Abstract
Sickle cell disease (SCD) is a classic example of a disorder with recessive Mendelian inheritance, in which each parent contributes one mutant allele to an affected offspring. However, there are exceptions to that rule. We describe here the first reported case of conversion of inherited sickle cell trait to SCD by uniparental disomy (UPD) resulting in mosaicism for SS and AS erythrocytes. A 14-year-old boy presented with splenomegaly and hemolysis. Although his father has sickle cell trait, his mother has no abnormal hemoglobin (Hb). DNA sequencing, performed to rule out Hb S/β-thalassemia, detected homozygous Hb SS. Further studies revealed mosaic UPD of the β-globin locus, more SS erythroid progenitors than AS, but a reverse ratio of erythrocytes resulting from the survival advantage of AS erythrocytes. This report exemplifies non-Mendelian genetics wherein a patient who inherited sickle cell trait has mild SCD resulting from postzygotic mitotic recombination leading to UPD.
Introduction
Sickle cell disease (SCD) is the first molecular disease described associated with a mutated protein.1 SCD most commonly results from homozygosity for the hemoglobin S (Hb S) mutation in the β-globin gene, HBB (on chromosome 11p15.4) that substitutes valine for glutamic acid at codon 6. SCD can also be caused by compound heterozygosity for Hb S and either a β-thalassemia mutation or one of several β-globin variants that support Hb S polymerization and sickle cell formation. The presence of sickle cells on a blood smear is the sine qua non of all genotypes constituting SCD and results in vaso-occlusive clinical phenomena,2 including painful crises and splenic sequestration crises. Although most patients with SCD undergo autosplenectomy within the first year of life, some retain an enlarged spleen that increases the risk of splenic sequestration.3
SCD is a prototypical autosomal recessive disease with Mendelian inheritance wherein each parent contributes one mutation to an affected offspring. There are, however, rare instances in which a child may develop such a disorder when only one parent carries a mutation. Most commonly, uniparental disomy (UPD) may unmask a recessive mutation, or a second mutation may occur de novo in a person who has inherited one mutation. In UPD, both copies of all or part of a chromosome are received from the same parent.4 If the copies are from the same chromosome (isodisomy), then any mutation present on that chromosome becomes homozygous and can cause recessive disease. Whole chromosome UPD is most commonly the result of trisomic rescue (exclusion of one of 3 chromosome copies from a trisomic embryo), whereas segmental UPD results from postzygotic mitotic recombination or gene conversion of a segment of a chromosome.5
Here we report a patient with mild SCD who inherited Hb S from his father and a normal β-globin gene from his mother. Postzygotic mitotic recombination led to mosaic segmental paternal isodisomy of chromosome 11, resulting in a subpopulation of erythroid progenitors homozygous for Hb S. This is the first report of SCD resulting from UPD in a person with inherited sickle cell trait.
Methods
Patient history
The patient, a 14-year-old Dominican boy of African ancestry, presented to the pediatric emergency department at the Columbia University Medical Center with abdominal pain and jaundice. He had an enlarged, tender spleen, and a computed tomography scan confirmed a spleen size of 20 cm with areas of infarct. His past history was significant for mild chronic hemolytic anemia, elevated bilirubin, and cholelithiasis. He was also reported to have had an acute hemolytic crisis with splenomegaly at the age of 4 years. He has had no other SCD complications, such as painful crises, acute chest syndrome, pneumonias, or priapism. Further studies were performed with written informed consent from the patient and his family in accordance with the Declaration of Helsinki. All experiments were approved by the University of Utah Institutional Review Board.
HPLC and flow cytometric analyses
Ion-exchange high-performance liquid chromatography (HPLC) was performed using a PolyCATA column (3.54CT0510; Poly LC). Fluorescence-activated cell sorter (FACS) analysis for detecting Hb S and Hb A in erythrocytes was performed as described.6 One million red blood cells from each sample were stained separately with fluorescein isothiocyanate (FITC) Hb S antibody (code MBS-F, lot 009138) and FITC Hb A antibody (code MBA-F, lot 105880) from PerkinElmer Life and Analytical Sciences. Specificity for hemoglobin antibodies was evaluated with peripheral blood from patients with Hb AA, Hb SS, Hb AS, and Hb SS patients on a chronic transfusion regimen.
BFU-E colony culture
In vitro assay of erythroid progenitors' erythroid burst-forming unit (BFU-E) was performed as previously described.7 Single BFU-E colonies were picked after 14 days in culture. Their genomic DNA was isolated from the single colonies using the Puregene DNA purification kit (Gentra).
β-globin sequencing assay and fluorescence resonance energy transfer probe assay for the Hb S mutation
Genomic DNA was extracted from peripheral blood mononuclear cells, and the coding and regulatory regions of HBB were amplified by polymerase chain reaction (PCR; primers available on request) using High Fidelity PCR Master (Roche Applied Science). PCR products were bidirectionally sequenced with the BigDye Terminator Cycle Sequencing Kit (Applied Biosystems), and the reactions were analyzed by capillary electrophoresis. Extracted DNA from each BFU-E colony was genotyped for the Hb S mutation using a fluorescence resonance energy transfer probe assay.8
UPD analysis on chromosome 11
UPD analysis was performed on the DNA extracted from peripheral blood mononuclear cells using a panel of 18 polymorphic short tandem repeat (STR) genotyping markers (Applied Biosystems Linkage Mapping Set, Version 2.5-MD10) spaced evenly across chromosome 11. Four additional markers (D11S1318, D11S1923, D11S1999, and D11S2071) were added to better define the extent of the UPD. Fluorescently labeled PCR products were electrophoresed using an Applied Biosystems 3130XL Genetic Analyzer and analyzed with GeneMapper software, Version 3.7.
Image analysis
Wright-Giemsa–stained peripheral smear was reviewed with an Olympus (4E/19753) microscope using lens of S Plan 100 (100×/1.25 oil). The image was acquired using a digital camera (Olympus DP71) and was processed with DP Controller.
Results
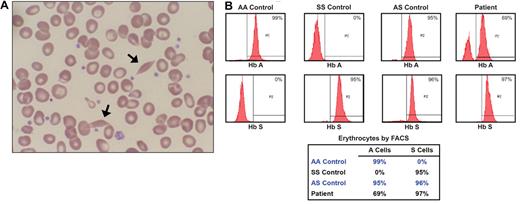
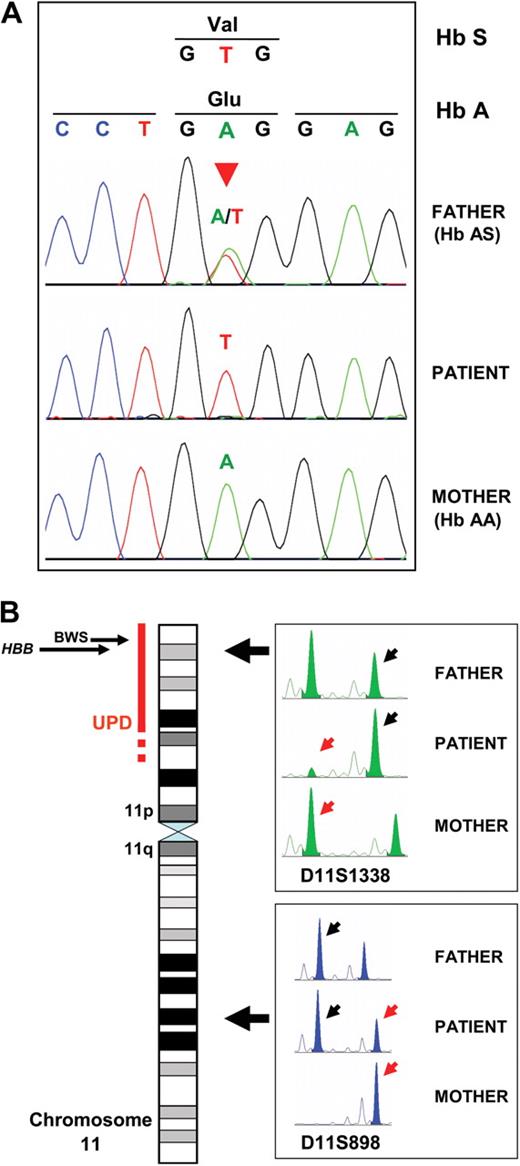
Laboratory data of the propositus and his parents and their hemoglobin evaluation by HPLC are shown in Table 1. Occasional sickle cells were present in the peripheral smear of the propositus (Figure 1A). β-globin DNA sequencing showed the propositus to be homozygous and his father heterozygous for Hb S, whereas his mother had only wild-type β-globin (Figure 2A). DNA extracted from 16 of 19 BFU colonies was genotyped as Hb SS, and 3 were genotyped as Hb AS (84% SS and 16% AS), indicating the frequency of the maternal (Hb A) allele in erythroid precursors to be 8%.
CBC and Hb HPLC results on the patient and his parents
| Test . | Patient . | Mother . | Father . |
|---|---|---|---|
| CBC | |||
| Erythrocytes, 106/μL | 3.51 | 4.12 | 4.59 |
| Hematocrit, percentage | 29.9 | 37.5 | 39.1 |
| Hemoglobin, g/dL | 10.4 | 12.6 | 14.8 |
| Leukocytes, 103/μL | 5.5 | 5.9 | 4.5 |
| Platelets, 103/μL | 384 | 256 | 193 |
| MCV, fL | 85.1 | 91 | 85.1 |
| Reticulocyte count, percentage | 4.5 | N/A | N/A |
| HPLC | |||
| Hb A2, percentage | 3.8 | 2.6 | 3.7 |
| Hb F, percentage | 0.9* | 0.3 | 0.0 |
| Hb S, percentage | 52.1 | 0 | 37.9† |
| Hb A, percentage | 43.2 | 97.1 | 58.4 |
| Test . | Patient . | Mother . | Father . |
|---|---|---|---|
| CBC | |||
| Erythrocytes, 106/μL | 3.51 | 4.12 | 4.59 |
| Hematocrit, percentage | 29.9 | 37.5 | 39.1 |
| Hemoglobin, g/dL | 10.4 | 12.6 | 14.8 |
| Leukocytes, 103/μL | 5.5 | 5.9 | 4.5 |
| Platelets, 103/μL | 384 | 256 | 193 |
| MCV, fL | 85.1 | 91 | 85.1 |
| Reticulocyte count, percentage | 4.5 | N/A | N/A |
| HPLC | |||
| Hb A2, percentage | 3.8 | 2.6 | 3.7 |
| Hb F, percentage | 0.9* | 0.3 | 0.0 |
| Hb S, percentage | 52.1 | 0 | 37.9† |
| Hb A, percentage | 43.2 | 97.1 | 58.4 |
CBC indicates complete blood count; HPLC, high-performance liquid chromatography; MCV, mean corpuscular volume; and N/A, not available.
The patient's low Hb F level may have contributed to the severity of his phenotype.
The father is heterozygous for a common α4.2 African α-thalassemia deletional mutation, explaining the lower than average Hb S percentage (data not given).
Peripheral smear and FACS analysis results. (A) Wright-stained peripheral smear (original magnification ×100) from the patient with occasional sickle cells (arrows). (B) Results of FACS analysis for Hb A and Hb S in peripheral erythrocytes from the patient and controls with Hb AA, Hb SS, and Hb AS. The propositus' chimerism of 69% AS cells and 31% SS cells is consistent with the 43.2% Hb A and 52.1% Hb S found through hemoglobin HPLC. The small fraction of peripheral blood cells that remain unstained by antibodies to either Hb A or Hb S probably represents nonerythroid cells or erythrocytes that were insufficiently permeabilized to antibody.
Peripheral smear and FACS analysis results. (A) Wright-stained peripheral smear (original magnification ×100) from the patient with occasional sickle cells (arrows). (B) Results of FACS analysis for Hb A and Hb S in peripheral erythrocytes from the patient and controls with Hb AA, Hb SS, and Hb AS. The propositus' chimerism of 69% AS cells and 31% SS cells is consistent with the 43.2% Hb A and 52.1% Hb S found through hemoglobin HPLC. The small fraction of peripheral blood cells that remain unstained by antibodies to either Hb A or Hb S probably represents nonerythroid cells or erythrocytes that were insufficiently permeabilized to antibody.
Genotyping results for the patient and his parents. (A) DNA sequencing for the c.20A>T (Hb S) mutation of propositus and parents. The propositus' 8% mosaicism for the wild-type allele (Hb A) was below the detection limit of the assay, and he appeared homozygous Hb SS. The mutation is marked by the red arrowhead. (B) Results of genotyping with chromosome 11 polymorphic STR markers to define the patient's UPD. The region of mosaic UPD is marked by the red bar next to the chromosome 11 ideogram. The dashed portion of the bar represents uncertainty in which the UPD begins. The locations of the HBB gene (11p15.4) and the BWS locus (11p15.5) are marked. The panels at right show electropherograms of 2 STR markers amplified by PCR from leukocyte DNA of the patient and his parents. Large left-facing arrows mark the positions of the markers on chromosome 11. For each marker, small black arrows point to the allele transmitted from the father to the patient, and small red arrows point to the allele transmitted from the mother. D11S898, an STR outside the region of UPD (11q22.1), shows normal biparental inheritance. At D11S1338, within the region of mosaic UPD (11p15.4), the patient has a significantly reduced maternal allele.
Genotyping results for the patient and his parents. (A) DNA sequencing for the c.20A>T (Hb S) mutation of propositus and parents. The propositus' 8% mosaicism for the wild-type allele (Hb A) was below the detection limit of the assay, and he appeared homozygous Hb SS. The mutation is marked by the red arrowhead. (B) Results of genotyping with chromosome 11 polymorphic STR markers to define the patient's UPD. The region of mosaic UPD is marked by the red bar next to the chromosome 11 ideogram. The dashed portion of the bar represents uncertainty in which the UPD begins. The locations of the HBB gene (11p15.4) and the BWS locus (11p15.5) are marked. The panels at right show electropherograms of 2 STR markers amplified by PCR from leukocyte DNA of the patient and his parents. Large left-facing arrows mark the positions of the markers on chromosome 11. For each marker, small black arrows point to the allele transmitted from the father to the patient, and small red arrows point to the allele transmitted from the mother. D11S898, an STR outside the region of UPD (11q22.1), shows normal biparental inheritance. At D11S1338, within the region of mosaic UPD (11p15.4), the patient has a significantly reduced maternal allele.
FACS analysis on the patient's peripheral erythrocytes was performed using FITC-labeled antibodies specific for Hb A and Hb S (Figure 1B). AA control cells showed the expected staining with anti-Hb A and no staining with anti-Hb S, and an SS control sample showed the opposite pattern. Sickle trait (AS) erythrocytes showed staining with both antibodies. Virtually all of the patient's erythrocytes stained with Hb S antibody, but 31% were negative for Hb A, indicating that they contained only Hb S. The remaining 69% of the cells stained for Hb A and thus must represent cells containing both Hb A and Hb S. Therefore, the patient's blood is composed of 69% of AS cells and 31% SS cells. Assuming that Hb AS cells contain 60% Hb A and 40% Hb S, this ratio is consistent with the 43.2% Hb A and 52.1% Hb S detected by hemoglobin HPLC (Table 1; 69% AS cells × 60% Hb A per cell = 41.4% Hb A).
UPD analysis on chromosome 11 revealed a normal biparental inheritance from 11q25 to 11p13, whereas from 11p14.2 to 11p15.5 (near the p telomere) the propositus had decreased levels of the maternal allele. This is consistent with mosaic segmental paternal isodisomy that extends from 11p13-11p14.2 to the 11p telomere (Figure 2B). Based on calculation of peak areas from STRs in the region of UPD, the maternal allele composes 7% to 9% of the total genotype in mononuclear cells from peripheral blood, closely analogous to the 8% frequency of the Hb A (maternal) allele found in sampled BFU-E colonies.
Discussion
We describe a 14-year-old boy who, although inheriting sickle cell trait, has mild SCD with a mosaic population of SS and AS erythroid cells resulting from postzygotic mitotic recombination. Autosomal recessive disorders, such as SCD, typically occur through classic Mendelian inheritance; however, it is rarely possible for a person who has inherited a mutation from only one parent to be affected. Here we describe the first reported case of conversion of inherited sickle cell trait to SCD by UPD resulting in mosaicism for SS and AS erythrocytes.
In analysis of BFU-E colonies, the majority (∼ 84%) of erythroid progenitors had the Hb SS genotype, consistent with the results of genotyping from leukocyte DNA. However, FACS analysis of the patient's peripheral erythrocytes demonstrated a reverse proportion of populations with 69% Hb AS cells and 31% Hb SS cells, consistent with the results of hemoglobin HPLC. This demonstrates that, although Hb AS progenitors are less in number, their erythrocyte progeny have a survival advantage and persist longer in the peripheral circulation. The mosaicism of Hb SS and AS erythrocytes explains the absence of severe sickle cell complications in the propositus, yet the easily detectable circulating Hb SS erythrocytes account for his mild anemia, chronic hemolysis, and cholelithiasis. Analogous to this patient, persons with SCD who develop stable mixed hematopoietic chimerism of different levels after bone marrow transplantation have also been reported to have amelioration of symptoms.9
The role of mitotic recombination in tumor loss of heterozygosity is well established10,11 ; however, its role in nonneoplastic disease is less known. Interestingly, mosaic segmental paternal isodisomy affecting a locus at chromosome 11p15.5, near HBB (Figure 2B), is responsible for approximately 20% of Beckwith-Wiedemann syndrome (BWS).12 BWS is a disorder of growth regulation associated with symptoms that include macrosomia, hemihyperplasia, and risk of embryonal tumors.13 Genes at the BWS locus are imprinted and differentially expressed from maternally and paternally derived chromosomes; BWS results from specific alterations to this normal pattern of expression. The patient's paternal isodisomy extends through the BWS locus and appears identical to the UPD associated with BWS. Clinically, however, he has no findings of the disorder, probably because of his mosaic, tissue-specific distribution of UPD. He is past the age of risk for embryonal tumors. Considering the known incidence of UPD at the BWS locus and the proximity of the HBB gene to this locus, β-globin disorders similar to this patient's probably occur at some frequency. Indeed, there are 2 previous reports that describe persons with conversion of β-thalassemia trait to thalassemia major through paternal chromosome 11 isodisomy. One case had classic β-thalassemia major accompanied by BWS, whereas the other was diagnosed with β-thalassemia major at a later age and was not reported to have features of BWS.14,15
This report demonstrates the importance of considering non-Mendelian modes of inheritance when evaluating a patient for a possible recessive disorder. Accurate interpretation of genetic testing in such cases requires correlation with results from other family members. We also suggest that there may be more examples of β-globin disorders in affected persons not exhibiting expected patterns of non-Mendelian inheritance.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.J.S. performed and analyzed the β-globin sequencing assay and UPD analysis and wrote the manuscript; A.M.A. analyzed the clinical and laboratory data and wrote the manuscript; M.L. and J.M.E. suspected the patient had a clinically significant hemoglobinopathy, referred the subject for further studies, analyzed the clinical data, and contributed to writing of the manuscript; S.S. performed experiments (colony-forming assays) and contributed to writing of the manuscript; A.P. and C.H.J. performed and analyzed the flow cytometric analysis of the hemolysate, analyzed the clinical data, and contributed to writing of the manuscript; D.H. performed the HPLC experiments; G.P.-K. performed the fluorescence resonance energy transfer probe assay and analyzed the data; E.L. analyzed data and contributed to writing of the manuscript; and J.T.P. designed the study, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Josef T. Prchal, Department of Medicine, Genetics and Pathology. Hematology Division, University of Utah, 30 North 1900 East, SOM 5C402, Salt Lake City, UT 84132; e-mail: josef.prchal@hsc.utah.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal