously into (CD45.1+) B6 mice. Twelve hours later, we detected BALB/c MR-DC-derived apoptotic bodies (CD45.2+ TUNEL+) in B6 splenic DCs (CD11c+ CD45.2−; Figure 5D). Thus, intravenously injected donor-derived MR-DCs rapidly die in vivo, at least partly by NK cell-mediated killing, and are internalized as apoptotic cells by host splenic DCs.
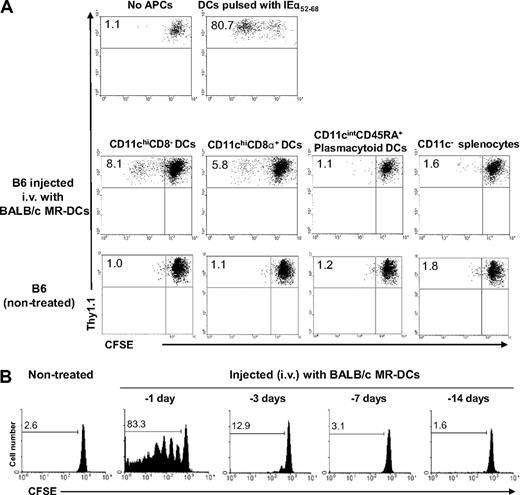
Donor-derived MR-DCs are processed and presented to antidonor T cells by recipient CD11chi DCs
To identify the splenic APC(s) responsible for presenting donor-Ag acquired from systemically administered immunosuppressive DCs, B6 mice were injected intravenously (or not, control) with 107 BALB/c MR-DCs. Twenty hours later, the following host (H2-Kb) splenic cells were FACS-sorted: (1) CD11chiCD8− DCs, (2) CD11chiCD8α+ DCs, (3) CD11cintCD45RA+ plasmacytoid DCs, and (4) CD11c− cells. Only CD11chiCD8− and CD8α+ DCs induced proliferation of CFSE-labeled 1H3.1 CD4+ T cells (specific for BALB/c IEα52-68-B6 IAb) ex vivo (Figure 6A). To determine how long host splenic DCs present the donor-Ag, B6 mice were injected with BALB/c MR-DCs 14, 7, 3, or 1 day before the adoptive transfer of CFSE-labeled 1H3.1 T cells. Presentation of the BALB/c IEα52-68 peptide by host DCs was limited in time, as only minor 1H3.1 cell proliferation was detected after the 3-day lag period between administration of MR-DCs and 1H3.1 cells (Figure 6B). Thus, systemically injected donor-derived MR-DCs are processed/presented to indirect CD4+ T cells by splenic CD8− and CD8α+ DC only briefly in vivo.
Donor-derived MR-DCs injected systemically are presented for a short-time by host CD11chi DCs. (A) Different subsets of splenic APCs from B6 mice nontreated (control) or injected 20 hours earlier with BALB/c MR-DCs were isolated by FACS sorting, then γ-irradiated and used as stimulators of CFSE-labeled 1H3.1 CD4+ T cells in 5-day MLCs. CFSE-labeled 1H3.1 T cells cultured alone or stimulated with splenic CD11c+ CD8− DCs prepulsed with IEα52-68 were included as negative and positive controls, respectively. Dilution of CFSE in 1H3.1 T cells (gated on CD4+ cells) was analyzed by flow cytometry. Numbers are percentages of dividing 1H3.1 CD4+ T cells. (B) Assessment of duration of presentation by host (B6) splenic APCs of the BALB/c IEα52-68 allopeptide derived from reprocessing of BALB/c MR-DCs on various days, before adoptive transfer of CFSE-labeled 1H3.1 CD4+ T cells. Numbers are percentages of dividing 1H3.1 CD4+ T cells in vivo. (A-B) Representative results from 2 or more independent experiments are shown.
Donor-derived MR-DCs injected systemically are presented for a short-time by host CD11chi DCs. (A) Different subsets of splenic APCs from B6 mice nontreated (control) or injected 20 hours earlier with BALB/c MR-DCs were isolated by FACS sorting, then γ-irradiated and used as stimulators of CFSE-labeled 1H3.1 CD4+ T cells in 5-day MLCs. CFSE-labeled 1H3.1 T cells cultured alone or stimulated with splenic CD11c+ CD8− DCs prepulsed with IEα52-68 were included as negative and positive controls, respectively. Dilution of CFSE in 1H3.1 T cells (gated on CD4+ cells) was analyzed by flow cytometry. Numbers are percentages of dividing 1H3.1 CD4+ T cells. (B) Assessment of duration of presentation by host (B6) splenic APCs of the BALB/c IEα52-68 allopeptide derived from reprocessing of BALB/c MR-DCs on various days, before adoptive transfer of CFSE-labeled 1H3.1 CD4+ T cells. Numbers are percentages of dividing 1H3.1 CD4+ T cells in vivo. (A-B) Representative results from 2 or more independent experiments are shown.
Donor-derived therapeutic immunosuppressive DCs function as Ag-transporting cells
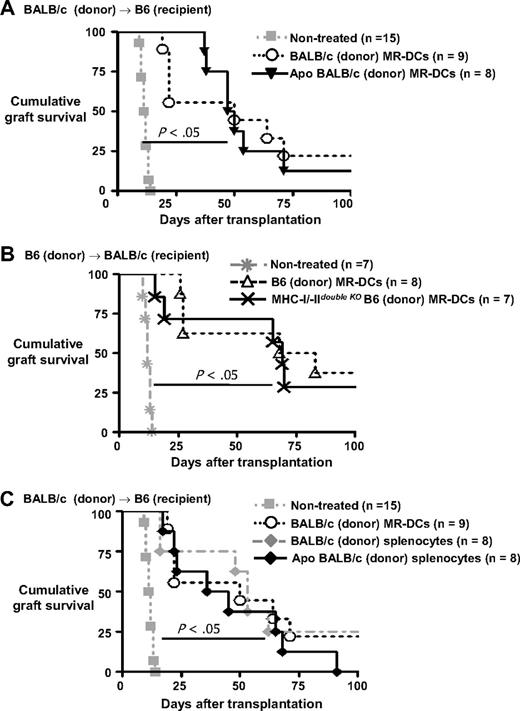
If donor-derived MR-DCs injected intravenously fail to modulate directly donor-reactive T cells, but rather serve as donor-Ag-transporting cells reprocessed into alloAg by recipient APCs, administration of donor MR-DCs incapable of functioning as APCs, but able to deliver donor-Ag to recipient quiescent APCs, should prolong allograft survival in our model. To test this idea, B6 mice were injected intravenously with apoptotic (UVB-irradiated) BALB/c MR-DCs (5 × 106 DCs) 7 days before transplantation of BALB/c hearts. Administration of donor-derived apoptotic MR-DCs prolonged allograft survival as efficiently as MR-DCs viable at the time of injection (MST = 56.0 ± 20.7 days and 52.2 ± 33.4 days, respectively, Figure 7A). Using an alternative approach, we demonstrated that systemic administration of B6 MHC I/IIdouble KO MR-DCs into wild-type BALB/c mice prolongs survival of B6 cardiac allografts as efficiently as therapeutic wild-type B6 MR-DCs (MST = 62.6 ± 31.8 and 66.4 ± 32.4 days, respectively, Figure 7B). B6 MHC I/IIdouble KO cells express little, if any, MHC I/II molecules on the surface but are able to produce MHC I and MHC II IAα and IEβ chains, which are retained intracellularly and constitute the source of donor-Ag in our model.12 Thus, therapeutic immunosuppressive DCs unable to function as APCs, but capable of delivering donor-Ag to recipient APCs, significantly prolong cardiac allograft survival in our system.
Comparative effects of therapies with donor-derived immunosuppressive MR-DCs and DST in mice. (A) Survival of BALB/c cardiac allografts in recipient B6 mice treated intravenously (or not, control), 7 days before transplantation with BALB/c MR-DCs alive or apoptotic. (B) Survival of B6 cardiac transplantations in recipient BALB/c mice untreated or injected intravenously 7 days before transplantation with MR-DCs generated from wild-type or MHC I/IIdouble KO B6 mice. (C) Comparison of survival of BALB/c cardiac allografts in recipient B6 mice pretreated (intravenously) 7 days before transplantation with viable BALB/c MR-DCs, or with BALB/c splenocytes alive or apoptotic.
Comparative effects of therapies with donor-derived immunosuppressive MR-DCs and DST in mice. (A) Survival of BALB/c cardiac allografts in recipient B6 mice treated intravenously (or not, control), 7 days before transplantation with BALB/c MR-DCs alive or apoptotic. (B) Survival of B6 cardiac transplantations in recipient BALB/c mice untreated or injected intravenously 7 days before transplantation with MR-DCs generated from wild-type or MHC I/IIdouble KO B6 mice. (C) Comparison of survival of BALB/c cardiac allografts in recipient B6 mice pretreated (intravenously) 7 days before transplantation with viable BALB/c MR-DCs, or with BALB/c splenocytes alive or apoptotic.
Finally, we compared the effect on allograft survival of donor-derived MR-DCs with that of DST splenocytes, the latter previously shown to be a much simpler method of regulating the antidonor response via the indirect pathway.10-12,37,38 Surprisingly, DSTs with alive or apoptotic splenocytes prolonged cardiac allograft survival in mice comparable with therapy with donor-derived MR-DCs (56.0 ± 32.1 days, 45.9 ± 26.5 days, and 52.2 ± 33.4 days, respectively, Figure 7C).
Discussion
Understanding the mechanisms by which DCs maintain peripheral tolerance and the development of methods to propagate DCs in vitro has paved the way for the use of immunosuppressive (also known as tolerogenic) DC-based therapies in experimental models to prevent/ameliorate graft-versus-host disease and allograft rejection.13-27 These therapies could potentially promote donor-specific tolerance, thus reducing dependence on chronic pharmacologic immunosuppression. Despite promising results, the mechanism(s) by which therapeutic DCs down-modulate alloimmunity in vivo remain unknown.
The prevailing dogma states that therapeutic DCs regulate the antidonor response by interacting physically with donor-reactive T cells in lymphoid organs, a concept that has not been tested in vivo because previous studies have assessed the effect of therapeutic DCs in vitro or in ex vivo assays after transplantation.13-27 To address this issue, we selected as prototypic therapeutic DCs, donor-derived MR-DCs generated in vitro with VD3, which restrain the antidonor response and prolong survival of mouse cardiac allografts with efficacy comparable with previously described immature, MR, or alternatively-activated DCs generated by alternative methods.13,14,16-19,21-23
In situ targeting of quiescent DCs in secondary lymphoid organs has demonstrated that presentation of model or allo-Ag by immature/semimature DCs promotes abortive T-cell activation followed by deletional tolerance,34-39 and in some cases, expansion of Treg.36-38 The prevailing idea predicts that a similar phenomenon should occur after systemic administration of immunosuppressive DCs bearing donor-Ag. By contrast, our results indicate that MR-DCs injected intravenously fail to trigger these events through direct interaction with antidonor T cells in vivo. Conversely, we found that injection of therapeutic MR-DCs results in presentation of alloAg by recipient DCs in the context of self-MHC II, triggering deficient activation and deletion of effector T cells, and increasing the percentage but not absolute number of indirect CD4+ FoxP3+ T cells. The latter suggests that naturally occurring CD4+ Tregs may be resistant to deletion when allo-Ag is presented by quiescent DCs. Similar down-regulation of the antidonor response was detected after administration of donor-derived apoptotic MR-DCs or Im-DCs (not exposed to VD3), supporting that the injected DCs function as Ag-transporting cells rather than APCs.
This sequence of events requires transfer of donor-Ag from the injected MR-DCs to recipient DCs in lymphoid organs. It has been shown that DCs acquire Ag from living or dying cells, via internalization of patches of plasma membrane, exosomes, or apoptotic cells.28,40-42 Our findings indicate that donor-derived MR-DCs survive briefly in vivo, in part because of targeting by recipient NK cells. Accordingly, we found that apoptotic cell fragments derived from the injected MR-DCs constitute a source of donor-Ag for recipient DCs. This does not exclude the transfer of donor-Ag by therapeutic DCs by other mechanisms. Our results show that host splenic conventional (CD11chi) DCs, unlike plasmacytoid DCs and CD11c− APCs, present allo-peptides acquired from the injected MR-DCs, agreeing with the fact that sustained MHC II–peptide complex formation, ubiquitination, and recycling render plasmacytoid DCs, unlike conventional DCs, inefficient at presentation of exogenous Ag.43
Recipient CD11chi DCs, but not B cells or plasmacytoid DCs, are required for modulation of the antidonor response after negative vaccination with donor-derived MR-DCs. In contrast, control LPS-matured DCs activated donor-reactive T cells in MHC−/− hosts, suggesting that for positive vaccination, therapeutic DCs are capable of directly activating T cells in vivo. However, even in this case, it has been shown that host DCs enhance several-fold T-cell proliferation and effector function.44 Transfer of Ag between therapeutic and endogenous DCs may constitute an amplification mechanism by which small amounts of exogenous Ag are spread over a large population of host DCs for induction of tolerance or immunity.45,46 The finding that systemic administration of donor-derived apoptotic MR-DCs, or viable MR-DCs lacking surface MHC I/II molecules, prolonged cardiac allograft survival as efficiently as wild-type viable MR-DCs, serves as evidence that donor-derived MR-DCs function mainly as Ag-transporting cells.
Evidence in mice suggests that the beneficial effect on cardiac allograft survival induced by DST is achieved through recipient APCs.10-12,37,38 Our results indicate that even the more recently developed therapies using recipient-derived immunosuppressive DCs pulsed with donor-Ag22-24 could also function via endogenous DCs because host (B6) MR-DCs pulsed with the BALB/c IEα52-68 peptide also failed to interact directly with T cells in vivo (Figure 3B). Our results also suggest that DST and DC-based therapies may function through a shared mechanism of reprocessing/presentation by recipient quiescent DCs in secondary lymphoid organs.
The mechanisms of action of therapeutic donor-derived immunosuppressive DCs and DST through recipient APCs encompass the following paradox: if the injected cells fail to interact directly with donor-reactive T cells, how do they down-regulate the direct pathway T-cell response in graft recipients? Although this difficult problem has not been the focus of our study, it could occur through regulation of the indirect CD4+ T cell help (via clonal deletion or Treg generation) required for induction of direct pathway T cells. It has been shown that indirect pathway Tregs mediate linked suppression in a mouse skin graft model47 and that Tregs attenuate antidonor CD8+ T-cell priming in lymphoid organs and prevent rejection on homing to the graft.48 There is also evidence that indirect pathway CD4+ T cells provide help to direct pathway CD8+ T cells in a skin transplantation model in mice49 and that cardiac allografts transplanted into CD80/86−/− mice result in long-term survival that is abrogated with treatment of agonistic CD28 Ab, suggesting that indirect pathway costimulation is required for acute rejection.50
In conclusion, our findings indicate that DC-based therapies modulate the antidonor response through recipient APCs. Comparing efficacy in larger animal models is yet to be performed, and our study does not preclude future generation of immunosuppressive DCs capable of directly mediating T-cell suppression. But given the cost, time, and risk of DC-based therapies, our data raise some concern on the potential benefits of current DC-based therapies in transplantation over previously tested and simpler methods, such as DST.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank L. D. Falo for generously providing the B6 CD11c-DTR-eGFP mice, G. Chalasani for the 2C TCRtg mice, and Gregory Gibson for providing technical assistance with confocal microscopy.
This work was supported by the National Institutes of Health (grants T32 AI074490 and F30 DK082131, S.J.D.; and grants R01 HL075512 and R01 HL077545, A.E.M.).
National Institutes of Health
Authorship
Contribution: S.J.D., Z.W., W.J.S., Q.L., O.A.T., A.M., G.E., A.T.L., and A.E.M. performed experiments; S.J.D., A.T.L., and A.E.M. analyzed results and made the figures; and S.J.D. and A.E.M designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Adrian E. Morelli, E1546 Biomedical Science Tower, 200 Lothrop St, Pittsburgh, PA 15213-2582; e-mail: morelli@imap.pitt.edu.

![Figure 2. Therapy with donor-derived MR-DCs regulates the antidonor response and prolongs allograft survival in mice. (A) Survival of BALB/c hearts transplanted into B6 mice nontreated or injected intravenously 7 days before transplantation with (1) 5 × 106 donor (BALB/c)-derived MR-DCs, (2) 5 × 106 donor (BALB/c)-derived immature (not MR) DCs, (3) 5 × 106 recipient (B6)-derived MR-DCs, or (4) 5 × 106 third-party (C3H)-derived MR-DCs. (B) Representative images of sections of BALB/c heart grafts, 7 days after transplantation in B6 recipients nontreated or injected intravenously (7 days before transplantation) with donor-derived MR-DCs. The arrow indicates an area with leukocyte infiltration and edema. Hematoxylin and eosin (original magnification ×200). (C) Microscopic analysis and quantification of graft-infiltrating CD4+ and CD8+ T lymphocytes (as average number of cells per 10 low-powered fields [LPF]) on sections of BALB/c hearts collected 7 days after transplantation in B6 recipients. *Tissue areas detailed in insets. Fluorescence microscopy (original magnifications ×200 and ×400). (D) Assessment of the direct and indirect pathway T-cell responses by IFN-γ ELISPOT assay, 7 days after transplantation, using as responders purified T cells from spleens of naive B6 mice or B6 recipients of BALB/c hearts pretreated (or not) with BALB/c MR-DCs. Data represent 2 or more independent experiments with 3 or more animals per group (mean ± SD shown; values shown for direct pathway).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/15/10.1182_blood-2009-10-251058/4/m_zh89991057950002.jpeg?Expires=1763551637&Signature=2IyTw6FB4AjTUMDDch2E30NYJ4Ww3FOPEwTuIUgDiTR964-5wsHUyAwejRaKARoUoa7krW5F5lEJMCc7kl2dJR7ppiQiuxrcvo8RBsjMZu~H50JfGCa1L7EEBdwLl5kGZApFjzKEJArPjJeQqUFuYqVQFRhdC6ffQ~Itr1UzOb6zaAiHA~nDEDb0wuvqeRFxqrFmrKuZsDwirebyfrbsf7~VjK9EKp6RS4BMERFHEi2N14MRikwRG1VRRftLT8zNCo6ZNtfuRJA77PG8IJcxVMAqwEktjbr9ET4bipnZzhbRuUvplE02BabKBFhQexfr4I7iM0bAp8w76~cU~-Ro3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal