In this issue of Blood, Stables et al provide new insight into how immunoregulation by prostaglandins influences 2 important challenges to human health: severe infections causing sepsis and the rise of antimicrobial drug resistance in bacterial pathogens.11
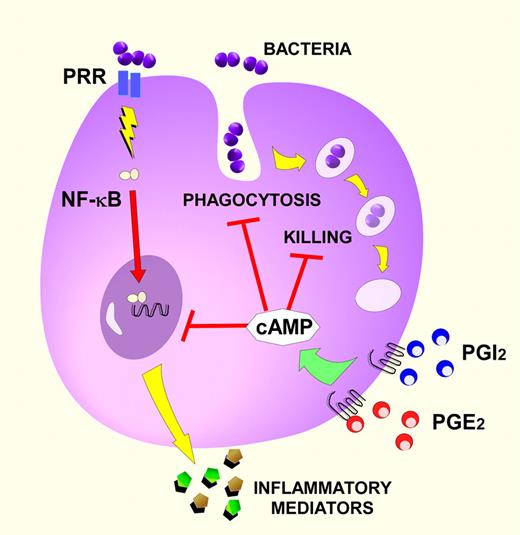
Prostaglandin signaling suppresses innate defense functions of monocytes and macrophages. In this depiction, the prostaglandins PGE2 and prostacyclin (PGI2) trigger the intracellular production of the second messenger cAMP through the activation of Gs-coupled receptors on monocytes or macrophages. PGE2 evokes cAMP through EP2 and EP4 receptors while PGI2 does so through the IP receptor. cAMP-signaling cascades impair 3 primary functions of these innate immune cells: phagocytosis, intracellular killing, and the induction of inflammatory mediators (cytokines, chemokines, and lipids) by cells infected with bacterial pathogens. NF-κB, the transcription factor nuclear factor κB; and PRR, pathogen recognition receptor (eg, Toll-like receptors). Red bars indicate inhibitory signaling pathways.
Prostaglandin signaling suppresses innate defense functions of monocytes and macrophages. In this depiction, the prostaglandins PGE2 and prostacyclin (PGI2) trigger the intracellular production of the second messenger cAMP through the activation of Gs-coupled receptors on monocytes or macrophages. PGE2 evokes cAMP through EP2 and EP4 receptors while PGI2 does so through the IP receptor. cAMP-signaling cascades impair 3 primary functions of these innate immune cells: phagocytosis, intracellular killing, and the induction of inflammatory mediators (cytokines, chemokines, and lipids) by cells infected with bacterial pathogens. NF-κB, the transcription factor nuclear factor κB; and PRR, pathogen recognition receptor (eg, Toll-like receptors). Red bars indicate inhibitory signaling pathways.
Bacterial infections are a major cause of morbidity and mortality worldwide. Innovative approaches to their prevention and management are needed. New treatments have focused on discovering antibiotics but this is problematic given the rise of antimicrobial drug resistance in common bacterial pathogens. Recent attention has been placed on identifying immunomodulatory agents that enhance innate and/or adaptive immune defenses of the infected host.2 The present work by Stables et al advances this immunopharmacology paradigm as it pertains to bacterial infections.1 Their work suggests that one solution may lie within the biology of aspirin.
Stables and collaborators used pharmacologic and genetic techniques to determine whether prostaglandin (PG) synthesis and signaling alters host immune responses to infections caused by either group B Streptococcus (GBS) or Streptococcus pneumoniae. Through elegant human and murine studies, Stables et al found that the inhibition of the PG-synthesizing cyclooxygenase-1 (COX-1) and COX-2 enzymes significantly improved innate immune defenses against common streptococcal pathogens. In so doing, they have brought several previously (and disparately) characterized immunomodulatory actions of PGs together. Their studies characterized several PG receptors and the intracellular signaling molecule (cAMP) involved in suppressing host defenses against infection.
Strengths of the experimental design by Stables et al include the combined use of rodent and human infection models to explore host-microbial interactions.1 Notably, their findings were reproducible when studying either antibiotic-susceptible or -resistant S pneumoniae. This is interesting and important because the class of COX inhibitors used in these studies, the nonsteroidal anti-inflammatory drugs (NSAIDs), is in common clinical use, raising the question of whether such medications might one day be used as adjuvant therapy in the treatment of antibiotic-resistant bacterial infections.
The PGs, oxygenated metabolites of the cell-membrane phospholipid component arachidonic acid, are generated rapidly in the face of physiologic or pathophysiologic perturbation. Unlike proteins, PGs are produced almost immediately upon cell stimulation, without relying on gene transcription and translation. They are important in many nonimmune physiologic processes, explaining the utility of aspirin in preventing arterial thrombosis (by reducing platelet thromboxane A2 production) and the adverse effects of NSAIDs such as gastric ulceration and renal toxicity. During infection, PGs have complex actions, both driving and relaxing host responses. PGE2, the archetype immunoregulatory PG, promotes inflammation by inducing endothelial cell–mediated vasodilatation (producing warmth, erythema, and edema), and supporting Th17 adaptive immune responses.3 However, as supported by Stables et al, PGE2 has potent antiinflammatory and immunosuppressive properties including the direct inhibition of: neutrophil chemotaxis and activation; leukocyte reactive oxygen intermediate production; phagocytosis; bacterial killing; and the generation of myriad proinflammatory cytokines, chemokines, and lipids.4 Conversely, PGE2 enhances the production of the antiinflammatory cytokine interleukin-10 and stimulates the expression of the suppressor of cytokine signaling-3 protein.4 In general, the inhibitory effects of PGE2, and the closely related PGI2, result from cAMP-dependent signaling processes triggered by EP2 and EP4 receptor binding, and IP receptor activation for PGI2 (see figure). These immunosuppressive actions of PGs likely evolved to prevent inflammatory tissue damage and promote the resolution of inflammation.5
Aspirin and other NSAIDs have been used in patients with febrile infections for thousands of years, if one considers salicylate-containing botanicals. In 1962, Northover and Subramanian reported that NSAIDs ameliorated the lethal effects of bacterial endotoxin in dogs.6 There has since been great interest in targeting COX pathways for augmenting host defenses against infection, yet adjunctive therapies involving PG biology have not entered the anti-infective pharmacopeia. The reasons for this are complex, possibly reflecting the fact that PGs are a diverse class of mediators that regulate myriad important physiologic and pathophysiologic processes. For example, PGs mediate hemodynamic responses to infection and fever, which itself may augment immune responses to infection.7 Thus, ablating all downstream COX metabolites might not be beneficial.
Stables and colleagues shed a light on this aspect of using NSAIDs to treat severe infections. They reported a shift toward a more “proinflammatory” phenotype at the cellular and systemic levels when PG synthesis was blocked. Because certain PGs limit inflammatory responses and drive the resolution of inflammation, untoward effects could result if these actions are derailed. In fact, the authors noted that mice treated with COX inhibitors before peritoneal infection exhibited enhanced production of tumor necrosis factor-α (TNF-α) and increased mortality compared with untreated mice infected with GBS.1 This excess mortality was prevented if animals received a TNF-α inhibitory agent, suggesting that PGs protected the infected host from a cytokine storm. However, when the COX inhibitors were applied after GBS infection, this excessive mortality was not observed, suggesting that PG-blocking approaches might best be used in the treatment of infection rather than in their prevention.1
The failure of COX inhibitors to emerge as therapeutically useful in eradicating infections could reflect a lack of appropriate clinical studies, or the need for a more targeted approach (to the pathogen, the patient, or the specific PG). The present work suggests that particular pathogens (eg, streptococci) might be best suited for PG-inhibitory therapy.1 In addition, PG-inhibitor therapy might provide the greatest benefit to patients who are immunocompromised due to exaggerated PG synthesis or signaling.4 Examples of clinical diseases and physiologic conditions associated with increased PGE2 production and enhanced susceptibility to infection include cancer, aging, HIV infection, malnutrition, solid organ/bone marrow transplantation, and pregnancy.4 It is also probable that new drugs will need to target particular PG molecules (or their downstream signaling networks). Previous work has suggested that inhibiting specific EP receptors for PGE2 might be beneficial to host defense8 and the data from Stables and collaborators drive home this point. They found significant enhancement of leukocyte killing of antibiotic-susceptible and -resistant pneumococci when human whole blood was pretreated with antagonists of the EP2 and IP receptors.1
While much has been learned about the roles of PGs in modulating immune defenses against bacterial infection, more research is needed to identify the ideal pharmacologic targets for clinical use. Perhaps then healthcare providers will urge infected patients to “take 2 EP2 inhibitors and call me in the morning.”
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
Acknowledgment
The author thanks Dr Marc Peters-Golden for his critical reading of this manuscript.
National Institutes of Health
REFERENCES
Author notes
Contribution: D.M.A. is the sole author of this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal