In this issue of Blood, Sun and collaborators report the existence of a population of hemangioblasts in the adult mouse uterus, adding substance to the long-lasting debate on the persistence of these ancestral progenitor cells in the developed organism.
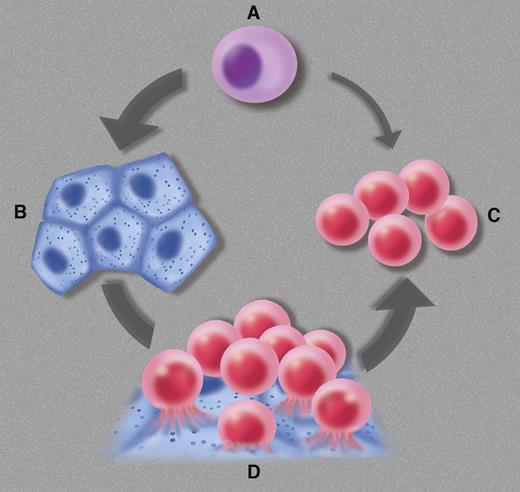
The hemangioblast (A) is defined as a mesodermal progenitor cell committed to the generation of endothelial cells (B) and blood cells (C), sometimes—perhaps always—via a hematogenous endothelium intermediate (D). Professional illustration by Marie Dauenheimer.
The hemangioblast (A) is defined as a mesodermal progenitor cell committed to the generation of endothelial cells (B) and blood cells (C), sometimes—perhaps always—via a hematogenous endothelium intermediate (D). Professional illustration by Marie Dauenheimer.
Florence Sabin, the first woman to be elected to the American National Academy of Sciences, needed no more than a primitive microscope and chicken embryos kept alive in culture to conclude in 1917 the existence of a unique origin for both emerging endothelial and blood cells.1 The term hemangioblast was coined 15 years later to name such progenitors, but did not gain wide popularity until the end of the 20th century when the existence of angio-hematopoietic stem cells became one of the most challenging concepts in developmental hematology. Sabin's brilliant intuition was confirmed when hemangioblasts were identified among the progeny of mouse and human embryonic stem cells2,3 and in early mouse and fish embryos.4,5
Genuine hemangioblasts are commonly defined as founders of the blood and vascular systems in early embryonic life. Hemangioblasts derived from the extraembryonic mesoderm are at the origin of coupled vasculogenesis and primitive blood formation in the yolk sac, whereas those born to intraembryonic (splanchnopleural) mesoderm generate definitive hematopoietic cells in the dorsal aorta through a hemogenic endothelium intermediate, a sequence that may also apply to yolk sac hemangioblasts (see figure).6,7 Besides these documented key roles in the incipient blood and vascular systems, some investigators have evoked the possibility that hemangioblasts persist and function at later stages of development, and even during postnatal and adult life. Candidate hemangioblasts marked by the coexpression of CD34 and KDR (the receptor 2 for vascular endothelial growth factor) were identified in human umbilical cord blood and adult bone marrow, albeit at the infinitesimal frequency of approximately 1 in 2 × 105 mononucleated cells.8 Donor adult bone marrow cells regenerated both blood cell lineages and retinal blood vessels, at the clonal level, in a mouse model of diabetic ischemia.9 Detection of the BCR/ABL fusion gene in endothelial cells grown in culture from the blood or bone marrow of chronic myelogenous leukemia patients was also taken as an indication that hemangioblasts persist in adult life.10 These results have remained anecdotal, due in part to technical difficulties in reproducing some of them. In the absence of more recent confirmation, no consensus has been reached regarding the persistence of angio-hematopoietic cells beyond embryonic life.
Now, Sun et al11 have used the seminal functional test for embryonic hemangioblasts, the blast colony-forming cell assay,2 to detect rare progenitor cells in the adult mouse uterus which in culture yield both endothelial and hematopoietic cells. Approximately 0.15% of CD34+c-kit− uterine cells are described as forming blast colonies, although the purity of the immunomagnetically sorted starting cell population was not mentioned. Green fluorescent protein (GFP)–tagged individual uterine blast colonies transplanted together with total bone marrow helper cells engrafted 29 of 32 lethally irradiated host mice and yielded, up to at least 12 months, minor but consistent populations of both hematopoietic cells in bone marrow, blood, and spleen and blood vessel lining cells in multiple organs. Interestingly, bone marrow from short-term (12 weeks) and long-term (12 months) chimeric mice transplanted into secondary recipients generated 8-day spleen colonies containing GFP+ cells, and yielded GFP+ hematopoietic cells in the marrow, blood, and spleen after 2 months. This is the first attempt to directly document in vivo the self-renewal potential of hemangioblasts, which are usually regarded as short-lived, ephemeral cells. The results indicate that at least some of the transplanted blast colony cells were renewed in the primary host, although this self-renewal may have been limited to hematopoiesis-committed descendents of the original blast cells, because the presence of GFP+ endothelial cells in secondary recipients was not reported.
These provocative observations suggest the presence of angio-hematopoietic cells in the uterus. Sun and collaborators propose, in addition, that these uterine hemangioblasts are not of bone marrow origin because no donor-derived blast colony forming cells were detected in the uterus of bone marrow radiation chimeras. Therefore, the data they report may reflect the dissemination and sustained residence of embryonic hemangioblasts in multiple organs. The authors state in the discussion that the uterus had the highest potential for blast cell formation compared with blood, bone marrow, heart, and skeletal muscle, but have not quantified this potential in the latter tissues. Alternatively, this adult stock of progenitor cells may be specifically harbored in the uterus. Although the dramatic, cyclic regeneration of the uterine wall implies robust local progenitor cell activity, also for angiogenesis, the presence of blood-forming cells in this organ remains to be justified. In this respect, the authors propose, as an audacious hypothesis, that maternal hemangioblasts may be involved in the formation of the trophoblast and yolk sac blood islands. Such uterine hemangioblasts should play no role in placental hematopoiesis, though well-documented to be embryo-derived (reviewed in Dzierzak and Speck6 ).
Overall, these intriguing observations revive a continuing debate and should stimulate further experimentation to confirm and document the presence of angio-hematopoietic stem cells in the female genital tract and other organs of the adult. As a final note, adherence to the strictest definition of a hemangioblast would require the demonstration that these cells can form endothelial and blood cells, but no other cell lineage, otherwise these should merely belong to a multipotent stem cell pool, the identification of which in the uterus would be of no less interest than that of committed hemangioblasts.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal