In this issue of Blood, Castillo and colleagues conclude from a meta-analysis of observational studies that blood transfusions are associated with a 20% increase in the risk of developing NHL.1
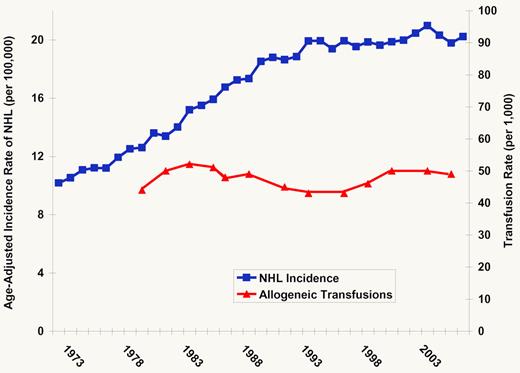
The rapid increase followed by a leveling off in the incidence of non-Hodgkin lymphoma (NHL) in the United States and Europe in the last half of the 20th century4,5 has largely remained unexplained, a situation that has fascinated and frustrated a generation of lymphoma researchers. This state of affairs is likely due in part to a multifactorial etiology and the heterogeneous nature of this malignancy with its many subtypes. A flurry of epidemiologic work starting in the 1990s has provided many new leads, including the observation that blood transfusions might be associated with an increased risk of developing NHL.6,7 Several biologic mechanisms provided a plausible explanation for the association,8 although bias, confounding by indication or some other factor, and chance all remain viable alternative explanations. Although the number of transfusions and the amount of blood transfused increased dramatically after World War II until the early 1980s,9 concerns of transmitting infectious agents led to a decline in allogeneic transfusion, which has only recently rebounded.3
Rate of NHL and transfusion since 1973. Blue line indicates the age-adjusted incidence of NHL in the United States, 1973-2007.2 Red line indicates the estimated rate of allogeneic transfusion in the United States, 1980-2006.3
What is the current evidence for an association of transfusion history and NHL risk? In their article, Castillo and colleagues report the first meta-analysis of the observational data on this topic, using a comprehensive search strategy to identify relevant studies and a state-of-the-art meta-analysis approach.10 Their main finding, based on 9 case-control and 5 cohort studies, was that blood transfusions were associated with a 20% increase in the risk of NHL. These results were similar for men and women and for transfusions given before or after 1992. The latter finding is of particular interest because there have been major changes in transfusion practice over the last 30 years that have dramatically decreased the risk of pathogen transmission.11 Thus, any mechanism explaining a transfusion and NHL association will need to account for the impact of extensive screening, changes in the use of blood components, the advent of leukodepletion, and other changes in transfusion practices over this timeframe, as well as the leveling off of NHL incidence (see figure).
There were 2 important sources of heterogeneity specifically identified in the meta-analysis that warrant discussion. The first was by study design, such that cohort studies observed an association of transfusion and NHL risk (meta–relative risk [meta-RR] of 1.34) while case-control studies did not (meta-RR of 1.05). Castillo et al offered several potential explanations for this discrepancy, including small sample size, failure to take into account confounding variables, and recall bias in case-control studies. However, small sample size is not likely to explain the discrepancy (there were more total cases contributed from case-control compared with cohort studies in the meta-analysis), and there has been little evidence from individual studies for strong confounding factors beyond confounding by indication, which could not be directly addressed in the meta-analysis. Recall bias could play a role, although one would predict that this would inflate (not attenuate) any association based on the presumption that cases would be more likely to ruminate and better report on prior transfusions relative to controls. Other explanations include selection bias due to low response rates among controls (which could enrich for a positive history of transfusion), genuine differences among populations studied, or other sources of heterogeneity. Finally, although the cohort study is the strongest observational study design, the meta-analysis was based on only 5 cohort studies.
The second source of heterogeneity identified was by NHL subtype. NHL is known to be biologically and clinically heterogeneous, and the impact of this heterogeneity for etiologic risk factors is receiving renewed attention. Castillo et al found that of the common subtypes, an association with transfusion history was only observed for chronic lymphocytic leukemia/small lymphocytic lymphoma (meta-RR = 1.66) and not diffuse large B-cell (meta-RR = 1.06) or follicular (meta-RR = 1.02) lymphomas. It is important to note that these exploratory results were based on a much smaller subset of the studies.
One of the main limitations of this report relates to the limitations of meta-analysis itself relative to a pooled analysis of primary data. Beyond the statistical concerns of meta-analysis,11 a pooled study allows for more detailed analyses based on different exposure scenarios and selected subgroups, as well as adjustment for potential confounding factors across studies, evaluation of interactions, and a more robust and complete assessment of NHL subtypes. In this study, it was not possible to estimate meta-RRs based on latency, number of transfusions, types of transfusion (eg, specific component(s) transfused; autologous versus allogeneic transfusion), or indication for transfusion. Assessment of subtypes was limited to the most common ones for studies that published estimates; studies that did not include subtype estimates had to be excluded, and evaluation of rarer subtypes was not possible. However, even pooled analysis cannot overcome limitations in primary data collection, and many studies could not specifically document what was actually transfused.
Is the association of transfusion with NHL risk likely to be causal? There are insufficient data at this time to decide. Although a randomized trial would be the strongest study design to directly assess this question, such a study would be both unethical and impractical. Thus, we will need to rely on observational data in humans supplemented by laboratory insights. The meta-analysis here raises more questions than it “meta-answers,” but it does move the field forward by identifying where we are and the types of additional data that we need. Castillo and colleagues' conclusion supporting a conservative approach to transfusion is prudent. Ultimately, any association of transfusion and NHL risk is more likely to impact our understanding of lymphomagenesis than it is to specifically impact transfusion practice, where risk and benefit considerations remain extremely complex.12
Conflict-of-interest disclosure: The author declares no competing financial interests. ■