Abstract
G6PC3 deficiency, characterized by neutropenia and neutrophil dysfunction, is caused by deficiencies in the endoplasmic reticulum (ER) enzyme glucose-6-phosphatase-β (G6Pase-β or G6PC3) that converts glucose-6-phosphate (G6P) into glucose, the primary energy source of neutrophils. Enhanced neutrophil ER stress and apoptosis underlie neutropenia in G6PC3 deficiency, but the exact functional role of G6Pase-β in neutrophils remains unknown. We hypothesized that the ER recycles G6Pase-β–generated glucose to the cytoplasm, thus regulating the amount of available cytoplasmic glucose/G6P in neutrophils. Accordingly, a G6Pase-β deficiency would impair glycolysis and hexose monophosphate shunt activities leading to reductions in lactate production, adenosine-5′-triphosphate (ATP) production, and reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity. Using annexin V–depleted neutrophils, we show that glucose transporter-1 translocation is impaired in neutrophils from G6pc3−/− mice and G6PC3-deficient patients along with impaired glucose uptake in G6pc3−/− neutrophils. Moreover, levels of G6P, lactate, and ATP are markedly lower in murine and human G6PC3-deficient neutrophils, compared with their respective controls. In parallel, the expression of NADPH oxidase subunits and membrane translocation of p47phox are down-regulated in murine and human G6PC3-deficient neutrophils. The results establish that in nonapoptotic neutrophils, G6Pase-β is essential for normal energy homeostasis. A G6Pase-β deficiency prevents recycling of ER glucose to the cytoplasm, leading to neutrophil dysfunction.
Introduction
There are 2 enzymatically active glucose-6-phosphatases (G6Pases), the liver/kidney/intestine–restricted G6Pase-α (or G6PC)1,2 and the ubiquitously expressed G6Pase-β (also known as G6PC3).3,4 Both enzymes are transmembrane endoplasmic reticulum (ER) proteins, with a similar topology, that places their active site on the luminal side of the ER membrane.5,6 Both have similar kinetic properties2,4 and hydrolyze glucose-6-phosphate (G6P) to glucose and phosphate when coupled with the ubiquitously expressed G6P transporter (G6PT) that translocates G6P from the cytoplasm into the lumen of the ER.7,8 The primary role of the G6Pase/G6PT complex is to provide glucose and phosphate to the ER lumen. The G6Pase-α/G6PT complex maintains blood glucose homeostasis between meals by hydrolyzing G6P to glucose in the terminal step of gluconeogenesis and glycogenolysis.1,2 Deficiencies in G6Pase-α cause glycogen storage disease type Ia (GSD-Ia) and deficiencies in G6PT result in GSD type Ib (GSD-Ib).1,2,9 Both GSD-Ia and GSD-Ib patients manifest a phenotype of disturbed blood glucose homeostasis with GSD-Ib patients also suffering neutropenia and neutrophil dysfunction,1,9 reflecting a role of G6PT in tissues beyond the liver and kidney.
The biologic roles of G6Pase-β and the G6Pase-β/G6PT complex are poorly defined. Neutrophils, which express both G6Pase-β and G6PT,10 are capable of endogenous production of glucose, the primary energy for neutrophils,11 which might suggest that G6Pase-β and G6PT play a role in neutrophil homeostasis and function. To examine this hypothesis, we generated mouse lines deficient in either G6Pase-β10 or G6PT12 and showed that both G6Pase-β–deficient (G6pc3−/−) and G6PT-deficient (GSD-Ib) mice manifest neutropenia and neutrophil dysfunction that mimic the symptoms in human GSD-Ib patients.1,9 We further showed that neutrophils deficient in either G6Pase-β10 or G6PT13 undergo enhanced ER stress and apoptosis, demonstrating that endogenous glucose production in the ER is critical for neutrophil homeostasis. This is consistent with the observation that human GSD-Ib patients manifest a phenotype of congenital neutropenia.1,9 Providing further support for the vital role of G6Pase-β/G6PT in normal neutrophil function, a recent linkage analysis identified the G6PC3 gene as the disease candidate for a severe congenital neutropenia syndrome.14 Moreover, neutrophils from G6PC3-deficient patients also exhibited enhanced ER stress and apoptosis.14
Neutrophils play a key role in the early inflammatory response to infection. Because neutrophils cannot produce glucose via gluconeogenesis,15 their glucose is supplied either by the breakdown of stored glycogen, or by uptake from the blood via facilitated diffusion across the plasma membrane mediated by the glucose transporters (GLUTs).16 Within the neutrophil, glucose is metabolized by hexokinase to G6P.11 There are multiple competing cytoplasmic pathways that use intracellular glucose/G6P, including glycogen formation,11,17 glycolysis,11,18 the hexose monophosphate shunt (HMS),11,19 and G6PT-mediated ER uptake. Glycogen use in neutrophils is considered primarily restricted to phagocytic activity.20 Previous views held that the primary competing glucose/G6P pathways in neutrophils are glycolysis and HMS. Glycolysis supplies the necessary energy for neutrophil locomotion and chemotaxis.11,18 HMS generates the reduced nicotinamide adenine dinucleotide phosphate (NADPH) substrate for NADPH oxidase, which facilitates the production of superoxide anions and hence other reactive oxygen species (ROS).21,22 However, more recent studies show that G6P transported into the ER is metabolized by at least 2 different enzymes: G6Pase-β to produce glucose4,5 and hexose-6-phosphate dehydrogenase (H6PDH) to generate NADPH that is important for in vivo function of 11β-hydroxysteroid dehydrogenase-1.23 H6PDH-deficient mice are not reported to have neutrophil abnormalities.24,25 In contrast, G6Pase-β–deficient mice do manifest neutrophil dysfunction10 that mimic those in GSD-Ib patients.1,9 This suggests that G6Pase-β provides the primary pathway of glucose metabolism in the ER and represents an additional G6P-utilization pathway that regulates neutrophil function.
We have demonstrated that endogenous glucose production in the ER via the G6Pase-β/ G6PT complex is critical for normal neutrophil function10 but the exact role and mechanism of action of G6Pase-β in phagocytes remains to be elucidated. We now propose that ER recycling of G6P is a third major competing pathway regulating cytoplasmic glucose/G6P in neutrophils. We hypothesize that ER uptake of G6P by the G6PT provides substrate to G6Pase-β, which then releases glucose back to the cytoplasm, as a rapid and responsive mechanism for regulating the amount of available cytoplasmic glucose/G6P. Accordingly, we predict that neutrophils lacking G6Pase-β will be unable to release glucose from the ER into the cytoplasm. As a result, cytoplasmic glucose/G6P levels in G6Pase-β–deficient neutrophils will fall, if not countered by an appropriate increase in blood glucose uptake. Such falling levels of intracellular G6P are predicted to impair glycolysis and HMS activities, which will lead to decreases in neutrophil lactate and adenosine-5′-triphophate (ATP) levels and neutrophil dysfunction charcterized by impaired respiratory burst, chemotaxis, calcium flux, and phagocytosis.
In this report we present evidence consistent with this hypothesis. Because G6PC3 deficiency is characterized by neutropenia and enhanced neutrophil apoptosis,10,14 functional assessments were conducted in neutrophils purified by annexin V depletion of apoptotic cells. We show that the intracellular levels of G6P, lactate, and ATP in neutrophils of G6pc3−/− mice and human G6PC3-deficient patients are markedly reduced compared with the controls. Likewise, NADPH oxidase expression and activation are impaired in both human and murine G6PC3-deficient neutrophils. In normal neutrophils, increasing cytoplasmic lactate and ATP levels stimulate uptake of blood glucose by inducing the translocation of GLUT1 to the plasma membrane.26-28 Consistent with this we show that the GLUT1 translocation to the plasma membrane is impaired in human and murine G6PC3-deficient neutrophils. Moreover, glucose uptake is impaired in G6pc3−/− neutrophils. Taken together, our results support the hypothesis that the underlying cause of neutrophil dysfunction in G6PC3 deficiency is a disturbance in ER energy homeostasis caused by a loss of the endogenous glucose/G6P reservoir in neutrophils.
Methods
Isolation of murine bone marrow and human blood neutrophils
Animal studies were conducted under a protocol approved by the National Institute of Child Health and Human Development Animal Care and Use Committee. The 2 G6PC3-deficient patients, both under granulocyte-colony stimulating factor (G-CSF) therapy, were studied, after written informed consent was received, under a National Institute of Allergy and Infectious Diseases Institutional Review Board. The congenic G6pc3−/− mice were obtained by backcrossing G6pc3−/− mice10 to C57BL/6J mice for 12 generations. Bone marrow (BM) cells were isolated from the femurs and tibiae of 6- to 8-week-old G6pc3−/− and control littermates, and neutrophils were purified from the BM cells using the EasySep negative selection system (StemCell Technologies). The antibody selection cocktail contained CD5/CD4/CD45R for thymocytes, T and B lymphocytes; TER119 for erythroid cells; and F4/80 for monocytes/macrophages. The purity of BM neutrophils was monitored by Wright staining and confirmed by immunocytochemistry using a rat monoclonal antibody against Gr-1 (BD Pharmingen). Neutrophil viability was determined by trypan blue staining and the viable neutrophils were counted using the Countess Automated Cell Counter (Invitrogen).
Human peripheral blood neutrophils were isolated from heparinized blood using EasySep human neutrophil enrichment kit (StemCell Technologies). The antibody cocktail is composed of CD2, CD3, CD9, CD19, CD36, CD56, glycophorin A, and dextran, to remove lymphocytes, monocytes, erythrocyte, platelets, NK, and dendritic cells. The purity of human neutrophils was monitored by Wright staining.
Annexin V binding and caspase-3 activity assays
Neutrophil apoptosis was assessed using the annexin V–FITC apoptosis detection kit (BioVision) and analyzed by flow cytometry as described previously,13 using the Guava EasyCyte Mini System (Millipore). Caspase-3 activity in neutrophil lysates was measured using labeled Asp-Glu-Val-Asp-p-nitroanilide (DEVD-pNA) and the caspase-3 colorimetric assay kit (BioVision) as described previously.10
Depletion of apoptotic cells from neutrophils
The Annexin V Microbead kit (Miltenyi Biotec) was used to deplete apoptotic cells in the isolated neutrophils. Briefly, freshly isolated control and G6PC3-deficient neutrophils were incubated with Annexin V Microbeads for 15 minutes at 6°C and the mixture passed through a MACS cell separation column (Miltenyi Biotec). The annexin V-negative cells were pelleted, resuspended in the appropriate buffer, and used for all assays except ER stress and apoptosis.
Respiratory burst, chemotaxis, calcium flux and phagocytosis measurements
Respiratory burst, chemotaxis, and calcium flux analysis were performed as previously described.10,12
The phagocytic activity of neutrophils was examined by measuring the uptake of pHrodo Escherichia coli bioparticles (Invitrogen) as described by Wan et al29 The number of neutrophils with phagocytosed bioparticles was visualized using the Axioskop2 plus fluorescence microscope, quantified by Adobe Photoshop CS3 (Adobe Systems), and expressed as [(number of cells with phagocytosed particles)/(total cell numbers analyzed) × 100].
Glucose uptake
Glucose was measured by the rate of 2-deoxy-D-[1,2-3H]-glucose (2-DG) uptake as described by Bashan and coworkers.30 Briefly, annexin V–depleted BM neutrophils (2 × 106) were incubated at 37°C for 2 to 10 minutes in 1 mL of Kreb's Ringer phosphate (KRP) buffer containing 5 μCi of 2-DG (33 Ci/mmol; MP Biomedicals). The reaction was stopped with 1 mL of ice-cold KRP buffer and centrifuged. The washed pellets were lysed in 0.5 mL of 1% (vol/vol) Triton-X100 in phosphate-buffered saline (PBS) and protein content and radioactivity in cell lysates were determined.
Quantitative real-time RT-PCR and Western blot analyses
Total RNAs were isolated from annexin V–depleted neutrophils using the TRIzol Reagent (Invitrogen). The mRNA expression was quantified by real-time reverse-transcription–polymerase chain reaction (RT-PCR) in an Applied Biosystems 7300 Real-Time PCR System. The following TagMan probes were used: gp91phox, Mm00514478_m1; p22phox, Mm00514478_m1; p47phox, Mm00447921_m1; Glut1, Mm00441480_m1; and β-actin, Mm00607939_s1. Data were analyzed using SDS Version 1.3 software (Applied Biosystems) and normalized to β-actin RNA.
Western blot analysis was performed as previously described.10,12 The following antibodies used were: a goat polyclonal antibody against gelatinase (or MMP-9; Santa Cruz Biotechnologies), a mouse monoclonal antibody against KDEL (Assay Designs), protein disulfide isomerase (PDI; Novus Biologicals), or gp91phox (BD Biosciences); a rabbit polyclonal antibody against GLUT1, p22phox, or p47phox (Santa Cruz Biotechnologies), or GRP17031 ; a mouse polyclonal antibody against β-actin (Santa Cruz Biotechnologies), or a rat monoclonal antibody against Gr-1 (BD Pharmingen).
Immunofluorescence microscopic analyses
Mouse BM neutrophils or human blood neutrophils were deposited on glass slides by cytospin, fixed for 10 minutes in 3.75% formaldehyde, permeabilized for 10 minutes at 25°C in 0.2% Triton X-100, incubated with Image-iT FX signal enhancer (Invitrogen) for 30 minutes at 25°C, then blocked for 1 hour at 25°C in PBS containing 5% goat serum. The permeabilized neutrophils were incubated for 1 hour at 25°C with a mouse polyclonal antibody against gp91phox or pan Cadherin (Abcam), a rabbit polyclonal antibody against GLUT1, p22phox, or p47phox in PBS supplemented with 5% goat serum. After washes with PBS, neutrophils were incubated for 1 hour at 25°C in the dark with an appropriate secondary antibody and conjugated with either Alexa Fluor 488 or 555 (Invitrogen). The labeled cells were mounted with an antifade, water-based mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) and visualized using a Zeiss Axiovert 200M inverted confocal microscope equipped with 40×/1.3 numeric aperture (NA) or 63×/1.4 NA oil objectives at room temperature (Carl Zeiss MicroImaging). The images were acquired using LSM 5 acquisition software (Carl Zeiss Microimaging).
G6P, lactate, and glycogen determination
Annexin V–depleted neutrophils were incubated for 30 minutes at 37°C in 5.6mM glucose-containing RPMI-1640 medium. For intracellular G6P, lactate, and glycogen determination, 107 neutrophils in 500 μL of ice-cold PBS were deproteinized on ice for 10 minutes with 125 μL of perchloric acid (14%, wt/vol). After removing the denatured proteins by centrifugation, the acid supernatants (500 μL) were neutralized with 133 μL of 2M KOH/0.2M MOPS, incubated on ice for 5 minutes, and precipitates removed by centrifugation. The supernatants were stored at −80°C.
To measure G6P, 10 μL of deproteinized neutrophil lysate was incubated for 1 hour at 25°C in a 96-well microplate (Greiner Bio-One) in 100 uL of Hanks buffered salt solution containing 1 unit of yeast G6P dehydrogenase (G6PDH; Sigma-Aldrich) and 4mM NADP+ (Sigma-Aldrich). Oxidation of G6P to 6-phosphogluconlactone reduced NADP+ to NADPH, which was measured by excitation at 340 nm and emission at 450 nm32 in a Flexstation II Fluorimeter.
Lactate in deproteinized neutrophil lysates was analyzed using a kit obtained from BioVision and the fluorescence intensities were measured by excitation at 535 nm and emission at 575 nm using a Flexstation II Fluorimeter.
Glycogen in the deproteinized neutrophil lysates was measured as released glucose, using a glucose assay kit obtained from BioVision, after hydrolysis in 0.1M sodium acetate buffer, pH 4.5 containing 1 mg/mL amyloglucosidase (38 U/mg; Sigma-Aldrich) for 1 hour at 55°C. Fluorescence intensities were measured by excitation at 530 nm and emission at 590 nm using a Flexstation II Fluorimeter. A standard curve was generated by measuring released glucose after hydrolysis of various amounts of glycogen (rabbit liver, type III; Sigma-Aldrich) by amyloglucosidase under the same conditions.
Total ATP determination
Annexin V–depleted neutrophils (5 × 105) were incubated in 5.6mM glucose-containing RPMI-1640 medium at 37°C for 30 minutes. Total ATP was determined using the ATP fluorometric assay kit (BioVision). Briefly, neutrophils were pelleted, lysed in 50 μL of ATP assay buffer, and centrifuged. The supernatant (5 μL) was mixed with ATP Probe, ATP Converter, and Developer Mix and incubated for 30 minutes at room temperature in the dark. The fluorescence intensity was determined by excitation at 535 nm and emission at 587 nm using a Flexstation II Fluorimeter.
Statistical analysis
The unpaired t test was performed using the GraphPad Prism Program, Version 4 (GraphPad Software). Values were considered statistically significant at P less than .05.
Results
G6pc3−/− bone marrow is neutropenic and exhibits neutrophil ER stress and apoptosis
Previous studies have shown that G6pc3−/− mice are neutropenic, with differential peripheral blood neutrophil counts in 6- to 7-week-old G6pc3−/− mice averaging 67% of the counts in age-matched unaffected littermates.10 We examined neutrophil counts in total BM cells, isolated from the femur and tibia of 6- to 8-week-old G6pc3−/− and control (G6pc3+/−/G6pc3+/+) littermates. The total cells recovered in the BM aspirates for G6pc3−/− mice (4.2 × 107 ± 0.04 × 107 cells) were, on average, 85% of those in control mice (5.0 × 107 ± 0.09 × 107 cells). Morphologically mature neutrophils, including band and segmented nucleophilic cells that were more than 95% pure, were isolated from G6pc3−/− and control mice (Figure 1A) by adopting a negative immunomagnetic system.33 Both populations expressed similar levels of Gr-1 (Figure 1B), a marker of neutrophil maturation,34 and gelatinase (Figure 1B), a marker of terminal neutrophil differentiation,35 indicating that the majority of G6pc3−/− and control BM neutrophils were of similar biochemical maturity. Flow cytometric analysis using anti–Gr-1 and anti-CD11b antibodies showed that neutrophils in G6pc3−/− BM averaged only 58.5% of the counts in the BM of age-matched control littermates (Figure 1C). Therefore, neutropenia in G6pc3−/− mice is present within the BM before extravasation.
G6pc3−/− BM neutrophils display enhanced ER stress and apoptosis. Bone marrow (BM) neutrophils were isolated from 6- to 8-week-old unaffected (+/+) and G6pc3−/− (−/−) littermates as described in “Isolation of murine bone marrow and human blood neutrophils.” (A) Hema 3–stained cytospins of BM neutrophils. (B) Western blot analysis of protein extracts of neutrophils using antibodies against Gr-1, gelatinase, or β-actin. Data from 2 pairs of littermates are shown and each lane contains 80 μg of protein. (C) A plot of BM neutrophil counts in control and G6pc3−/− mice determined by flow cytometry analysis using anti–Gr-1 and anti-CD11b antibodies (n = 4). (D) Western blot analysis of protein extracts of neutrophils using antibodies against GRP78, GRP170, PDI, or β-actin. Data from 2 pairs of littermates are shown and each lane contains 50 μg of protein. (E) Quantification of apoptotic cells (annexin V+) in BM neutrophils of control and G6pc3−/− mice determined by flow cytometric analysis. At least 5000 cells were used for each determination (n = 4). (F) The DEVD-cleaving activity of active caspase-3 in protein extracts of BM neutrophils. Data represent the mean ± SEM of 3 independent experiments. *P < .05, **P < .005.
G6pc3−/− BM neutrophils display enhanced ER stress and apoptosis. Bone marrow (BM) neutrophils were isolated from 6- to 8-week-old unaffected (+/+) and G6pc3−/− (−/−) littermates as described in “Isolation of murine bone marrow and human blood neutrophils.” (A) Hema 3–stained cytospins of BM neutrophils. (B) Western blot analysis of protein extracts of neutrophils using antibodies against Gr-1, gelatinase, or β-actin. Data from 2 pairs of littermates are shown and each lane contains 80 μg of protein. (C) A plot of BM neutrophil counts in control and G6pc3−/− mice determined by flow cytometry analysis using anti–Gr-1 and anti-CD11b antibodies (n = 4). (D) Western blot analysis of protein extracts of neutrophils using antibodies against GRP78, GRP170, PDI, or β-actin. Data from 2 pairs of littermates are shown and each lane contains 50 μg of protein. (E) Quantification of apoptotic cells (annexin V+) in BM neutrophils of control and G6pc3−/− mice determined by flow cytometric analysis. At least 5000 cells were used for each determination (n = 4). (F) The DEVD-cleaving activity of active caspase-3 in protein extracts of BM neutrophils. Data represent the mean ± SEM of 3 independent experiments. *P < .05, **P < .005.
We have previously shown that thioglycollate-stimulated peritoneal neutrophils from G6pc3−/− mice exhibit enhanced ER stress and apoptosis.10 We therefore examined the expression of molecular chaperones in the unfolded protein response signal transduction pathway between BM neutrophils of G6pc3−/− and control mice. Western blot analysis showed that the production of molecular chaperones, GRP78/Bip,36 GRP170,36 and PDI,36 were increased in BM neutrophils of G6pc3−/− mice relative to their control littermates (Figure 1D), implying that resting G6pc3−/− neutrophils also undergo enhanced ER stress.
Flow cytometric analysis showed that significantly elevated numbers of BM neutrophils from G6pc3−/− mice stained positive for annexin V37 compared with neutrophils from the control mice (Figure 1E), suggesting there was an enhanced rate of apoptosis in G6pc3−/− BM neutrophils. Consistent with this, activity assays demonstrated that levels of active caspase-338 were increased in G6pc3−/− BM neutrophils (Figure 1F).
In summary, both peritoneal10 and BM neutrophils from G6pc3−/− mice as well as peripheral blood neutrophils of G6PC3-deficient patients14 exhibit enhanced apoptosis, which underlies neutropenia in G6PC3 deficiency. Therefore, to measure neutrophil function accurately, we removed apoptotic cells from purified BM neutrophils by annexin V binding. The annexin V–depleted neutrophils from control and G6pc3−/− mice were of similar viability (Figure 2A). Moreover, cell viabilities were unchanged after incubation in 5.6mM glucose for 30 minutes (Figure 2A). The experiments described in the remaining subsections were all conducted in annexin V–depleted BM neutrophils.
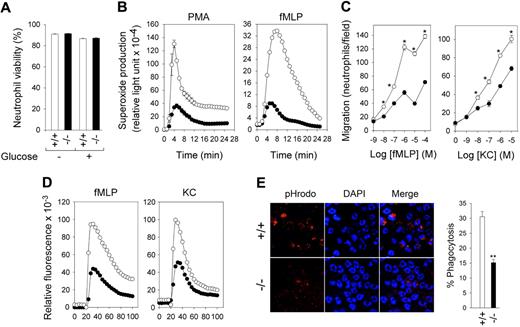
Annexin V–depleted BM neutrophils from G6pc3−/− mice exhibit impaired function. The annexin V–depleted BM neutrophils were isolated from 6- to 8-week-old unaffected (+/+, ○) and G6pc3−/− (−/−, ●) littermates as described in “Depletion of apoptotic cells from neutrophils.” (A) Neutrophil viability. The viability of annexin V–depleted BM neutrophils before (Glucose −) or after incubation for 30 minutes in 5.6mM glucose (Glucose +) was estimated by trypan blue exclusion. Results represent the mean ± SEM of quadruplet determinations. (B) Neutrophil respiratory burst activity in response to 200 ng/mL PMA. (C) Neutrophil concentration–dependent chemotaxis in response to fMLP and KC. *P < .05. (D) Calcium flux in response to 10−6M fMLP or KC. Representative experiments are shown. (E) Neutrophil phagocytosis activity. Representative immunofluorescence of cells with phagocytosed pHrodo E coli bioparticles (red fluorescence) and DAPI nuclei staining (blue fluorescence) at 400× magnification, and quantification of bioparticle-positive neutrophils incontrol and G6pc3−/− mice. Data represent the mean ± SEM of 3 independent experiments. **P < .005.
Annexin V–depleted BM neutrophils from G6pc3−/− mice exhibit impaired function. The annexin V–depleted BM neutrophils were isolated from 6- to 8-week-old unaffected (+/+, ○) and G6pc3−/− (−/−, ●) littermates as described in “Depletion of apoptotic cells from neutrophils.” (A) Neutrophil viability. The viability of annexin V–depleted BM neutrophils before (Glucose −) or after incubation for 30 minutes in 5.6mM glucose (Glucose +) was estimated by trypan blue exclusion. Results represent the mean ± SEM of quadruplet determinations. (B) Neutrophil respiratory burst activity in response to 200 ng/mL PMA. (C) Neutrophil concentration–dependent chemotaxis in response to fMLP and KC. *P < .05. (D) Calcium flux in response to 10−6M fMLP or KC. Representative experiments are shown. (E) Neutrophil phagocytosis activity. Representative immunofluorescence of cells with phagocytosed pHrodo E coli bioparticles (red fluorescence) and DAPI nuclei staining (blue fluorescence) at 400× magnification, and quantification of bioparticle-positive neutrophils incontrol and G6pc3−/− mice. Data represent the mean ± SEM of 3 independent experiments. **P < .005.
G6pc3−/− murine bone marrow neutrophils exhibit impaired function
We have previously shown that thioglycollate-stimulated peritoneal neutrophils from G6pc3−/− mice exhibit impaired respiratory burst, chemotaxis, and Ca2+ flux activities.10 We examined neutrophil function in resting BM neutrophils purified by annexin V depletion of apoptotic cells. In control BM neutrophils, superoxide production was markedly increased by exposure to phorbol 12-myristate 13-acetate (PMA) or formyl-methionyl-leucyl-phenylalanine (fMLP) while in G6pc3−/− BM neutrophils, the PMA- or fMLP-stimulated superoxide production was reduced (Figure 2B). BM neutrophils from unaffected mice exhibited a greater dose-dependent chemotactic response to fMLP and keratinocyte chemoattractant (KC) than BM neutrophils from G6pc3−/− mice (Figure 2C). Similarly, mobilization of calcium in response to fMLP and KC was impaired in G6pc3−/− BM neutrophils relative to controls (Figure 2D). Therefore, BM and peritoneal neutrophils from G6pc3−/− mice behave in a similar manner, both exhibiting impaired respiratory burst, chemotaxis, and calcium flux activities.
The phagocytic activity of nonapoptotic G6pc3−/− and control BM neutrophils was examined by measuring the uptake of pHrodo E coli bioparticles.29 In control mice, 30.5% (± 1.8%) of BM neutrophils ingested the bioparticles (Figure 2E). In contrast in G6pc3−/− mice only 15.2% (± 1.1%) of BM neutrophils harbored ingested bioparticles (Figure 2E), consistent with an impairment in phagocytosis.
Impaired glucose uptake and reduced G6P levels in G6pc3−/− neutrophils
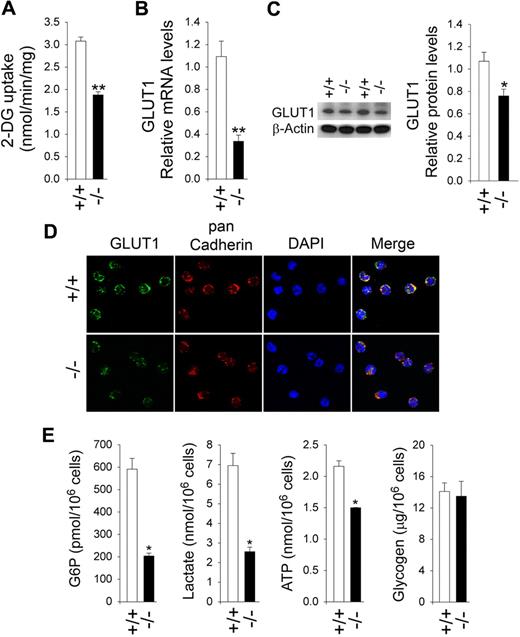
We hypothesized that the role of G6Pase-β is to cycle and regulate the amount of available cytoplasmic glucose/G6P. We predict that G6pc3−/− neutrophils will have lower levels of G6P, lactate, and ATP, which in turn will lead to impaired GLUT1 membrane translocation and reduced glucose uptake, the rate-limiting step in glucose metabolism.39 The nonapoptotic neutrophils from unaffected mice took up 2-DG at a rate 1.6-fold greater than neutrophils from G6pc3−/− mice (Figure 3A). As expected, the higher level of glucose uptake by BM neutrophils from control mice was associated with higher levels of GLUT1 expression and increased GLUT1 membrane-association. Quantitative real-time RT-PCR analysis showed that neutrophil GLUT1 mRNA levels in G6pc3−/− mice are, on average, 31% of the levels in their age-matched control littermates (Figure 3B). Western blot analysis confirmed the reduction in GLUT1 protein (Figure 3C). Immunofluorescence analysis using an antibody to GLUT1 showed that the expression of membrane associated GLUT1 was markedly decreased in G6pc3−/− neutrophils, compared with the controls (Figure 3D). Glucose uptake can also be increased by translocation of hexokinase to the plasma membrane.40 We observed no difference in membrane association of hexokinase between control and G6pc3−/− neutrophils (data not shown).
Analysis of 2-DG uptake, the expression of GLUT1, and intracellular G6P, lactate, ATP, and glycogen levels in G6pc3−/− neutrophils. Annexin V–depleted BM neutrophils were isolated from 6- to 8-week-old unaffected (+/+) and G6pc3−/− (−/−) littermates as described in “Depletion of apoptotic cells from neutrophils.” Freshly isolated annexin V–depleted neutrophils were used for 2-DG uptake, quantitative RT-PCR, and Western blot analyses. Immunofluorescence analysis of GLUT1 and measurement of G6P, lactate, ATP, and glycogen were conducted in annexin V–depleted neutrophils that were incubated for 30 minutes at 37°C in glucose-containing RPMI-1640 medium as described in “Immunofluorescene microscopic analyses”; “G6P, lactate, and glycogen determination”; and “Total ATP determination.” For 2-DG uptake and quantitative RT-PCR, the data represent the mean ± SEM of 3 independent experiments. **P < .005. For Western blot and immunofluorescence analysis, at least 3 separate experiments were conducted in which each mouse was assessed individually. (A) Uptake of 2-DG. (B) Quantification of GLUT1 mRNA by real-time RT-PCR. (C) Western blot analysis of protein extracts of annexin V–depleted BM neutrophils using antibodies against GLUT1 or β-actin. Each lane contains 50 μg of protein. The relative GlUT1 protein levels were quantified by densitometry of 4 separate pairs of Western blots. *P < .05. (D) Representative immunofluorescence of GLUT1 staining (green fluorescence), pan Cadherin membrane staining (red fluorescence), and DAPI nuclei staining (blue fluorescence) at 400× magnification. (E) G6P, lactate, ATP, and glycogen levels. Data represent the mean ± SEM of 4 independent experiments. *P < .05.
Analysis of 2-DG uptake, the expression of GLUT1, and intracellular G6P, lactate, ATP, and glycogen levels in G6pc3−/− neutrophils. Annexin V–depleted BM neutrophils were isolated from 6- to 8-week-old unaffected (+/+) and G6pc3−/− (−/−) littermates as described in “Depletion of apoptotic cells from neutrophils.” Freshly isolated annexin V–depleted neutrophils were used for 2-DG uptake, quantitative RT-PCR, and Western blot analyses. Immunofluorescence analysis of GLUT1 and measurement of G6P, lactate, ATP, and glycogen were conducted in annexin V–depleted neutrophils that were incubated for 30 minutes at 37°C in glucose-containing RPMI-1640 medium as described in “Immunofluorescene microscopic analyses”; “G6P, lactate, and glycogen determination”; and “Total ATP determination.” For 2-DG uptake and quantitative RT-PCR, the data represent the mean ± SEM of 3 independent experiments. **P < .005. For Western blot and immunofluorescence analysis, at least 3 separate experiments were conducted in which each mouse was assessed individually. (A) Uptake of 2-DG. (B) Quantification of GLUT1 mRNA by real-time RT-PCR. (C) Western blot analysis of protein extracts of annexin V–depleted BM neutrophils using antibodies against GLUT1 or β-actin. Each lane contains 50 μg of protein. The relative GlUT1 protein levels were quantified by densitometry of 4 separate pairs of Western blots. *P < .05. (D) Representative immunofluorescence of GLUT1 staining (green fluorescence), pan Cadherin membrane staining (red fluorescence), and DAPI nuclei staining (blue fluorescence) at 400× magnification. (E) G6P, lactate, ATP, and glycogen levels. Data represent the mean ± SEM of 4 independent experiments. *P < .05.
Consistent with these findings, intracellular G6P levels in BM neutrophils from G6pc3−/− mice were only 34.8% of the control mice (Figure 3E).
Reduced levels of lactate and ATP in G6pc3−/− neutrophils
Glycolysis, a primary source of energy in the neutrophils, generates ATP by converting G6P to lactate.11,18 Therefore, a reduction in available glucose/G6P is expected to impair glycolysis. We showed that lactate levels in G6pc3−/− neutrophils were 37% of the levels in control neutrophils (Figure 3E). These results were supported by measurements of total cellular ATP. In G6pc3−/− mice, neutrophil ATP levels were 69% of the levels in control mice (Figure 3E). We also examined glycogen levels and showed that glycogen levels were similar between control and G6pc3−/− BM neutrophils incubated both in the presence (Figure 3E) or absence of glucose (data not shown).
In summary, G6pc3−/− neutrophils exhibited impaired glucose uptake and harbored reduced intracellular levels of G6P, lactate, and ATP compared with control neutrophils.
Impaired expression and activation of NADPH oxidase in G6pc3−/− neutrophils
NADPH oxidase is a multicomponent enzyme system composed of 2 transmembrane proteins, gp91phox and p22phox, and several cytosolic proteins.21,22 The enzyme derives its substrate, NADPH, from the HMS pathway.11 We examined the expression of gp91phox and p22phox in nonapoptotic BM neutrophils from G6pc3−/− and unaffected littermates. Quantitative real-time RT-PCR analysis showed that gp91phox mRNA levels in neutrophils of G6pc3−/− mice were, on average, 55.5% of the levels in neutrophils of their age-matched control littermates (Figure 4A).
Analysis of the expression of NADPH oxidase in G6pc3−/− neutrophils. Annexin V–depleted BM neutrophils were isolated from 6- to 8-week-old unaffected (+/+) and G6pc3−/− (−/−) littermates as described in “Depletion of apoptotic cells from neutrophils.” (A) Quantification of gp91phox, p22phox, and p47phox mRNA by real-time RT-PCR. Data represent the mean ± SEM of 3 independent experiments. *P < .05, **P < .005. (B) Representative immunofluorescence analysis of gp91phox (red fluorescence) or p22phox (green fluorescence) and DAPI nuclei staining (blue fluorescence) at 400× magnification. (C) Western blot analysis of protein extracts using antibodies against gp91phox, p22phox, p47phox or β-actin. Data from 2 pairs of littermates are shown and each lane contains 50 μg of protein. The relative protein levels of gp91phox, p22phox, p47phox were quantified by densitometry of 4 separate pairs of Western blots. *P < .05; **P < .005. (D) Representative immunofluorescence analysis of p47phox (green fluorescence), pan Cadherin membrane staining (red fluorescence), and DAPI nuclei staining (blue fluorescence) at 400× magnification.
Analysis of the expression of NADPH oxidase in G6pc3−/− neutrophils. Annexin V–depleted BM neutrophils were isolated from 6- to 8-week-old unaffected (+/+) and G6pc3−/− (−/−) littermates as described in “Depletion of apoptotic cells from neutrophils.” (A) Quantification of gp91phox, p22phox, and p47phox mRNA by real-time RT-PCR. Data represent the mean ± SEM of 3 independent experiments. *P < .05, **P < .005. (B) Representative immunofluorescence analysis of gp91phox (red fluorescence) or p22phox (green fluorescence) and DAPI nuclei staining (blue fluorescence) at 400× magnification. (C) Western blot analysis of protein extracts using antibodies against gp91phox, p22phox, p47phox or β-actin. Data from 2 pairs of littermates are shown and each lane contains 50 μg of protein. The relative protein levels of gp91phox, p22phox, p47phox were quantified by densitometry of 4 separate pairs of Western blots. *P < .05; **P < .005. (D) Representative immunofluorescence analysis of p47phox (green fluorescence), pan Cadherin membrane staining (red fluorescence), and DAPI nuclei staining (blue fluorescence) at 400× magnification.
Immunofluorescence analysis using an antibody to gp91phox showed that the expression of membrane associated gp91phox was also markedly decreased in G6pc3−/− neutrophils, compared with the controls (Figure 4B). In parallel, quantitative real-time RT-PCR analysis showed that p22phox mRNA levels (Figure 4A) and the expression of the membrane associated p22phox subunit (Figure 4B) were also down-regulated in G6pc3−/− neutrophils. Western blot analysis confirmed the reduction in gp91phox and p22phox (Figure 4C), consistent with quantitative real-time RT-PCR and immunofluorescence results.
Activation of NADPH oxidase requires the translocation of the p47phox subunit to the plasma membrane.41 Quantitative real-time RT-PCR (Figure 4A) and Western blot (Figure 4C) analyses showed that the expression of p47phox was decreased in G6pc3−/− neutrophils, compared with the controls. In the absence of agonists, immunofluorescence analysis showed that the p47phox subunit was primarily localized in the cytosol in control and G6pc3−/− neutrophils (Figure 4D), consistent with a resting state of NADPH oxidase. In control neutrophils, PMA activation induced p47phox translocation to the plasma membrane evidenced by the colocalization of p47phox with the plasma membrane marker pan Cadherin (Figure 4D). In contrast, in G6pc3−/− neutrophils, the majority of p47phox remained in the cytosol in the presence of PMA (Figure 4D). Therefore, the impaired respiratory burst inherent of the G6pc3−/− neutrophils is associated with impairment of both the expression and activation of NADPH oxidase.
Reduced levels of G6P, lactate, ATP, GLUT1, and NADPH oxidase in human G6PC3-deficient neutrophils
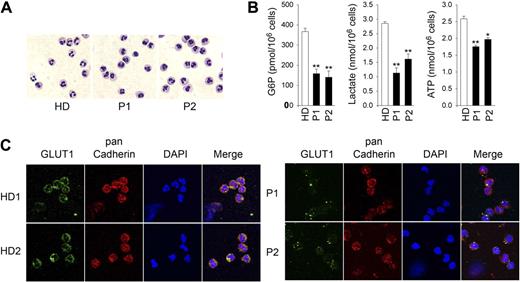
We recently described 2 cases of G6PC3 deficiency, a 13-year-old boy (P1) and his 9-year-old sister (P2), who both harbor the homozygous G6PC3 mutation G260R.41 If the role of G6Pase-β is to cycle and regulate the amount of available cytoplasmic glucose/G6P, neutrophils from human G6PC3-deficient patients should also exhibit impaired glycolysis. Morphologically mature neutrophils from control health donors (HD) and G6PC3-deficient patients (Figure 5A) were isolated by a negative immunomagnetic selection system and purified by annexin V depletion. The intracellular G6P levels in nonapoptotic neutrophils of P1 and P2 were on average 43.2% and 38.3%, respectively, of the levels in the control subjects (Figure 5B). In parallel, intracellular lactate levels in P1 and P2 were 39.6% and 56.6%, respectively of the HD levels (Figure 5B). Likewise, cellular ATP levels in P1 and P2 were 67.9% and 76.3%, respectively, of the levels in the control subjects (Figure 5B). Taken together, human G6PC3-deficient neutrophils also harbored reduced intracellular levels of G6P, lactate, and ATP.
Analysis of levels of G6P, lactate, and ATP in neutrophils of human G6PC3-deficient patients. Annexin V–depleted blood neutrophils were isolated from HD (n = 5; ages 23 to 37 years) and 2 G6PC3-deficient patients, P1 and P2, incubated for 30 minutes at 37°C in glucose-containing RPMI-1640 medium, and G6P, lactate, and ATP were determined as described in “G6P, lactate, and glycogen determination” and “Total ATP determination.” (A) Hema 3-stained cytospins of neutrophils. (B) G6P, lactate, and ATP levels. Data represent the mean ± SEM of 2 independent experiments, each done in duplicates. *P < .05; **P < .005. (C) Representative immunofluorescence of GLUT1 staining (green fluorescence), pan Cadherin membrane staining (red fluorescence), and DAPI nuclei staining (blue fluorescence) at 630× magnification.
Analysis of levels of G6P, lactate, and ATP in neutrophils of human G6PC3-deficient patients. Annexin V–depleted blood neutrophils were isolated from HD (n = 5; ages 23 to 37 years) and 2 G6PC3-deficient patients, P1 and P2, incubated for 30 minutes at 37°C in glucose-containing RPMI-1640 medium, and G6P, lactate, and ATP were determined as described in “G6P, lactate, and glycogen determination” and “Total ATP determination.” (A) Hema 3-stained cytospins of neutrophils. (B) G6P, lactate, and ATP levels. Data represent the mean ± SEM of 2 independent experiments, each done in duplicates. *P < .05; **P < .005. (C) Representative immunofluorescence of GLUT1 staining (green fluorescence), pan Cadherin membrane staining (red fluorescence), and DAPI nuclei staining (blue fluorescence) at 630× magnification.
Consistent with these findings, immunofluorescence analysis using an antibody to GLUT1 showed that the expression of membrane associated GLUT1 was markedly decreased in human G6PC3-deficient neutrophils, compared with that in the control subjects (Figure 5C).
We then examined the expression of gp91phox and p47phox subunits of NADPH oxidase in neutrophils of G6PC3-deficient patients and control subjects. Immunofluorescence analysis using an antibody to gp91phox (Figure 6A) or p47phox (Figure 6B) showed that the expression of the membrane associated NADPH oxidase subunits was markedly decreased in neutrophils of both P1 and P2, compared with the HD.
Analysis of the expression of NADPH oxidase in neutrophils of human G6PC3-deficient patients. Annexin V–depleted blood neutrophils were isolated from HD (n = 5; ages 23 to 37 years) and 2 G6PC3-deficient patients, P1 and P2 as described in “Depletion of apoptotic cells from neutrophils.” Two separate experiments were conducted, each analyzed in duplicates. (A) Representative immunofluorescence analysis of gp91phox (red fluorescence) and DAPI nuclei staining (blue fluorescence) at magnifications of 630×. (B) Representative immunofluorescence analysis of p47phox (green fluorescence), pan Cadherin membrane staining (red fluorescence), and DAPI nuclei staining (blue fluorescence) at 630× magnification.
Analysis of the expression of NADPH oxidase in neutrophils of human G6PC3-deficient patients. Annexin V–depleted blood neutrophils were isolated from HD (n = 5; ages 23 to 37 years) and 2 G6PC3-deficient patients, P1 and P2 as described in “Depletion of apoptotic cells from neutrophils.” Two separate experiments were conducted, each analyzed in duplicates. (A) Representative immunofluorescence analysis of gp91phox (red fluorescence) and DAPI nuclei staining (blue fluorescence) at magnifications of 630×. (B) Representative immunofluorescence analysis of p47phox (green fluorescence), pan Cadherin membrane staining (red fluorescence), and DAPI nuclei staining (blue fluorescence) at 630× magnification.
Discussion
Neutrophils require a constant supply of glucose for function and survival, but have increased demand for glucose when responding to immune threats requiring increased mobility, phagocytosis, and ROS production. The current view is that there are 2 primary pathways that compete for intracellular glucose/G6P in neutrophils: glycolysis and HMS.11 Within this view, the finding that there are significant neutrophil defects in the absence of an ER compartmentalized G6Pase-β enzyme is not readily explained. Therefore we hypothesized that ER uptake of G6P, to generate endoluminal glucose by G6Pase-β, must be a third major pathway, previously overlooked, that competes for the utilization of cytoplasmic glucose/G6P in neutrophils. We envisage that in a normal neutrophil, cytoplasmic G6P is transported into the ER, where it accumulates until it is hydrolyzed by G6Pase-β to glucose, which is then released back into the cytoplasm, either to be recycled to the ER or converted by hexokinase into G6P for the glycolytic, HMS, and/or glycogen synthetic pathways (Figure 7). Accordingly, in G6PC3-deficient neutrophils, disruption of this recycling would lead to accumulation of G6P in the ER, but prevent release of glucose back to the cytoplasm (Figure 7). The resulting limitation of cytoplasmic glucose/G6P availability would impact the cytoplasmic pathways of glucose metabolism and in turn impair additional blood glucose uptake. To examine this hypothesis we examined neutrophils extracted from G6pc3−/− mice and their control littermates and correlated those results to findings from neutrophils from 2 human G6PC3-deficient patients. Both human and mouse G6PC3-deficient neutrophils show similar results, suggesting they are representative of the human disorder.
Proposed pathways for G6P metabolism in normal and G6PC3-deficient neutrophils. Glucose transported into the cytoplasm via GLUT1 is metabolized by hexokinase to G6P which can participate in glycolysis, hexose monophosphate shunt (HMS), glycogen synthesis, or be translocated into the lumen of the ER by the G6PT. In normal neutrophils, G6P localized within the ER lumen can be hydrolyzed by G6Pase-β and the resulting glucose transported back into the cytoplasm to reenter any of the previously mentioned cytoplasmic pathways. However, in G6PC3-deficient neutrophils, which lack a functional G6Pase-β, ER-localized G6P cannot be recycled to the cytoplasm. The GLUT1 transporter, responsible for the transport of glucose in and out of the cell, is shown embedded in the plasma membrane. The G6PT, responsible for the transport of G6P into the ER and G6Pase-β, responsible for hydrolyzing G6P to glucose and phosphate, are shown embedded in the ER membrane.
Proposed pathways for G6P metabolism in normal and G6PC3-deficient neutrophils. Glucose transported into the cytoplasm via GLUT1 is metabolized by hexokinase to G6P which can participate in glycolysis, hexose monophosphate shunt (HMS), glycogen synthesis, or be translocated into the lumen of the ER by the G6PT. In normal neutrophils, G6P localized within the ER lumen can be hydrolyzed by G6Pase-β and the resulting glucose transported back into the cytoplasm to reenter any of the previously mentioned cytoplasmic pathways. However, in G6PC3-deficient neutrophils, which lack a functional G6Pase-β, ER-localized G6P cannot be recycled to the cytoplasm. The GLUT1 transporter, responsible for the transport of glucose in and out of the cell, is shown embedded in the plasma membrane. The G6PT, responsible for the transport of G6P into the ER and G6Pase-β, responsible for hydrolyzing G6P to glucose and phosphate, are shown embedded in the ER membrane.
In the absence of G6Pase-β, neutrophils were unable to acquire sufficient exogenous glucose to meet cytoplasmic demand. Glucose uptake measurements confirmed this and correlated the lower glucose uptake, reduced GLUT1 expression, and an impairment of translocation of GLUT1 to the plasma membrane. As expected, the lower glucose uptake resulted in a lower intracellular G6P concentration in both human and murine G6PC3-deficient neutrophils. With less cytoplasmic glucose/G6P available, glycolysis would be expected to be reduced, and this was also demonstrated by the lower levels of lactate and ATP in human and murine G6PC3-deficient neutrophils, which in turn further impair GLUT1 translocation and glucose uptake. Similarly the lower level of available G6P would be expected to impact the HMS, leading to a reduction in NADPH oxidase activity, as was also observed in human and murine G6PC3-deficient neutrophils.
It is not clear to what relative extent the glycolytic, HMS, and our proposed ER pathway compete for available cytoplasmic glucose/G6P. Indeed, the relative importance of these pathways might depend upon the needs of the cell at any given time. Existing data suggest that 85% of the exogenous glucose taken up by the neutrophils is metabolized via glycolysis to lactate.11,18 In this study, we showed that the levels of lactate in murine and human G6PC3-deficient neutrophils were 37% and 40% to 57%, respectively, of those in control neutrophils, which might suggest that the ER pathway competes for similar amounts of G6P as glycolysis. This is strengthened by the observation that the levels of G6P, lactate, and ATP in G6pc3−/− neutrophils, which were 35%, 37%, and 69%, respectively, of the levels in the control neutrophils, are all coordinately reduced. Supporting this, the levels of G6P, lactate, and ATP in human G6PC3-deficient neutrophils were 38% to 43%, 40% to 57%, and 68% to 76%, respectively, of the levels in neutrophils of control subjects, are also coordinately reduced.
The G6PC3-deficient patients reported in this study were identified based on clinical presentation of neutropenia and increased visibility of superficial veins, followed by sequence confirmation that the G6PC3 gene is mutated. There have been conflicting reports about the bone marrow in G6PC3-deficient patients. The human and murine G6PC3-deficient bone marrows reported in this study contain abundant mature neutrophils, which contrasts to the G6PC3-deficient patients reported by Boztug et al14 showing an absence of mature neutrophils in the bone marrow. Whether this reflects a true variability in the genotype-phenotype relationship in the newly described disorder of G6PC3 deficiency is very difficult to access until more patients are characterized. The differences could reasonably be a reflection of the patients' ages. Bone marrows of the patients of Boztug et al14 were 1 to 2 months old when characterized while G6PC3-deficient patients reported here were significantly more mature—9 and 13 years of age. There are many differences that could emerge during maturation of the immune system that may contribute to the apparent heterogeneity of the myeloid phenotype of G6PC3-deficient patients. On the other hand, phenotypic heterogeneity in humans could account for such disparities. It is also important to bear in mind that the G6PC3-deficient patients we studied were being treated by G-CSF therapy, while the G6pc3−/− mice did not receive G-CSF treatment. To delineate the mechanism underlying cellular function of G6PC3-deficient neutrophils, it would be ideal to compare neutrophil populations that have not been exposed to G-CSF, however this is not ethically acceptable for human patients given the current clinical standard of care. Therefore, it remains a possibility that G-CSF treatment might also impact cellular functions in a severe congenital neutropenia syndrome like G6PC3 deficiency. Given these precautions, it is important to note that the present study focused on the functional analysis of neutrophils, rather than the nature of the bone marrow composition. In this respect, the functional characteristics of human and mouse G6PC3-deficient neutrophils are similar. We show that in nonapoptotic human and mouse G6PC3-deficient neutrophils, G6Pase-β is essential for normal energy homeostasis and a G6Pase-β deficiency prevents recycling of ER glucose to the cytoplasm, leading to neutrophil dysfunction.
The phagocyte NADPH oxidase and ROS production play a key role in host defense against microbial pathogens. The oxidase, which is in a resting state in circulating blood neutrophils, can be activated by a large variety of soluble and particulate agents.21,22 This activation process depends upon the phosphorylation and membrane translocation of p47phox.42 G6P is absolutely required for neutrophil respiratory burst activity, serving as the substrate of G6PDH for NADPH production.11,19 Moreover, activation of NADPH oxidase by agonists requires energy generated by glycolysis.43,44 We showed that the expression of gp91phox and p47phox is markedly reduced in human and murine G6PC3-deficient neutrophils, compared with the controls. While PMA induces membrane translocation of p47phox in control neutrophils, this agonist fails to activate p47phox in G6pc3−/− neutrophils. In conclusion, the decrease in the expression and activation of NADPH oxidase results in impairment of the respiratory burst activity in G6pc3−/− neutrophils.
The expression of NADPH oxidase subunits can be stimulated by glutamine45,46 and peroxisome proliferators-activated receptor-alpha (PPAR-α).47 In neutrophils, glutamine stimulates the expression of NADPH oxidase subunits gp91phox, p22phox, and p47phox and is important for ROS production.45 Glutamine is a highly abundant amino acid in the blood, largely synthesized in the skeletal muscle by glutamine synthetase from glutamate, ammonia, and ATP.46,48 The G6Pase-β/G6PT complex is also expressed in high levels in the skeletal muscle, and generates endogenous glucose,49 a precursor of ATP. In G6PC3 deficiency, inactivation of G6Pase-β could hamper muscle glucose production, leading to reduced glutamine in both the muscle and the blood. This in turn would decrease glutamine levels in neutrophils, resulting in reduced expression of gp91phox, p22phox, and p47phox. Our preliminary results showed that blood glutamine levels in wild type (n = 6) and G6pc3−/− (n = 6) mice were 1.25mM (± 0.12mM) and 0.88mM (± 0.05mM), respectively, suggesting that inactivation of G6Pase-β reduced glutamine in the blood. PPAR-α is a nuclear receptor belonging to a superfamily of ligand-activated transcription factors that control lipid, glucose and energy metabolism.50 In macrophages, PPAR-α agonists induce the expression of gp91phox and p47phox mRNA expression and membrane p47phox protein.47 In pancreatic β cells, high glucose reduces PPAR-α mRNA expression.51 In future studies we will investigate if reduced intracellular G6P/glucose in neutrophils can decrease PPAR-α expression, resulting in down-regulation of the expression of NADPH oxidase subunits, and the role of glutamine in regulation of the expression of NADPH oxidase in G6PC3 deficiency.
ATP plays a critical role in chemotaxis. Neutrophils release ATP from the leading edge of the cell surface to amplify chemotactic signals and direct cell orientation by feedback through P2 purinergic receptors.52 The released ATP can be hydrolyzed by ecto-ATPases to generate adenosine that regulates chemotaxis of neutrophils by activating purinergic P1 receptors that are recruited to the leading edge, to promote cell migration.53 ATP can increase intracellular calcium levels in neutrophils, potentiating the oxidative burst and enhancing neutrophil adhesion to the endothelium.54 Moreover, ATP-mediated calcium sequestration by the ER or sarcoplasmic reticulum is enhanced by G6P.55 Taken together the ER-associated glucose/G6P reservoir plays a critical for energy homeostasis and normal function of neutrophils.
The coupled action of G6Pase-β and G6PT to regulate the cytoplasmic pathways of glycolysis and HMS suggests that GSD-Ib neutrophils should exhibit similar defects. Indeed, the rate of 2-DG uptake into human GSD Ib neutrophils is impaired30 and their intracellular G6P levels are markedly lower, compared with the control neutrophils,56 indicating that G6PT-deficient neutrophils also manifest disrupted energy homeostasis.
In conclusion, we have shown that ER-localized G6Pase-β expression is important for exogenous glucose uptake across the plasma membrane in annexin V–depleted nonapoptotic neutrophils and is linked to their demand for cytoplasmic glucose/G6P. G6Pase-β inactivation disrupts neutrophil energy homeostasis, leading to impaired neutrophil respiratory burst, chemotaxis, calcium flux, and phagocytosis. Our findings support the hypothesis that G6Pase-β–dependent glucose/G6P recycling occurs in the neutrophil and is a critical regulatory pathway for intracellular glucose homeostasis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This research was supported by the Intramural Research Programs of the National Institute of Child Health and Human Development and the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
National Institutes of Health
Authorship
Contribution: H.S.J. designed and performed research, analyzed data, and contributed to writing the paper; Y.M.L., Y.Y.C., D.H.M., and S.S.D. performed research; P.M.M. contributed to writing the paper; B.C.M. analyzed data and contributed to writing the paper; and J.Y.C. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Janice Y. Chou, Bldg 10, Rm 9D42, NIH, 10 Center Dr, Bethesda, MD 20892-1830; e-mail: chouja@mail.nih.gov.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal