Abstract
The incidence of non-Hodgkin lymphoma (NHL) has increased steadily for the past few decades. Previous studies have suggested an association between blood transfusions and NHL. The main objective of this study was to evaluate this relationship with a meta-analysis of observational studies. A literature search was undertaken, looking for case-control and cohort studies evaluating the risk of developing NHL in persons who received allogeneic blood transfusions; 14 studies were included. Outcome was calculated and reported as relative risk (RR). Heterogeneity was assessed with Cochrane Q and I2 statistics. Dissemination bias was evaluated by funnel plot visualization and trim-and-fill analysis. Quality assessment was performed with the Newcastle-Ottawa scale. Our analysis showed a RR of developing NHL of 1.05 (95% CI, 0.89-1.25; P = .42) and 1.34 (95% CI, 1.15-1.55; P < .01) in case-control and cohort studies, respectively. When pooling all studies, RR was 1.2 (95% CI, 1.07-1.35; P < .01). In subset analysis, RR of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) was 1.66 (95% CI, 1.08-2.56; P = .02). The RR of NHL was elevated in both men and women and in persons receiving transfusions either before or after 1992. Blood transfusions appear to increase the risk of developing NHL; however, the risk of CLL/SLL appears higher than for other NHL subtypes.
Introduction
Non-Hodgkin lymphoma (NHL) is the seventh most common cancer diagnosed in the United States, with approximately 66 000 new cases diagnosed in 2009.1 The incidence of NHL has increased at a compound rate of 2% to 3% per year since the mid-1970s, making NHL one of the fastest rising cancers in incidence in the United States, second only to melanoma.1 Even after accounting for an aging population, longer survival in patients infected with HIV, or the increased prevalence of autoimmune conditions, the reasons for the progressively increased incidence of NHL are largely unexplained.
Within the past decades, significant progress has been made to produce safer blood products. Not surprisingly, with the improvement on blood safety and handling and the availability of relatively large donor pools, the rate of transfusion of blood components has also increased. In 2006, 13.2 million units of allogeneic red blood cells (RBCs) were transfused in the United States alone.2 Because of their immunologic effects, RBC transfusions might be associated with the development or progression of malignancies.
Several epidemiologic studies have evaluated the relationship between the transfusion of allogeneic RBC concentrates and the development of NHL with conflicting results. Because this question is unlikely to be answered by randomized controlled trials, the main objective of our study was to evaluate the potential relationship between RBC transfusion and the development of NHL with a meta-analysis of observational studies.
Methods
Literature search
Two authors (J.J.C. and S.D.) independently performed a computer-based literature search with PubMed/MEDLINE and the Cochrane Database of Systematic Reviews through December 2009. The initial PubMed search with the term “non-Hodgkin lymphoma” generated 30 370 returns. The term “blood transfusion” generated 66 472 returns. When both search terms were combined, 166 returns were obtained. The titles and abstracts of the selected articles were examined. Full-text articles were retrieved; if a paper was selected for inclusion, the bibliographic references were scrutinized to look for additional studies. A similar strategy was used to search the Cochrane database, which did not generate any unique returns.
Inclusion and exclusion criteria
An article was deemed relevant to our study if the article reported original data in English and originated from epidemiologic observational studies, either case-control or cohort, that reported an association between allogeneic RBC transfusions and the development of NHL. Because of a different physiopathology, studies on Hodgkin lymphoma or multiple myeloma were not included. Studies that evaluated autologous RBC transfusions and nonpublished studies available only in abstract form were also excluded. Any discrepancies between the 2 reviewers on inclusion or exclusion of a study were resolved through consensus in all cases. If there were multiple publications from the same study, only the most recent was selected, using the older publications only to clarify methodology or main characteristics of the studied population, if necessary.
Data extraction
The data extraction was performed independently by 2 reviewers (J.J.C. and S.D.) and included author, year of publication, country of origin, sample size, inclusion and exclusion criteria, method of ascertainment of RBC transfusion, and method of diagnosis of NHL. For case-control studies, we extracted years of inclusion and the source and definition of cases and controls, the outcome measured with 95% confidence intervals (CIs), and the variables used for adjustment. For cohort studies, we extracted the source of the cohort, years of follow-up, the source of the expected incidence of NHL, the outcome measured with 95% CIs, and the variables used for adjustment. Any discrepancies between reviewers were addressed by a joint reevaluation of the original article. For missing information, attempts were made to contact the authors of the original studies. The characteristics and quality of the studies included in this meta-analysis, as well as their outcomes, will be presented in accordance to the checklist proposed by the Meta-analysis Of Observational Studies group.3
Quality assessment
The quality of each study was assessed independently by 2 reviewers (S.K.P. and J.J.C.) with the Newcastle-Ottawa Scale (NOS). The NOS uses 2 different tools for case-control and cohort studies and consists of 3 parameters of quality: selection, comparability, and exposure/outcome assessment. The NOS assigns a maximum of 4 points for selection, a maximum of 2 points for comparability, and a maximum of 3 points for exposure/outcome. Therefore, 9 points is the highest score, reflecting the highest quality. Any discrepancies between reviewers were addressed by a joint reevaluation of the original article.
Data synthesis and analysis
The primary outcome measured was relative risk (RR) and 95% confidence interval (95% CI) of developing NHL in patients who have received RBC transfusions. To measure the outcome, we have used 2 different models; the Mantel-Haenszel or fixed-effects model and the DerSimonian-Laird or random-effects model (REM). The REM accounts for heterogeneity between studies, which is expected in an analysis of this nature. All analyses were reported with the use of the REM, unless otherwise noted. Subset analyses were performed by sex, lymphoma subtype, and year of transfusion. We assessed for heterogeneity with the use of 2 methods, the Cochrane Q statistic, which gave a qualitative value and was considered statistically significant for heterogeneity if a P value of less than .1 was obtained, and the I2 statistic, which gives a quantitative measurement; I2 values higher than 50% were considered a reflection of severe heterogeneity. Publication bias was also addressed in 2 ways, by direct observation of funnel plots representing RR by sample size, and by the use of the trim-and-fill method, which estimated and adjusted for the potential effect that nonpublished studies might have on the measured outcome. All calculations and graphs were obtained with MIX Version 1.7,4 which is a free statistical software for meta-analysis that has been validated against STATA (Stata Corporation) and Comprehensive Meta-Analysis (Biostat Inc).
Results
Search results
The results of our search strategy are summarized in Figure 1. A total of 166 articles was identified during our initial search from which 150 were ineligible because they were written in a language other than English, they were reviews or case reports, or they did not pertain to either RBC transfusions or NHL. Sixteen articles were selected for full-text retrieval, and, after reviewing their reference lists, 3 additional articles were deemed potentially relevant. After a detailed evaluation of the 19 articles, 5 were rejected because they were duplicated or did not pertain to NHL incidence or the use of RBC transfusions. Finally, 9 case-control5-13 and 5 cohort studies14-18 were included in our analysis.
Characteristics of the case-control studies
The main characteristics of the published case-control studies that evaluated the association between RBC transfusions and NHL are shown in Table 1. Studies were published between 1996 and 2008. Six studies originated from the United States7-10,12,13 and 3 studies from Europe.5,6,11 A total of 5904 cases and 10 107 controls were included in this meta-analysis. There was a male predominance of 64% and 63% in cases and controls, respectively. Most of the studies did not show a statistically significant association between RBC transfusions and NHL, with exception of one study.6 Exposure to transfusion was assessed by medical records in 3 studies,5,9,11 by interviews in 4 studies,8,10,12,13 and by mailed questionnaires in 2 studies.6,7 Five studies assessed the diagnosis of NHL from medical records6,7,9,11,13 and 4 studies from an independent pathology review.5,8,10,12
Main characteristics of case-control studies evaluating the association between blood transfusions and non-Hodgkin lymphoma
| Author . | Year . | Country . | Ascertainment period . | Source cases, no. . | Source controls, no. . | Assessment NHL diagnosis . | Assessment RBC transfusion . | Matching and adjustments . |
|---|---|---|---|---|---|---|---|---|
| Brandt6 | 1996 | Sweden | 1991-1995 | Southern Swedish Regional Tumor Registry, 280 | Population-based, General Population Registry, 1827 | Registry records | Questionnaire | Age, sex, residence |
| Adami5 | 1997 | Sweden | Varied-1991 | Swedish Inpatient Register, 361 | Swedish Inpatient Register, 705 | Independent pathology review | Medical records | Age, sex, diagnosis, procedure, date of discharge, hospital |
| Nelson10 | 1998 | United States | 1989-1992 | Los Angeles County Cancer Surveillance Program, 378 | Population-based, Los Angeles County, 378 | Independent pathology review | Interviews | Age, sex, race, IV drug use |
| Maguire-Boston9 | 1999 | United States | 1975-1993 | Mayo Clinic records, Olmsted County, MN, 37 | Population-based, Mayo Clinic records, 174 | Pathology records | Medical records | Age, sex, clinic number |
| Tavani11 | 1999 | Italy | 1983-1998 | Newly hospitalized patients, Milan and Porderone hospitals, 385 | Newly hospitalized patients, Milan and Porderone hospitals, 1297 | Pathology records | Medical records | Age, sex, area of residence, education |
| Chow8 | 2002 | United States | 1988-1995 | Northern California Cancer Center Case Ascertainment system, 1591 | Northern California Cancer Center Case Ascertainment system, 2515 | Independent pathology review | Face-to-face interviews | Age, sex, HIV status |
| Zhu13 | 2003 | United States | 1984-1988 | 8 cancer registries (Atlanta, Detroit, San Francisco, Seattle, Miami, Connecticut, Iowa and Kansas), 1511 | Population-based, Atlanta, Detroit, San Francisco, Seattle, Miami, Connecticut, Iowa. and Kansas, 1910 | Cancer Registries records | Telephone interviews | Age, cancer registry |
| Zhang12 | 2004 | United States | 1996-2000 | Yale Cancer Center Rapid Case Ascertainment Shared Resource, 600 | Population-based, Connecticut, 712 | Independent pathology review | Face-to-face interviews | Age, family history of NHL in first-degree relatives |
| Cerhan7 | 2008 | United States | 1998-2000 | Iowa, Seattle, Los Angeles, and Detroit SEER cancer registries, 759 | Population-based, Iowa, Seattle, Los Angeles and Detroit, 589 | Registry records | Computer-assisted and mailed questionnaires | Age, sex, race, study center |
| Author . | Year . | Country . | Ascertainment period . | Source cases, no. . | Source controls, no. . | Assessment NHL diagnosis . | Assessment RBC transfusion . | Matching and adjustments . |
|---|---|---|---|---|---|---|---|---|
| Brandt6 | 1996 | Sweden | 1991-1995 | Southern Swedish Regional Tumor Registry, 280 | Population-based, General Population Registry, 1827 | Registry records | Questionnaire | Age, sex, residence |
| Adami5 | 1997 | Sweden | Varied-1991 | Swedish Inpatient Register, 361 | Swedish Inpatient Register, 705 | Independent pathology review | Medical records | Age, sex, diagnosis, procedure, date of discharge, hospital |
| Nelson10 | 1998 | United States | 1989-1992 | Los Angeles County Cancer Surveillance Program, 378 | Population-based, Los Angeles County, 378 | Independent pathology review | Interviews | Age, sex, race, IV drug use |
| Maguire-Boston9 | 1999 | United States | 1975-1993 | Mayo Clinic records, Olmsted County, MN, 37 | Population-based, Mayo Clinic records, 174 | Pathology records | Medical records | Age, sex, clinic number |
| Tavani11 | 1999 | Italy | 1983-1998 | Newly hospitalized patients, Milan and Porderone hospitals, 385 | Newly hospitalized patients, Milan and Porderone hospitals, 1297 | Pathology records | Medical records | Age, sex, area of residence, education |
| Chow8 | 2002 | United States | 1988-1995 | Northern California Cancer Center Case Ascertainment system, 1591 | Northern California Cancer Center Case Ascertainment system, 2515 | Independent pathology review | Face-to-face interviews | Age, sex, HIV status |
| Zhu13 | 2003 | United States | 1984-1988 | 8 cancer registries (Atlanta, Detroit, San Francisco, Seattle, Miami, Connecticut, Iowa and Kansas), 1511 | Population-based, Atlanta, Detroit, San Francisco, Seattle, Miami, Connecticut, Iowa. and Kansas, 1910 | Cancer Registries records | Telephone interviews | Age, cancer registry |
| Zhang12 | 2004 | United States | 1996-2000 | Yale Cancer Center Rapid Case Ascertainment Shared Resource, 600 | Population-based, Connecticut, 712 | Independent pathology review | Face-to-face interviews | Age, family history of NHL in first-degree relatives |
| Cerhan7 | 2008 | United States | 1998-2000 | Iowa, Seattle, Los Angeles, and Detroit SEER cancer registries, 759 | Population-based, Iowa, Seattle, Los Angeles and Detroit, 589 | Registry records | Computer-assisted and mailed questionnaires | Age, sex, race, study center |
NHL indicates non-Hodgkin lymphoma; RBC, red blood cell; IV, intravenous; and SEER, Surveillance, Epidemiology and End Results Program.
Characteristics of the cohort studies
The main characteristics of the published cohort studies that evaluated the association between RBC transfusions and NHL are shown in Table 2. Studies were published between 1993 and 2009. Three studies originated from Europe14,17,18 and 2 from the United States.15,16 A total of 3883 cases in a cohort of 1 134 089 persons were included in this meta-analysis. Most of the studies showed a statistically significant increased risk of NHL with transfusions, with the exception of one study.18 The exposure to transfusions was assessed by registry data in 3 studies14,17,18 and by mailed questionnaire in 2.15,16 The diagnosis of NHL was assessed through national or regional registries in all studies.
Main characteristics of cohort studies evaluating the association between blood transfusions and non-Hodgkin lymphoma
| Author . | Year . | Country . | Source of cohort . | NHL assessment . | RBC transfusion assessment . | Mean follow-up . | Total cohort (total person-years) . | Cases observed (cases expected) . | Source of expected cases . | Adjustments . |
|---|---|---|---|---|---|---|---|---|---|---|
| Blomberg14 | 1993 | Sweden | Blood Centre Registry, Lund University Hospital | Regional tumor registry | Blood Centre Registry, Lund University Hospital | 10 y (1981-1991) | 1572 (8249) | 7 (1.7) | Malmohus County Council Registry | Age |
| Memon18 | 1994 | United Kingdom | 41 major hospitals and blood centers in England, Wales, and Scotland | National Health Service Central Registry | 41 major hospitals and blood centers in England, Wales, and Scotland | 25 y (1942-1990) | 12 690 (340 227) | 4 (1.85) | National Health Service Central Registry | NR |
| Cerhan15 | 2001 | United States | Iowa Women's Health Study | State Health Registry of Iowa | Mailed questionnaire | 12 y (1986-1997) | 37 934 (418 342) | 40 (NR) | NR | Age, marital status, residence, smoking, alcohol intake, diabetes, and diet |
| Hjalgrim17 | 2007 | Sweden, Denmark | Scandinavian Donation and Transfusion (SCANDAT) database | National Cancer Registry and Hospital Registers | Scandinavian Donation and Transfusion (SCANDAT) database | Varied (Sweden, 1968-2002; Denmark, 1982-2002) | 888 843 (5 652 918) | 2853 (NR) | Incidence rates from the general populations of Sweden and Denmark | Age, sex, and country |
| Erber16 | 2009 | United States (Los Angeles, Hawaii) | Multiethnic Cohort (MEC) study | Hawaii Tumor Registry, Los Angeles County Cancer Surveillance Program, State of California Cancer Registry | Mailed questionnaire | 10 y (started between 1993 and 1996) | 193 050 (NR) | 939 (NR) | NR | Ethnicity, education, body mass index and alcohol intake |
| Author . | Year . | Country . | Source of cohort . | NHL assessment . | RBC transfusion assessment . | Mean follow-up . | Total cohort (total person-years) . | Cases observed (cases expected) . | Source of expected cases . | Adjustments . |
|---|---|---|---|---|---|---|---|---|---|---|
| Blomberg14 | 1993 | Sweden | Blood Centre Registry, Lund University Hospital | Regional tumor registry | Blood Centre Registry, Lund University Hospital | 10 y (1981-1991) | 1572 (8249) | 7 (1.7) | Malmohus County Council Registry | Age |
| Memon18 | 1994 | United Kingdom | 41 major hospitals and blood centers in England, Wales, and Scotland | National Health Service Central Registry | 41 major hospitals and blood centers in England, Wales, and Scotland | 25 y (1942-1990) | 12 690 (340 227) | 4 (1.85) | National Health Service Central Registry | NR |
| Cerhan15 | 2001 | United States | Iowa Women's Health Study | State Health Registry of Iowa | Mailed questionnaire | 12 y (1986-1997) | 37 934 (418 342) | 40 (NR) | NR | Age, marital status, residence, smoking, alcohol intake, diabetes, and diet |
| Hjalgrim17 | 2007 | Sweden, Denmark | Scandinavian Donation and Transfusion (SCANDAT) database | National Cancer Registry and Hospital Registers | Scandinavian Donation and Transfusion (SCANDAT) database | Varied (Sweden, 1968-2002; Denmark, 1982-2002) | 888 843 (5 652 918) | 2853 (NR) | Incidence rates from the general populations of Sweden and Denmark | Age, sex, and country |
| Erber16 | 2009 | United States (Los Angeles, Hawaii) | Multiethnic Cohort (MEC) study | Hawaii Tumor Registry, Los Angeles County Cancer Surveillance Program, State of California Cancer Registry | Mailed questionnaire | 10 y (started between 1993 and 1996) | 193 050 (NR) | 939 (NR) | NR | Ethnicity, education, body mass index and alcohol intake |
NHL indicates non-Hodgkin lymphoma; RBC, red blood cell; and NR, not reported.
Quality assessment results
The quality assessment of all the published studies that evaluated the association between RBC transfusions and NHL are shown in Table 3.
Quality assessment according to the Newcastle-Ottawa scale
| Author . | Year . | Selection . | Comparability . | Outcome . | Exposure . | Total . |
|---|---|---|---|---|---|---|
| Cohort studies | ||||||
| Blomberg14 | 1993 | 4 | 1 | 2 | — | 7 |
| Memon18 | 1994 | 4 | 0 | 2 | — | 6 |
| Cerhan15 | 2001 | 4 | 2 | 2 | — | 8 |
| Hjalgrim17 | 2007 | 4 | 2 | 3 | — | 9 |
| Erber16 | 2009 | 4 | 2 | 3 | — | 9 |
| Average | 4 | 1.4 | 2.4 | — | 7.8 | |
| Case-control studies | ||||||
| Brandt6 | 1996 | 3 | 2 | — | 2 | 7 |
| Adami5 | 1997 | 4 | 2 | — | 3 | 9 |
| Nelson10 | 1998 | 4 | 2 | — | 2 | 8 |
| Maguire-Boston9 | 1999 | 3 | 2 | — | 3 | 8 |
| Tavani11 | 1999 | 3 | 2 | — | 2 | 7 |
| Chow8 | 2002 | 4 | 2 | — | 2 | 8 |
| Zhu13 | 2003 | 3 | 2 | — | 2 | 7 |
| Zhang12 | 2004 | 4 | 2 | — | 2 | 8 |
| Cerhan7 | 2008 | 3 | 2 | — | 3 | 8 |
| Average | 3.4 | 2 | — | 2.3 | 7.7 |
| Author . | Year . | Selection . | Comparability . | Outcome . | Exposure . | Total . |
|---|---|---|---|---|---|---|
| Cohort studies | ||||||
| Blomberg14 | 1993 | 4 | 1 | 2 | — | 7 |
| Memon18 | 1994 | 4 | 0 | 2 | — | 6 |
| Cerhan15 | 2001 | 4 | 2 | 2 | — | 8 |
| Hjalgrim17 | 2007 | 4 | 2 | 3 | — | 9 |
| Erber16 | 2009 | 4 | 2 | 3 | — | 9 |
| Average | 4 | 1.4 | 2.4 | — | 7.8 | |
| Case-control studies | ||||||
| Brandt6 | 1996 | 3 | 2 | — | 2 | 7 |
| Adami5 | 1997 | 4 | 2 | — | 3 | 9 |
| Nelson10 | 1998 | 4 | 2 | — | 2 | 8 |
| Maguire-Boston9 | 1999 | 3 | 2 | — | 3 | 8 |
| Tavani11 | 1999 | 3 | 2 | — | 2 | 7 |
| Chow8 | 2002 | 4 | 2 | — | 2 | 8 |
| Zhu13 | 2003 | 3 | 2 | — | 2 | 7 |
| Zhang12 | 2004 | 4 | 2 | — | 2 | 8 |
| Cerhan7 | 2008 | 3 | 2 | — | 3 | 8 |
| Average | 3.4 | 2 | — | 2.3 | 7.7 |
With regard to the case-control studies, 100% of the studies were of high quality (ie, NOS score higher than 6). The most common selection bias was the lack of independent pathologic assessment of NHL cases (55%). In terms of comparability bias, all the studies included adequate matching or adjustments (eg, age and sex). The most common exposure bias was the lack of reporting of nonresponse rates (67%).
In the cohort studies, 80% of the studies were of high quality; one study had a NOS score of 6. In terms of selection bias, 100% of the studies met all the high-quality criteria. The most common comparability bias was the lack of reporting of adjustments (40%). The most common outcome bias was the lack of reporting of persons lost to follow-up (60%).
Outcome results
In the case-control studies, the RR of developing NHL after RBC transfusions was 1.05 (95% CI, 0.89-1.25; P = .42; Figure 2). The funnel plot and the trim-and-fill analysis did not show evidence of dissemination bias. In the cohort studies, the RR of developing NHL was 1.34 (95% CI, 1.15-1.55; P < .01; Figure 2). On evaluation of the funnel plot, a slight asymmetry was observed which favored the association between transfusions and NHL. The trim-and-fill analysis showed 2 imputed studies, which would have not changed our results significantly (RR, 1.29; 95% CI, 1.11-1.5). When combining all the studies, the RR of developing NHL was 1.2 (95% CI, 1.07-1.35; P < .01; Figure 2). The visual evaluation of the funnel plot and the trim-and-fill analysis did not show evidence of dissemination bias.
Risk estimates of the relative risk of developing NHL for persons who received allogeneic RBC transfusions, by study design.
Risk estimates of the relative risk of developing NHL for persons who received allogeneic RBC transfusions, by study design.
Subset analyses
Sex.
Five studies7,9,12,16,17 reported outcomes in female participants. When evaluating the risk of developing NHL in female participants, the RR was 1.26 (95% CI, 1.09-1.45; P < .01; Figure 3 top). The trim-and-fill analysis showed 2 imputed studies, which would have not affected our results (RR, 1.33; 95% CI, 1.15-1.53). Four studies evaluated outcomes in men.9,13,16,17 The RR of NHL in male participants was 1.19 (95% CI, 1.03-1.38; P = .02; Figure 3 bottom). The trim-and-fill analysis showed 2 imputed studies, which would have not affected our results (RR, 1.26; 95% CI 1.1-1.44).
Risk estimates of the relative risk of developing NHL for persons who received allogeneic RBC transfusions, by sex.
Risk estimates of the relative risk of developing NHL for persons who received allogeneic RBC transfusions, by sex.
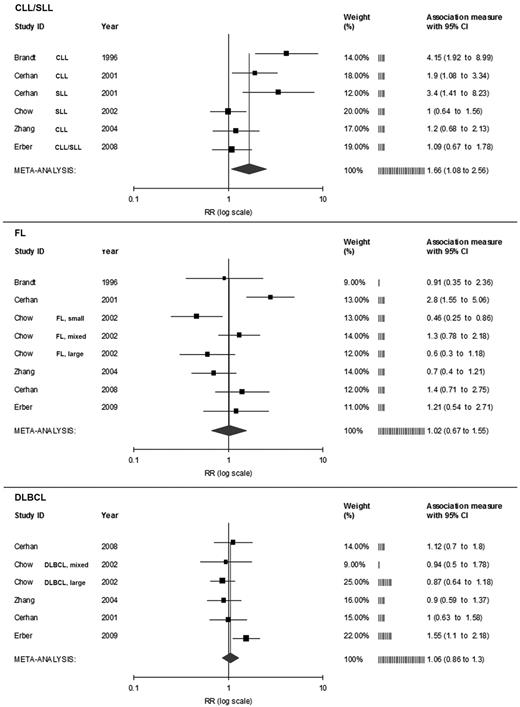
Lymphoma subtypes.
Five studies reported outcomes in association with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL).6-8,12,16 The RR of developing CLL/SLL was 1.66 (95% CI,1.08-2.56; P = .02; Figure 4 top). Six studies reported outcomes associated with the development of follicular lymphoma.6-8,12,15,16 The RR of developing follicular lymphoma was 1.02 (95% CI, 0.67-1.55; P = .94; Figure 4 center). Five studies reported risks of developing diffuse large B-cell lymphoma in patients receiving RBC transfusions.7,8,12,15,16 The RR of developing diffuse large B-cell lymphoma was 1.06 (95% CI, 0.86-1.3; P = .6; Figure 4 bottom).
Risk estimates of the relative risk of developing NHL for persons who received allogeneic RBC transfusions, by lymphoma subtype. FL indicates follicular lymphoma; and DLBCL, diffuse large B-cell lymphoma.
Risk estimates of the relative risk of developing NHL for persons who received allogeneic RBC transfusions, by lymphoma subtype. FL indicates follicular lymphoma; and DLBCL, diffuse large B-cell lymphoma.
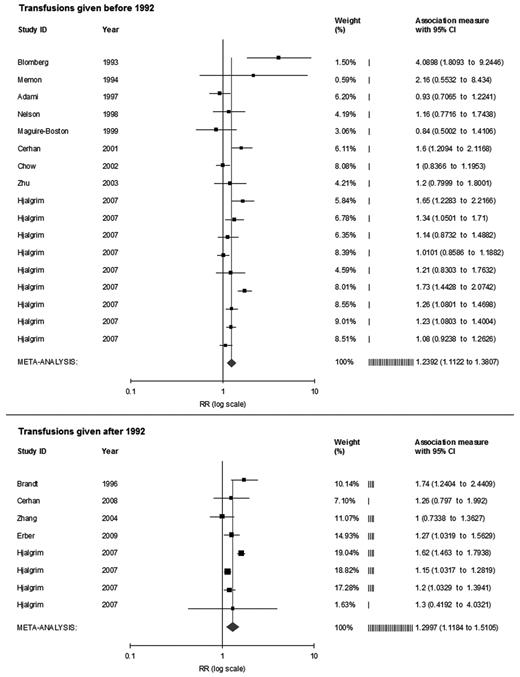
Year of transfusion.
Nine studies reported a risk of developing NHL in patients who received transfusions from 1957 to 19915,8-10,13-15,17,18 and 5 studies reported on patients who received transfusions from 1992 onward.6,7,12,16,17 When pooling the studies on patients who received transfusions before and after 1992, the RR of NHL was 1.24 (95% CI, 1.11-1.38; P < .001) and 1.3 (95% CI, 1.12-1.51; P < .001), respectively (Figure 5).
Risk estimates of the relative risk of developing NHL for persons who received allogeneic RBC transfusions, by year of transfusion.
Risk estimates of the relative risk of developing NHL for persons who received allogeneic RBC transfusions, by year of transfusion.
Transfusion latency.
Six studies reported the RR of developing NHL according to the time elapsed since the RBC transfusion5-8,15,17 ; however, the studies reported the time from transfusions in dissimilar lapses of time, not allowing a formal meta-analysis. One study excluded NHL cases 3 months after transfusion,11 2 studies excluded NHL cases 6 months after transfusion,9,17 6 studies excluded cases presenting within 1 year of transfusions,5-8,12,13 one study excluded the first 2 years,16 and another study excluded the first 3 years of follow-up.10 Two studies did not exclude NHL cases within a specific lapse of time after transfusions.14,15 However, Cerhan et al15 reported that 91% of the cases had transfusions more than 5 years before the diagnosis of NHL.
Number and type of transfusions.
Four studies reported separate RRs according to the number of transfusions received.5,8-10 However, the categories were not similar and did not permit a formal meta-analysis. The type of transfusions (ie, RBC concentrate vs whole blood) were reported by a minority of studies,5,17 not allowing further analysis. However, in those studies in which the type of transfusion was reported, more than 90% were RBC concentrate transfusions.
Reasons for transfusions.
Six studies reported the actual reasons for the administered transfusions. One study reported that 83% of the transfusions were given in surgical settings.6 Another study reported that 80% of the transfusions were given for nonmedical conditions; most of the remaining transfusions were given for gastrointestinal bleeding and only 30% were for anemia.7 A third study reported that 5% of the transfusions were for anemia, but most was for obstetrical and surgical indications.12 Cerhan et al15 reported that 92% of the transfusions were given for postsurgical blood loss or other causes of bleeding. One study reported that trauma, surgery, and gastrointestinal bleeding accounted for 15% to 33% of the indications for transfusion.17 Finally, one study reported that most of the transfusions were given because of hemolytic disease of the newborn.18
Discussion
Several conditions have been associated with an increased risk of developing NHL, such as autoimmune diseases, chronic bacterial and viral infections, and congenital and acquired immunodeficiency states. Diabetes mellitus and pesticides have also been implicated in the cause of NHL.19,20 The common ground is that disease states or environmental conditions associated with immune dysregulation may lead to the development of NHL. Allogeneic RBC transfusions are immunogenic and can provide a similar environmental challenge. Furthermore, improved collecting techniques and processing of blood components have led to a decrease in the rate of disease transmission, originating a more widespread acceptance of blood products, increasing the use of allogeneic RBC transfusions.21,22
Our study shows a statistical association between blood transfusions and the development of NHL. But, although cohort studies showed a statistically significant association with the development of NHL, the case-control studies alone did not. The disparity may be attributed to study-specific factors, including small sample size, failure to take into account confounding variables, and recall bias. Subgroup analyses showed that the risk of NHL in both men and women receiving blood transfusions was increased. These findings suggest that there is no association between sex, receiving a blood transfusion, and developing NHL. In the subgroup analysis for different subtypes of NHL, there is a stronger association between having a blood transfusion and developing CLL/SLL. This finding is hypothesis generating and deserves further attention. There could be a true association between transfusions and CLL/SLL or the patients may not have had the condition diagnosed before receiving a blood transfusion because CLL/SLL can present indolently, without B symptoms or apparent lymphadenopathy, and a mildly abnormal leukocyte count. Finally, the risk of developing NHL was similar in persons who received a transfusion before and after 1992. This finding probably reflects the immunomodulating effects of transfusions despite the advent of leukodepletion and viral screening.
Blood transfusions have been associated to immunologically mediated events, such as hemolytic transfusion reactions and transfusion-related graft-versus-host disease. The immunomodulation caused by blood transfusion is probably related to suppression of the function of T and natural killer cells,23,24 a process that can potentially increase the risk of developing a malignancy. Animal models have shown that allogeneic blood transfusions can have cancer-promoting effects and were associated with increase in tumor size and development of metastasis in mice with malignancy.25,26 Although the immunomodulating effects of transfusions are due to leukocyte-mediated actions related to the expression of human leukocyte antigens, the contributions from plasma, addition solutions, plasticizers, or erythrocytes contained within the blood bag have not been adequately evaluated.27,28 Interestingly, autologous transfusions have induced lesser degrees of immunosuppression than allogeneic transfusions in patients with gastric cancer.29 However, several studies have theorized that there may be an undiscovered viral or molecular vector that can potentially transmit through a blood transfusion.7,9,15,18
Data from the Surveillance, Epidemiology, and End Results program showed that in the 1970s and 1980s the overall age-adjusted incidence of NHL had increased by 3% to 4% yearly.30 Since 1991 the incidence has leveled off to 0.4% per year in men and 1.2% per year in women.31 With improvements in screening and leukodepleting of blood occurring concurrently with the stabilizing in the incidence of NHL, one would think that blood transfusions before1991 might have lead to a higher incidence of NHL. However, our study shows a similar risk of NHL when transfusions were given either before or after 1992. This shows that the leveling off of the incidence of NHL is probably due to factors that are yet to be determined.
The following are interesting observations of our study. First, patients who received transfusions because of anemia appear to have a higher risk of developing NHL8,12,15,17 ; however, anemia could be an independent risk factor for NHL or may just have represented an early sign of an indolent NHL. Because of the limited data on the reason for which patients received blood transfusions, it was impossible to determine a relationship between the reason for which a blood transfusion was given and the risk of developing NHL.
Second, many of the studies excluded NHL cases diagnosed shortly after a blood transfusion, mainly to minimize the risk of the transfusion being prompted by an undiagnosed NHL. Interestingly, we could observe that there was an increased risk of developing NHL in persons who received blood transfusions 3 to 6 months before lymphoma diagnosis.11,17 However, some studies showed that the risk of NHL remained elevated for several years after a blood transfusion.15,17,18 Because the transfusion latency periods were not subdivided in a standardized fashion, no further analysis could be made to determine whether the time from blood transfusion was related to developing NHL.
An aspect not evaluated by our study was the age of the RBC concentrate used for transfusion. A recently published study showed that RBCs stored for longer periods of time have thrombotic or inflammatory effects.32 RBC components are stored with a variety of solutions, which can potentially affect the aging process of the RBCs.33 Among the most common solutions are CPD (trisodium citrate, dextrose, and disodium phosphate) and acidic additive solutions. However, most of the studies did not specify the type of solution the RBC transfusions contained.
Our study has limitations that are based on the quality of the published studies. First, although some studies used registry data to confirm blood transfusion administration, many studies used self-reporting questionnaires to assess if patients received a blood transfusion. Patients with lymphoma might be more likely to recall a transfusion. Furthermore, a platelet or plasma transfusion can have been mistakenly reported as a blood transfusion. Second, latency and reasons for transfusions were not clearly stated in all studies. Similarly, in many of the studies, it could not be determined whether patients with a greater number of transfusions were more likely to develop NHL. In addition, because it is difficult to determine the exact time of a blood transfusion before the diagnosis of NHL, blood transfusions may have been required secondary to a developing NHL as opposed to other reasons. Finally, some but not all the studies controlled for other potential confounding variables, including congenital or acquired immunosuppression, HIV infection, and other autoimmune diseases.
Conclusion
Our analysis shows that there could be a mildly increased risk of developing NHL in those patients who received allogeneic RBC transfusions. The risk of NHL was elevated in men and women and in persons who received transfusions before and after 1992. The risk of developing CLL/SLL, however, was significantly higher than for other NHL subtypes. Although the risk is minimal, given the increasing number of blood transfusions administered every year, the potential number of incident cases of NHL because of allogeneic RBC transfusions could be considered nonnegligible. A conservative approach should be used in determining the need of a blood transfusion. Future studies should focus on the immunomodulating effects of blood transfusions and their lymphomagenic potential.
An Inside Blood analysis of this article appears at the front of this issue.
Preliminary findings from this study were presented at the 51st annual meeting of the American Society of Hematology, New Orleans, LA, December 5-8, 2009.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
We thank Dr Joseph Sweeney, Director of Transfusion Services and Coagulation at The Miriam Hospital (Providence, RI) for critically reviewing our manuscript.
Authorship
Contribution: J.J.C. designed the study; J.J.C. and S.D. performed the literature search and data gathering; J.J.C. and S.K.P. performed the quality assessment; J.J.C. performed the statistical analysis; and J.J.C. and S.D. wrote the manuscript. All authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jorge J. Castillo, The Miriam Hospital, 164 Summit Ave, Providence, RI 02906; e-mail: jcastillo@lifespan.org.