Abstract
The majority of cord blood transplantations (CBTs) have human leukocyte antigen (HLA) disparities. We investigated the impact that patients' pretransplantation anti-HLA antibodies have on the outcome of CBTs. Testing for anti-HLA antibody and its specificity was performed retrospectively at the Japanese Red Cross Tokyo Blood Center with sensitive solid-phase antibody detection assays. Among 386 CBTs, which were first myeloablative stem cell transplantations for malignancies and used a single unit of cord blood, 89 tested positive. Among the antibody-positive group, the cord blood did not have the corresponding HLA type for the antibody in 69 cases (ab-positive), while 20 cases had specificity against the cord blood HLA (positive-vs-CB). Cumulative incidence of neutrophil recovery 60 days after transplantation was 83% (95% confidence interval [CI], 79%-87%) for the antibody-negative group (ab-negative), 73% (95% CI, 61%-82%) for ab-positive, but only 32% (95% CI, 13%-53%) for the positive-vs-CB (P < .0001, Gray test). With multivariate analysis, the ab-positive showed significantly lower neutrophil recovery than the ab-negative (relative risk [RR] = 0.69, 95% CI, 0.49-0.96, p = .027). The positive-vs-CB had significantly lower neutrophil recovery (RR = 0.23, 95% CI, 0.09-0.56, P = .001) and platelet recovery (RR = 0.31, 95% CI, 0.12-0.81, P = .017) than the ab-negative. Patients' pretransplantation anti-HLA antibodies should be tested and considered in the selection of cord blood.
Introduction
The number of unrelated cord blood transplantations (CBTs) has increased, mainly because cord blood (CB) is more readily available than bone marrow, CB can be collected without burden or risk to the donors, and successful outcomes have been reported with less stringent requirements for human leukocyte antigen (HLA) compatibility.1-3 Comparable outcomes have been reported with analyses of unrelated bone marrow transplantations (BMTs) and CBTs, although there were lower neutrophil and platelet recoveries in CBTs.4-7 Graft failure, with a high mortality rate, has been noted as a problem.8,9
The role of anti-HLA antibodies in graft rejection of organ transplantations has been analyzed extensively.10,11 However, only a few studies have analyzed the significance of anti-HLA antibodies in stem cell transplantations,12-14 in which the recipient's immune system is taken over by the donor's cells and for which a great effort is made to match the recipient and donor HLA types at the allele level.
We investigated the impact that patients' pretransplantation anti-HLA antibodies have on the outcome of CBTs, for which the majority have HLA mismatches. We previously reported that anti-HLA antibodies, when the specificity corresponded to a mismatched antigen, had a negative effect on engraftment of CBTs.15 However, this finding was from a single cord blood bank study with limited samples. In this study, the number of cases is increased retrospectively, in cooperation with 7 of the public banks in Japan, with the intention of clarifying the significance of anti-HLA antibodies.
Methods
Patients
This study included patients with hematologic malignancies who received their first hematopoietic stem cell transplantation with a myeloablative conditioning regimen, using a single unit of CB from 1 of the 7 CB banks. All patients underwent CBT between 2001 and 2007. To be eligible for this study, patients' plasma/sera had to be available for analysis. Patients were excluded if they had not received conditioning, received reduced intensity conditioning or had not received graft-versus-host disease (GVHD) prophylaxis. The criteria were met by 386 patients, including the 153 cases analyzed in our previous report.15 As a standard procedure, the CB banks confirmed the HLA types of the patients and CB units before shipping, with samples being stored frozen, having obtained written consent from the patients in accordance with the Declaration of Helsinki. The procedures of our CB bank were approved by the institutional review board of the Japanese Red Cross Blood Service. HLA matching of CB and patient was performed using the antigen levels for HLA-A, -B and -DR. Each CB bank collected recipients' clinical information at 100 days after transplantation. Patient information on survival, disease status and long-term complications was updated annually with follow-up questionnaires.
Antibody testing
Patients' plasma/sera samples stored in each CB bank were sent to the Japanese Red Cross Tokyo Blood Center, where the plasma samples were treated with thrombin. All samples were tested with FlowPRA (One Lambda) for class I (ie, HLA-A/B/C) and class II (ie, HLA-DR/DP/DQ) anti-HLA antibodies. Samples of 20 μL were incubated with HLA class I–coated and HLA class II–coated microspheres, respectively, for 30 minutes in the dark under gentle agitation. The specimens were then washed before being incubated with anti–human immunoglobulin G–conjugated fluorescein isothiocyanate in the same conditions as in the first incubation. Next, the samples were analyzed with a flow cytometer. When the histogram showed more than a 10% shift in the positive gate or multiple peaks, the samples were further tested for the specificity of the antibody using LABScreen PRA and Single Antigen (One Lambda).16 Fluorescence was measured with the Luminex100 flow analyzer (Luminex) and the data were examined with an HLA software program (One Lambda). Generally, a median fluorescence intensity (MFI), adjusted for background signals, of 1000 or more was considered to be positive, and when there was a cross-reactive HLA antigen with an MFI of more than 800, it was also considered positive.
Definitions
A case was defined as being antibody-negative (ab-negative) when the screening test with FlowPRA was negative, or when the antibody specificity was not evident or was against the self-HLA type only. After identifying the specificity of the detected antibody, and when the designated specificity did not correspond to the recipient's own HLA, the case was defined as antibody-positive. The antibody-positive cases were further classified as either positive-vs-CB, when the specificity of the antibody corresponded to the mismatched antigen of the rejection direction for the donor-recipient HLA pair, or as ab-positive, when the mismatched donor antigen did not correspond to the antibody.
Neutrophil recovery was defined by an absolute neutrophil count of at least 500 cells/mm3 for 3 consecutive days, by 60 days after transplantation, and platelet recovery was defined by a count of at least 20 000 platelets/mm3 without transfusion support. An absence of neutrophil recovery by day 60 was defined as graft failure. Diagnosis and clinical grading of acute GVHD were performed according to the established criteria.17 Relapse was defined as a recurrence of an underlying hematologic malignant disease. Treatment-related death was defined as death during continuous remission. Event-free survival (EFS) was defined as survival with engraftment in a state of continuous remission.
Statistical analysis
Descriptive statistical analysis was performed to assess variables that are related to patient, disease and transplant characteristics. The 2-sided χ2 test was used for categorical variables, and the 2-sided Wilcoxon rank sum test was used for continuous variables. Cumulative incidence curves were used in a competing-risks setting to calculate the probability of neutrophil and platelet recovery, acute GVHD, relapse and transplant-related mortality (TRM).18 For neutrophil and platelet recovery, death before neutrophil or platelet recovery was the competing event; for GVHD, relapse and death without GVHD were the competing events; for relapse, death without relapse was the competing event; and, for TRM, relapse was the competing event. The Gray test was used for group comparisons of cumulative incidence.19 Overall survival (OS) and EFS were calculated using the Kaplan-Meier method. The log-rank test was used for group comparisons of OS and EFS.
The association of anti-HLA antibodies with outcomes was evaluated by multivariate analyses, with the use of the Cox proportional-hazards regression to adjust for OS and EFS, and with the use of the Fine and Gray proportional-hazards model for subdistribution of a competing risk for other outcomes.20 All models were adjusted for the variables that are known to be associated with outcome or for the variables differing in distribution between the groups (P < .10): patient's age at transplantation, patient's sex, donor-patient sex mismatch, donor-patient ABO mismatch, diagnosis, disease status at conditioning, the number of HLA mismatches by antigen level, the number of CD34 cells per patient weight, the type of prophylaxis against GVHD and the usage of granulocyte colony-stimulating factor (G-CSF). Variables with more than 2 categories were dichotomized for the final multivariate model. Variables were dichotomized as follows: patient age greater or less than 45 years at transplantation, recipient's sex, sex-mismatched donor-patient pair versus matched sex pair, donor-recipient ABO major mismatch versus others for ABO matching, myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) with multilineage dysplasia versus leukemia, lymphoproliferative disease (lymphoma or myeloma) versus leukemia, advanced versus standard risk of the disease, 2 loci HLA mismatches versus matched or 1 locus mismatch, CD34+ cell dose ≤ 0.85 × 105/kg versus > 0.85 × 105/kg, cyclosporine-based versus tacrolimus-based GVHD prophylaxis, GVHD prophylaxis including methotrexate (MTX) versus no MTX, and G-CSF usage versus no usage. Disease status at transplantation was categorized as standard risk for the first complete remission or the second complete remission of AML, the first complete remission of acute lymphoblastic leukemia (ALL), the first chronic phase of chronic myeloid leukemia (CML), refractory anemia of MDS or the first complete remission of lymphoproliferative disorders. No significant interactions were identified between each variable and anti-HLA antibody positivity. All P values were 2-sided.
Results
Anti-HLA antibodies
Of 386 cases tested, 89 (23.1%) were antibody-positive. Of the 89 antibody-positive cases, 69 were defined as ab-positive and 20 as positive-vs-CB. Among the 69 ab-positive cases, 45 had an antibody against an HLA class I antigen, 10 against an HLA class II, and 14 against both a class I and a class II. Among 20 positive-vs-CB cases, 15 had an antibody against an HLA class I antigen and 5 against an HLA class II.
Patient characteristics
The characteristics of the patients are shown in Table 1. There was a significant difference in recipients' age at transplantation, with the median age higher in the anti-HLA antibody-positive group than the antibody-negative group (P < .0001). The proportion of female patients in the anti-HLA antibody-positive group was larger than that in the negative group (75% vs 44%, P < .0001), and this resulted in a difference in the sex-matching proportion. In addition, the proportion of patients with MDS or AML with trilineage dysplasia after MDS was larger in the positive group (14% vs 6%). HLA-A, -B, and -DR (antigen level) were mismatched in the rejection direction in 88% of the cases. There were 46 pairs with 0 mismatches (6/6 HLAs matched), 157 pairs with 1 mismatch (5/6 HLAs matched), 182 pairs with 2 mismatches (4/6 HLAs matched), and 1 pair with 3 mismatches (3/6 HLAs matched). The CD34 dose was significantly lower in the anti-HLA antibody-positive group than the antibody-negative group (P = .041).
Characteristics of cord blood recipients for 297 patients with negative HLA antibody and 89 patients with positive HLA antibody
| . | Negative anti-HLA IgG . | Positive anti-HLA IgG . | P . | ||
|---|---|---|---|---|---|
| N . | % . | N . | % . | ||
| Number of transplants | 297 | 89 | |||
| Patient age at transplantation | < .0001 | ||||
| Median (range) | 33 | (0-69) | 46 | (0-68) | |
| Patient sex | < .0001 | ||||
| Male | 166 | (55.9) | 22 | (24.7) | |
| Female | 131 | (44.1) | 67 | (75.3) | |
| Sex matching | .001 | ||||
| Matched | 146 | (49.2) | 46 | (51.7) | |
| Male to female | 66 | (22.2) | 33 | (37.1) | |
| Female to male | 85 | (28.6) | 10 | (11.2) | |
| Disease | .002 | ||||
| ALL | 109 | (36.7) | 18 | (20.2) | |
| AML | 122 | (41.1) | 47 | (52.8) | |
| ATL | 11 | (3.7) | 4 | (4.5) | |
| MDS | 17 | (5.7) | 12 | (13.5) | |
| CML | 9 | (3.0) | 6 | (6.7) | |
| NHL | 28 | (9.4) | 2 | (2.2) | |
| MM | 1 | (0.3) | 0 | (0.0) | |
| Disease status* | .13 | ||||
| Standard | 124 | (41.8) | 44 | (49.4) | |
| Advanced | 168 | (56.6) | 41 | (46.1) | |
| Unknown | 5 | (1.7) | 4 | (4.5) | |
| Human leukocyte antigen matching (A, B, DR) | |||||
| No. of serologically mismatched loci | |||||
| Graft-versus-host disease direction | .27 | ||||
| 0 | 34 | (11.4) | 17 | (19.1) | |
| 1 | 131 | (44.1) | 38 | (42.7) | |
| 2 | 131 | (44.1) | 34 | (38.2) | |
| 3 | 1 | (0.3) | 0 | (0.0) | |
| Rejection direction | .75 | ||||
| 0 | 36 | (12.1) | 10 | (11.2) | |
| 1 | 124 | (41.8) | 33 | (37.1) | |
| 2 | 136 | (45.8) | 46 | (51.7) | |
| 3 | 1 | (0.3) | 0 | (0.0) | |
| ABO matching | .36 | ||||
| Matched | 98 | (33.0) | 36 | (40.4) | |
| Minor mismatch | 71 | (23.9) | 17 | (19.1) | |
| Major mismatch | 128 | (43.1) | 35 | (39.3) | |
| Unknown | 0 | (0.0) | 1 | (1.1) | |
| Number of nucleated cells infused, ×107/kg | .61 | ||||
| Median (range) | 2.58 | (0.64-20.8) | 2.56 | (1.65-13.9) | |
| Number of CD34+ cells infused, ×105/kg | .041 | ||||
| Median (range) | 0.88 | (0.06-9.44) | 0.80 | (0.17-3.82) | |
| GVHD prophylaxis | .66 | ||||
| Cyclosporine based | 174 | (58.6) | 48 | (53.9) | |
| Tacrolimus based | 111 | (37.4) | 38 | (42.7) | |
| Other | 12 | (4.0) | 3 | (3.4) | |
| MTX for GVHD prophylaxis | .72 | ||||
| No | 68 | (22.9) | 22 | (24.7) | |
| Yes | 229 | (77.1) | 67 | (75.3) | |
| G-CSF | .71 | ||||
| No | 20 | (6.7) | 7 | (7.9) | |
| Yes | 277 | (93.3) | 82 | (92.1) | |
| . | Negative anti-HLA IgG . | Positive anti-HLA IgG . | P . | ||
|---|---|---|---|---|---|
| N . | % . | N . | % . | ||
| Number of transplants | 297 | 89 | |||
| Patient age at transplantation | < .0001 | ||||
| Median (range) | 33 | (0-69) | 46 | (0-68) | |
| Patient sex | < .0001 | ||||
| Male | 166 | (55.9) | 22 | (24.7) | |
| Female | 131 | (44.1) | 67 | (75.3) | |
| Sex matching | .001 | ||||
| Matched | 146 | (49.2) | 46 | (51.7) | |
| Male to female | 66 | (22.2) | 33 | (37.1) | |
| Female to male | 85 | (28.6) | 10 | (11.2) | |
| Disease | .002 | ||||
| ALL | 109 | (36.7) | 18 | (20.2) | |
| AML | 122 | (41.1) | 47 | (52.8) | |
| ATL | 11 | (3.7) | 4 | (4.5) | |
| MDS | 17 | (5.7) | 12 | (13.5) | |
| CML | 9 | (3.0) | 6 | (6.7) | |
| NHL | 28 | (9.4) | 2 | (2.2) | |
| MM | 1 | (0.3) | 0 | (0.0) | |
| Disease status* | .13 | ||||
| Standard | 124 | (41.8) | 44 | (49.4) | |
| Advanced | 168 | (56.6) | 41 | (46.1) | |
| Unknown | 5 | (1.7) | 4 | (4.5) | |
| Human leukocyte antigen matching (A, B, DR) | |||||
| No. of serologically mismatched loci | |||||
| Graft-versus-host disease direction | .27 | ||||
| 0 | 34 | (11.4) | 17 | (19.1) | |
| 1 | 131 | (44.1) | 38 | (42.7) | |
| 2 | 131 | (44.1) | 34 | (38.2) | |
| 3 | 1 | (0.3) | 0 | (0.0) | |
| Rejection direction | .75 | ||||
| 0 | 36 | (12.1) | 10 | (11.2) | |
| 1 | 124 | (41.8) | 33 | (37.1) | |
| 2 | 136 | (45.8) | 46 | (51.7) | |
| 3 | 1 | (0.3) | 0 | (0.0) | |
| ABO matching | .36 | ||||
| Matched | 98 | (33.0) | 36 | (40.4) | |
| Minor mismatch | 71 | (23.9) | 17 | (19.1) | |
| Major mismatch | 128 | (43.1) | 35 | (39.3) | |
| Unknown | 0 | (0.0) | 1 | (1.1) | |
| Number of nucleated cells infused, ×107/kg | .61 | ||||
| Median (range) | 2.58 | (0.64-20.8) | 2.56 | (1.65-13.9) | |
| Number of CD34+ cells infused, ×105/kg | .041 | ||||
| Median (range) | 0.88 | (0.06-9.44) | 0.80 | (0.17-3.82) | |
| GVHD prophylaxis | .66 | ||||
| Cyclosporine based | 174 | (58.6) | 48 | (53.9) | |
| Tacrolimus based | 111 | (37.4) | 38 | (42.7) | |
| Other | 12 | (4.0) | 3 | (3.4) | |
| MTX for GVHD prophylaxis | .72 | ||||
| No | 68 | (22.9) | 22 | (24.7) | |
| Yes | 229 | (77.1) | 67 | (75.3) | |
| G-CSF | .71 | ||||
| No | 20 | (6.7) | 7 | (7.9) | |
| Yes | 277 | (93.3) | 82 | (92.1) | |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ATL, adult T-cell leukemia; MDS, myelodysplastic syndrome; CML, chronic myeloid leukemia; NHL, non-Hodgkin lymphoma; MM, multiple myeloma; GVHD, graft-versus-host disease; MTX, methotrexate; and G-CSF, granulocyte colony-stimulating factor.
Standard risk: 1st and 2nd complete remission of AML, 1st complete remission of ALL, 1st chronic phase of CML, refractory anemia of MDS, 1st complete remission of lymphoproliferative diseases.
Outcomes
Effect of anti-HLA antibodies on hematologic recovery
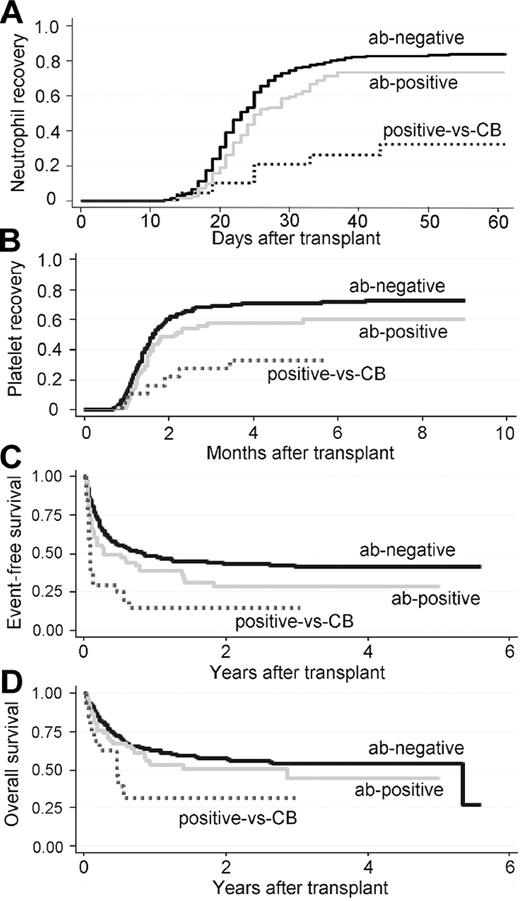
Anti-HLA antibodies showed a significant effect on neutrophil and platelet recovery (Figure 1A-B). Compared with the ab-negative group, the unadjusted cumulative incidence of neutrophil recovery at day 60 was significantly lower in the ab-positive and positive-vs-CB groups (83% vs 73% and 32%; P < .0001). The unadjusted cumulative incidence of platelet recovery at 9 months was also significantly different for the ab-negative, ab-positive and positive-vs-CB groups, at 72%, 60% and 33%, respectively (P = .0036). There was no difference recognized between the effect of anti–class I antibodies and anti–class II antibodies (data not shown).
Cumulative incidence of neutrophil/platelet recovery and survival for 386 CBT cases. ab-negative indicates patient does not have anti-HLA antibody; ab-positive, patient has anti-HLA antibody but the CB does not have the corresponding antigen for the antibody specificity; and positive-vs-CB, patient has anti-HLA antibody and the CB has the corresponding antigen for the antibody specificity. The cumulative incidences of neutrophil recovery (A) for the ab-negative, ab-positive and positive-vs-CB groups were 83% (95% CI, 79%-87%), 73% (95% CI, 61%-82%), and 32% (95% CI, 13%-53%), respectively, at day 60 (P < .0001). The cumulative incidence for the ab-positive group was significantly lower than that of the ab-negative group (P = .024). The cumulative incidence for the positive-vs-CB group was significantly lower than that of the ab-positive group (P = .005). The cumulative incidences of platelet recovery (B) for the ab-negative, ab-positive and positive-vs-CB groups were 72% (95% CI, 67%-77%), 60% (95% CI, 47%-71%), and 33% (95% CI, 14%-55%), respectively, at 9 months (P = .0036). The differences between the ab-negative and ab-positive groups (P = .05) and the ab-positive and positive-vs-CB groups (P = .062) were not significant. For the Kaplan-Meier estimate of event-free survival (EFS; C), the impact of anti-HLA antibodies was significant (P = .0001), with EFS at 2 years for the ab-negative, ab-positive and positive-vs-CB groups being 43% (95% CI, 37%-49%), 29% (95% CI, 17%-41%), and 15% (95% CI, 4%-33%), respectively. The differences between the ab-negative and ab-positive groups (P = .037) and the ab-positive and positive-vs-CB groups (P = .016) were significant. For overall survival (OS; D), the impact of anti-HLA antibodies was significant (P = .030) with the OS at 2 years for the ab-negative, ab-positive and positive-vs-CB groups being 57% (95% CI, 51%-63%), 50% (95% CI, 36%-63%), and 31% (95% CI, 10%-55%), respectively. The differences between the ab-negative and ab-positive (P = .25) and the ab-positive and positive-vs-CB groups (P = .13) were not significant.
Cumulative incidence of neutrophil/platelet recovery and survival for 386 CBT cases. ab-negative indicates patient does not have anti-HLA antibody; ab-positive, patient has anti-HLA antibody but the CB does not have the corresponding antigen for the antibody specificity; and positive-vs-CB, patient has anti-HLA antibody and the CB has the corresponding antigen for the antibody specificity. The cumulative incidences of neutrophil recovery (A) for the ab-negative, ab-positive and positive-vs-CB groups were 83% (95% CI, 79%-87%), 73% (95% CI, 61%-82%), and 32% (95% CI, 13%-53%), respectively, at day 60 (P < .0001). The cumulative incidence for the ab-positive group was significantly lower than that of the ab-negative group (P = .024). The cumulative incidence for the positive-vs-CB group was significantly lower than that of the ab-positive group (P = .005). The cumulative incidences of platelet recovery (B) for the ab-negative, ab-positive and positive-vs-CB groups were 72% (95% CI, 67%-77%), 60% (95% CI, 47%-71%), and 33% (95% CI, 14%-55%), respectively, at 9 months (P = .0036). The differences between the ab-negative and ab-positive groups (P = .05) and the ab-positive and positive-vs-CB groups (P = .062) were not significant. For the Kaplan-Meier estimate of event-free survival (EFS; C), the impact of anti-HLA antibodies was significant (P = .0001), with EFS at 2 years for the ab-negative, ab-positive and positive-vs-CB groups being 43% (95% CI, 37%-49%), 29% (95% CI, 17%-41%), and 15% (95% CI, 4%-33%), respectively. The differences between the ab-negative and ab-positive groups (P = .037) and the ab-positive and positive-vs-CB groups (P = .016) were significant. For overall survival (OS; D), the impact of anti-HLA antibodies was significant (P = .030) with the OS at 2 years for the ab-negative, ab-positive and positive-vs-CB groups being 57% (95% CI, 51%-63%), 50% (95% CI, 36%-63%), and 31% (95% CI, 10%-55%), respectively. The differences between the ab-negative and ab-positive (P = .25) and the ab-positive and positive-vs-CB groups (P = .13) were not significant.
With multivariate analysis, adjusted for other recipient- and transplant-related variables, neutrophil recoveries in the ab-positive and positive-vs-CB groups were significantly affected compared with that in the ab-negative group (Hazard ratio [HR] = 0.69, 95% confidence interval [CI], 0.49-0.96, P = .027 for the ab-positive group, HR = 0.23, 95% CI, 0.09-0.56, P = .001 for the positive-vs-CB group). Neutrophil recovery was also significantly better for the ab-positive group than the positive-vs-CB group (HR = 0.31, 95% CI, 0.12-0.80, P = .015). Platelet recovery in the positive-vs-CB group was significantly affected compared with that in the ab-negative group (HR = 0.31, 95% CI, 0.12-0.81, P = .017), but not with that in the ab-positive group (HR = 0.35, 95% CI, 0.11-1.10, P = .071; Table 2). Of 84 patients defined as having graft failure, 2 recipients showed neutrophil recovery 60 days after transplantation (at day 65 [ab-negative] and day 81 [positive-vs-CB]), 11 patients (13%) showed autologous recovery and 32 patients (38%) received a second transplantation.
Results of multivariate analysis of outcomes in 386 cord blood transplantations
| . | . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|---|
| Neutrophil recovery | ab-negative | 1.00 | ||
| ab-positive | 0.69 | (0.49-0.96) | .027 | |
| positive-vs-CB | 0.23 | (0.09-0.56) | .001 | |
| positive-vs-CB vs ab-positive | 0.31 | (0.12-0.80) | .015 | |
| Platelet recovery | ab-negative | 1.00 | ||
| ab-positive | 0.73 | (0.49-1.07) | .11 | |
| positive-vs-CB | 0.31 | (0.12-0.81) | .017 | |
| positive-vs-CB vs ab-positive | 0.35 | (0.11-1.10) | .071 | |
| Grade II-IV acute GVHD | ab-negative | 1.00 | ||
| ab-positive | 0.76 | (0.44-1.30) | .31 | |
| positive-vs-CB | 0.49 | (0.13-1.78) | .28 | |
| positive-vs-CB vs ab-positive | 0.79 | (0.19-3.20) | .74 | |
| Relapse | ab-negative | 1.00 | ||
| ab-positive | 1.98 | (1.14-3.43) | .015 | |
| positive-vs-CB | 1.75 | (0.73-4.21) | .21 | |
| positive-vs-CB vs ab-positive | 0.69 | (0.24-1.97) | .48 | |
| Transplant-related mortality | ab-negative | 1.00 | ||
| ab-positive | 0.94 | (0.55-1.60) | .81 | |
| positive-vs-CB | 2.06 | (0.96-4.43) | .064 | |
| positive-vs-CB vs ab-positive | 3.82 | (1.37-10.71) | .0011 | |
| Treatment failure (EFS) | ab-negative | 1.00 | ||
| ab-positive | 1.53 | (1.07-2.19) | .021 | |
| positive-vs-CB | 3.46 | (2.01-5.96) | < .001 | |
| positive-vs-CB vs ab-positive | 2.30 | (1.20-4.43) | .012 | |
| Overall mortality (OS) | ab-negative | 1.00 | ||
| ab-positive | 1.33 | (0.86-2.04) | .20 | |
| positive-vs-CB | 2.33 | (1.18-4.59) | .015 | |
| positive-vs-CB vs ab-positive | 1.99 | (0.85-4.70) | .12 |
| . | . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|---|
| Neutrophil recovery | ab-negative | 1.00 | ||
| ab-positive | 0.69 | (0.49-0.96) | .027 | |
| positive-vs-CB | 0.23 | (0.09-0.56) | .001 | |
| positive-vs-CB vs ab-positive | 0.31 | (0.12-0.80) | .015 | |
| Platelet recovery | ab-negative | 1.00 | ||
| ab-positive | 0.73 | (0.49-1.07) | .11 | |
| positive-vs-CB | 0.31 | (0.12-0.81) | .017 | |
| positive-vs-CB vs ab-positive | 0.35 | (0.11-1.10) | .071 | |
| Grade II-IV acute GVHD | ab-negative | 1.00 | ||
| ab-positive | 0.76 | (0.44-1.30) | .31 | |
| positive-vs-CB | 0.49 | (0.13-1.78) | .28 | |
| positive-vs-CB vs ab-positive | 0.79 | (0.19-3.20) | .74 | |
| Relapse | ab-negative | 1.00 | ||
| ab-positive | 1.98 | (1.14-3.43) | .015 | |
| positive-vs-CB | 1.75 | (0.73-4.21) | .21 | |
| positive-vs-CB vs ab-positive | 0.69 | (0.24-1.97) | .48 | |
| Transplant-related mortality | ab-negative | 1.00 | ||
| ab-positive | 0.94 | (0.55-1.60) | .81 | |
| positive-vs-CB | 2.06 | (0.96-4.43) | .064 | |
| positive-vs-CB vs ab-positive | 3.82 | (1.37-10.71) | .0011 | |
| Treatment failure (EFS) | ab-negative | 1.00 | ||
| ab-positive | 1.53 | (1.07-2.19) | .021 | |
| positive-vs-CB | 3.46 | (2.01-5.96) | < .001 | |
| positive-vs-CB vs ab-positive | 2.30 | (1.20-4.43) | .012 | |
| Overall mortality (OS) | ab-negative | 1.00 | ||
| ab-positive | 1.33 | (0.86-2.04) | .20 | |
| positive-vs-CB | 2.33 | (1.18-4.59) | .015 | |
| positive-vs-CB vs ab-positive | 1.99 | (0.85-4.70) | .12 |
CI indicates confidence interval; GVHD, graft-versus-host disease; ab-negative, patient does not have anti-HLA antibody; ab-positive, patient has anti-HLA antibody but the CB does not have the corresponding antigen for the antibody specificity; and positive-vs-CB, patient has anti-HLA antibody and the CB has the corresponding antigen for the antibody specificity.
For neutrophil recovery, other significant variables were CD34+ cell dose ≤ 0.85 × 105/kg, no usage of G-CSF, advanced disease status and diagnosis of MDS or AML with multilineage dysplasia.
For platelet recovery, other significant variables were advanced disease status, CD34+ cell dose ≤ 0.85 × 05/kg and GVHD prophylaxis without MTX.
For acute GVHD of grade II-IV, other significant variables were diagnosis of lymphoproliferative disease and CD34+ cell dose ≤ 0.85 × 105/kg.
For relapse, other significant variables were advanced disease status and patient's sex being male.
For transplant-related mortality, other significant variables were patient age more than 45 years at transplantation, GVHD prophylaxis without MTX and cyclosporin-based GVHD prophylaxis compared with tacrolimus-based GVHD prophylaxis.
For treatment failure (as a reverse of event-free survival), other significant variables were advanced disease status, patient age more than 45 years at transplantation, diagnosis of lymphoproliferative disease and GVHD prophylaxis without MTX.
For overall mortality (as a reverse of overall survival), other significant variables were advanced disease status, patient age more than 45 years at transplantation and GVHD prophylaxis without MTX.
Effect of anti-HLA antibodies on GVHD, relapse, and mortality
Anti-HLA antibodies did not have a significant effect on grade II-IV acute GVHD, relapse, or TRM. The EFS at 2 years after transplantation were 43%, 29%, and 15% for the ab-negative, ab-positive, and positive-vs-CB groups, respectively (P = .0001, log-rank test). The OS at 2 years after transplantation were 57%, 50%, and 31% for the ab-negative, ab-positive, and positive-vs-CB groups, respectively (P = .030, log-rank test; Figure 1C-D).
With multivariate analysis, adjusted for other recipient- and transplant-related variables, the positive-vs-CB group showed significantly inferior OS (HR = 2.33, 95% CI, 1.18-4.59, P = .015) and EFS (HR = 3.46, 95% CI, 2.01-5.96, P < .001) compared with the ab-negative group (Table 2). There was no significant increase in the risk of relapse (HR = 1.75, 95% CI, 0.73-4.21, P = .21) or TRM (HR = 2.06, 95% CI, 0.96-4.43, P = .064). The ab-positive group showed increased risks of relapse (HR = 1.98, 95% CI, 1.14-3.43, P = .015) and inferior EFS (HR = 1.53, 95% CI, 1.07-2.19, P = .021) compared with the ab-negative group, but there was no significant increase in the risk of TRM (HR = 0.94, 95% CI, 0.55-1.60, P = .81) or OS (HR = 1.33, 95% CI, 0.86-2.04, P = .20). No significant difference was shown in the risk of developing grade II-IV GVHD in the ab-positive and positive-vs-CB groups compared with that in the ab-negative group (HR = 0.76, 95% CI, 0.44-1.30, P = .31 for the ab-positive group, and HR = 0.49, 95% CI, 0.13-1.78, P = .28 for the positive-vs-CB group; Table 2).
A comparison of the positive-vs-CB group with the ab-positive group was also performed. The positive-vs-CB group showed increased risks of TRM (HR = 3.82, 95% CI, 1.37-10.71, P = .0011) and inferior EFS (HR = 2.30, 95% CI, 1.20-4.43, P = .012) compared with the ab-positive group (Table 2).
Risk modification according to CD34 cell dose
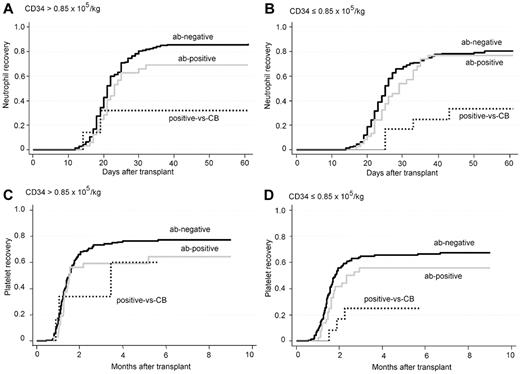
As the dichotomized CD34 dose was a significant factor in multivariate analysis of neutrophil recovery (RR 0.66, P = .0004) and platelet recovery (RR 0.73, P = .015), we proceeded to analyze the effect of anti-HLA antibodies in subgroups of CD34 cell dose. Cumulative incidences of neutrophil recovery at day 60 for the ab-negative, ab-positive and positive-vs-CB groups were 86%, 69%, and 32% (P = .058), respectively, for the higher CD34 cell dose group (CD34 cell dose more than the median, ie, > 0.85 × 105/kg), and 80%, 77%, and 33% (P = .0061) for the lower CD34 cell dose group. Cumulative incidences of platelet recovery at 9 months for the ab-negative, ab-positive and positive-vs-CB groups were 77%, 64%, and 60% (P = .36), respectively, for the higher CD34 cell dose group and 67%, 56%, and 25% (P = .020) for the lower CD34 cell dose group (Figure 2).
Cumulative incidence of neutrophil and platelet recovery in subgroups of CD34 cell dose. ab-negative indicates patient does not have anti-HLA antibody; ab-positive, patient has anti-HLA antibody but the CB does not have the corresponding antigen for the antibody specificity; and positive-vs-CB, patient has anti-HLA antibody and the CB has the corresponding antigen for the antibody specificity. Neutrophil (A) and platelet (C) recovery was not significantly affected by anti-HLA antibodies in the subgroup of CD34 cell dose > 0.85 × 105/kg. In the subgroup of CD34 cell dose ≤ 0.85 × 105/kg, the anti-HLA antibodies showed a significant impact on neutrophil (P = .0061; B) and platelet recovery (P = .020; D).
Cumulative incidence of neutrophil and platelet recovery in subgroups of CD34 cell dose. ab-negative indicates patient does not have anti-HLA antibody; ab-positive, patient has anti-HLA antibody but the CB does not have the corresponding antigen for the antibody specificity; and positive-vs-CB, patient has anti-HLA antibody and the CB has the corresponding antigen for the antibody specificity. Neutrophil (A) and platelet (C) recovery was not significantly affected by anti-HLA antibodies in the subgroup of CD34 cell dose > 0.85 × 105/kg. In the subgroup of CD34 cell dose ≤ 0.85 × 105/kg, the anti-HLA antibodies showed a significant impact on neutrophil (P = .0061; B) and platelet recovery (P = .020; D).
Multivariate analysis showed that neutrophil recovery was significantly affected for the ab-positive in the higher CD34 cell dose group, and for all antibody categories in the lower CD34 cell dose group, compared with the ab-negative in the higher CD34 cell dose group. For platelet recovery, the ab-positive and positive-vs-CB in the lower CD34 cell dose group were significantly affected (Table 3).
Multivariate analysis of anti-HLA antibody and CD34 cell dose
| . | CD34 cell dose . | Anti-HLA antibody . | Hazard ratio . | (95%CI) . | P . |
|---|---|---|---|---|---|
| Neutrophil recovery | > 0.85 × 105/kg | ab-negative | 1 | ||
| ab-positive | 0.55 | (0.31-0.98) | .042 | ||
| positive-vs-CB | 0.27 | (0.05-1.44) | .12 | ||
| ≤ 0.85 × 105/kg | ab-negative | 0.59 | (0.46-0.76) | < .0001 | |
| ab-positive | 0.52 | (0.37-0.74) | .0002 | ||
| positive-vs-CB | 0.17 | (0.07-0.42) | .0001 | ||
| Platelet recovery | > 0.85 × 105/kg | ab-negative | 1 | ||
| ab-positive | 0.78 | (0.45-1.36) | .38 | ||
| positive-vs-CB | 0.50 | (0.09-2.92) | .44 | ||
| ≤ 0.85 × 105/kg | ab-negative | 0.76 | (0.57-1.01) | .057 | |
| ab-positive | 0.51 | (0.32-0.82) | .0051 | ||
| positive-vs-CB | 0.19 | (0.06-0.61) | .0049 |
| . | CD34 cell dose . | Anti-HLA antibody . | Hazard ratio . | (95%CI) . | P . |
|---|---|---|---|---|---|
| Neutrophil recovery | > 0.85 × 105/kg | ab-negative | 1 | ||
| ab-positive | 0.55 | (0.31-0.98) | .042 | ||
| positive-vs-CB | 0.27 | (0.05-1.44) | .12 | ||
| ≤ 0.85 × 105/kg | ab-negative | 0.59 | (0.46-0.76) | < .0001 | |
| ab-positive | 0.52 | (0.37-0.74) | .0002 | ||
| positive-vs-CB | 0.17 | (0.07-0.42) | .0001 | ||
| Platelet recovery | > 0.85 × 105/kg | ab-negative | 1 | ||
| ab-positive | 0.78 | (0.45-1.36) | .38 | ||
| positive-vs-CB | 0.50 | (0.09-2.92) | .44 | ||
| ≤ 0.85 × 105/kg | ab-negative | 0.76 | (0.57-1.01) | .057 | |
| ab-positive | 0.51 | (0.32-0.82) | .0051 | ||
| positive-vs-CB | 0.19 | (0.06-0.61) | .0049 |
CI indicates confidence interval; ab-negative, patient does not have anti-HLA antibody; ab-positive, patient has anti-HLA antibody but the CB does not have the corresponding antigen for the antibody specificity; and positive-vs-CB, patient has anti-HLA antibody and the CB has the corresponding antigen for the antibody specificity.
Discussion
The short time needed to obtain a cord blood unit and the less rigorous HLA matching required have contributed to an increased number of CBTs. There are concerns, however, regarding the time taken for engraftment and the high rejection rates.4-9 An accumulation of HLA-mismatched CBT cases and graft failure drew our attention to anti-HLA antibodies. Although there were early reports on engraftment in haploidentical BMTs,12,21 the anti-HLA antibody test had not been routinely used unless a patient had become refractory to platelet transfusion, and such a condition was sometimes regarded to be a contraindication for CBT.1
By comparing patients with positive and negative anti-HLA antibody test results, we found that the positive group included a higher ratio of female and MDS patients, suggesting allosensitization in less immunocompromised circumstances. In this analysis, 23.1% of our samples tested positive for an anti-HLA antibody, which is higher than the 15% reported previously with data from a single CB bank.15 However, in this analysis, 2 of the participating CB banks chose samples with positive results from their own screening tests, and thus excluded some of the clearly negative samples. In addition, the test results by FlowPRA and using Luminex beads should be considered carefully. There is a report that describes the high positivity rates obtained by this test method as being due to natural antibodies,22 and others regard this test system as too sensitive and lacking clinical relevance.23 The discrepancy in positivity rates between laboratories (data not shown) led us to collect the samples in 1 laboratory for this analysis.
We have clearly shown that the patients' pretransplantation anti-HLA antibodies are a negative factor for engraftment, especially when the specificity corresponds to the donor antigens. In such cases, the probability of engraftment falls to only 38%. In this analysis there was an effect shown on neutrophil recovery in the ab-positive group as well as in the positive-vs-CB group. This is different from our preliminary report, in which the neutrophil recovery of the ab-positive group was similar to that of the ab-negative group.15 This discrepancy might be due to limiting the subjects to recipients of myeloablative conditioning, and to the increase in the number of subjects for analyses containing a higher proportion of alloimmunized cases, together with the difference between the cumulative incidence statistical method used in this study, which considers competing risks, and the Kaplan-Meier method that was used in the previous study. In addition, there is a report that shows that priming to 1 alloantigen results in the elimination of donor bone marrow of a different alloantigen.24
We confirmed the importance of the CD34 cell dose in engraftment, in accordance with the report by Wagner et al that showed that the CD34 cell dose has a significant impact on neutrophil recovery, TRM and survival.25 Earlier, Rubinstein et al26 reported that myeloid engraftment was associated with the cell number of the unit together with the degree of HLA compatibility, and suggested a role for HLA alloimmunization in some graft failures. In this study with multivariate analysis, the effect of the antibody on neutrophil recovery was significant for ab-positive cases in the CD34 subgroup of more than the median cell dose. The hazard ratio for positive-vs-CB in the lower CD34 cell dose group was especially low.
Ottinger et al showed that the OS for their crossmatch positive cases was significantly lower than that for the crossmatch negative control group among those receiving a transplant at an early stage of the disease,13 and also showed that graft failure is the dominant factor for low OS. In our study, patients with antibodies against the graft (positive-vs-CB) had a significantly higher graft failure rate, an approximately 4-fold increase, and had inferior OS and EFS compared with antibody-negative patients. They showed a tendency for increased TRM, yet the difference was marginal (P = .064). One possible reason for the TRM increase being marginal is the relatively high number of second transplantations. Among those who did not achieve neutrophil recovery, 38% received a second transplantation and 13% showed autologous recovery. The longer survival period under a highly immunosuppressed condition, provided by the second transplantation or autologous recovery, may have contributed to elevate the risk of relapse, which affected the OS. Another possible reason is the influence of other causes of TRM. Engraftment is important to control infection. However, infections often develop early. We have previously reported that in CB transplantations the median day of early infection was 8 days after transplantation, and bacterial infection in adults significantly affected mortality.27
For the long-term effect, we could not assess changes in the anti-HLA antibodies after the transplantation, as the CB banks do not have patients' blood samples taken after the CBT. In addition, the follow-up information does not include the effectiveness of the platelet transfusions from random donors.
Among variables used in this analysis, HLA disparity at the antigen level, 2 HLA mismatches versus matched or 1 mismatch, did not have a significant effect on the outcome. Previously, Rubinstein et al reported that an absence of HLA disparity was a significant factor for neutrophil recovery, together with cell dose.26 HLA disparity has been reported by the Eurocord group to be significant in neutrophil and platelet recovery and in relapse after CBT in a study in which 65% of the patients were 15 years old or younger.2 University of Minnesota researchers reported a significant effect of HLA disparity on survival but not on engraftment when the median age was 7.4 years,25 but no clear association with it for adult cases.3 One explanation for HLA disparity not having a significant effect in our study is that our cases consisted of pediatric and adult patients, and included a wide variety of GVHD prophylaxis, making the effect of HLA disparity unclear.
From the findings of our study, we simply suggest in clinical practice to determine the presence and specificity of any anti-HLA antibodies, and avoid the corresponding antigens when choosing a CB unit. There are already reports on successful engraftments after CBT by avoiding the corresponding antigens.28,29 The quality of the CB unit also has to be considered, as cell dose is a significant factor for engraftment.25,26 From the multivariate analysis shown in Table 3, it appears that the choice of a unit with an antigen(s) corresponding to the antibody specificity presents a higher risk of graft failure. As the test system is highly sensitive, it is possible that the result will show that a patient's antibodies have a wide range of specificity. For such an occurrence, instead of opting not to use CB for the transplantation, it would be beneficial for transplant centers to have access to confirmative tests for antibodies. A test method that targets antigens naturally expressed on the cell surface, such as immunocomplex capture fluorescence analysis30 or a lymphocyte indirect immunofluorescence test,31 or alternative crossmatch tests with HLA-typed cells could be helpful in clinical decision making.
We conclude that patients' pretransplantation anti-HLA antibodies should be identified for specificity and considered in the selection of cord blood units.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all of the physicians and staff at the hospitals in Japan who collaborated in this study.
This work was supported in part by a Research Grant for Tissue Engineering (H17-014) and a Research Grant for Allergic Disease and Immunology (H20-015) from the Japanese Ministry of Health, Labor and Welfare.
Authorship
Contribution: M.T. designed the study, analyzed data, and wrote the paper; Y.A. performed statistical analysis and co-wrote the paper; K.F., H.T. and A.O. performed research and analyzed the data; H.K., S. Kai, H.S., M.K., H.A. and S. Kato submitted samples and extracted data; and K.N. reviewed and oversaw the research design.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Minoko Takanashi, MD, PhD, Japanese Red Cross Tokyo Blood Center, 2-1-67 Tatsumi, Koto-ku, Tokyo 135-8639, Japan; e-mail: mi-takanashi@tokyo.bc.jrc.or.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal