Abstract
Transfusion-related acute lung injury (TRALI) is a serious complication of transfusion and has been ranked as one of the leading causes of transfusion-related fatalities. Nonetheless, many details of the immunopathogenesis of TRALI, particularly with respect to recipient factors are unknown. We used a murine model of antibody-mediated TRALI in an attempt to understand the role that recipient lymphocytes might play in TRALI reactions. Intravenous injection of an IgG2a antimurine major histocompatibility complex class I antibody (34-1-2s) into BALB/c mice induced moderate hypothermia and pulmonary granulocyte accumulation but no pulmonary edema nor mortality. In contrast, 34-1-2s injections into mice with severe combined immunodeficiency caused severe hypothermia, severe pulmonary edema, and approximately 40% mortality indicating a critical role for T and B lymphocytes in suppressing TRALI reactions. Adoptive transfer of purified CD8+ T lymphocytes or CD4+ T cells but not CD19+ B cells into the severe combined immunodeficiency mice alleviated the antibody-induced hypothermia, lung damage, and mortality, suggesting that T lymphocytes were responsible for the protective effect. Taken together, these results suggest that recipient T lymphocytes play a significant role in suppressing antibody-mediated TRALI reactions. They identify a potentially new recipient mechanism that controls the severity of TRALI reactions.
Introduction
Hemovigilance data from the Food and Drug Administration and the Serious Hazards of Transfusion study still rate transfusion-related acute lung injury (TRALI) as the most serious complication of transfusion today.1,2 A 1983 seminal TRALI report presented evidence linking leukocyte antibodies in the transfused donations with the pathology of TRALI.3 Since that publication, the number of TRALI reports associated with donor-derived leukocyte antibodies has grown significantly, adding support to the central role of leukocyte antibodies in the pathogenesis of TRALI.4-8 Leukocyte antibodies appear to be a key stimulus of neutrophils in both the proposed immune-mediated mechanism and the 2-event mechanism of TRALI.9-12 Despite this, some enigmas remain, as not all leukocyte antibodies cause TRALI in recipients with the cognate antigen13,14 and some antibodies (eg, anti–human neutrophil antigen-3a [HNA-3a] and anti–human leukocyte antigen-A2 [HLA-A2]) are associated with clinically more severe TRALI reactions.7,8,15,16
Animal models have contributed significantly to our understanding of the immune mechanisms of TRALI. For example, an ex vivo rabbit lung model of TRALI demonstrated that complement was required to induce anti–HNA-3a–mediated neutrophil activation and increased pulmonary vascular permeability.17 In contrast, an ex vivo rat model found that an anti-CD177 antibody could activate HNA-2a–positive neutrophils without the presence of complement,18 suggesting that different antibodies may have different mechanisms of action. On the other hand, it has been demonstrated in an in vivo rat model that human plasma and lipids from stored blood component together with anti–major histocompatibility complex (MHC) class I antibodies could cause neutrophil activation and acute lung injury.19-21 Taken together, these animal studies have shown that several immune mechanisms exist that can ultimately induce recipient neutrophil activation and TRALI induction.
A murine model of antibody-mediated TRALI was developed by Looney et al22 who observed that, when BALB/c mice were injected with a mouse monoclonal MHC class I antibody (mAb, 34-1-2s), a significant increase in excess lung water, lung vascular permeability, mortality, and neutropenia were observed within 2 hours after mAb infusion. This in vivo model has the advantage in that it will allow for studies on what recipient factors are responsible for antibody-mediated neutrophil accumulation, activation, and lung damage. We report here that the severity of anti–MHC class I antibody–mediated TRALI is significantly dependent on the presence of recipient lymphocytes, particularly CD8+ T cells and CD4+ T cells. This lymphocyte-mediated suppression of TRALI reactions appeared to be an active process and not the result of simple adsorption of the antibody on lymphocyte MHC molecules, thus acting like a sink. These results suggest that studies on recipient immune factors, particularly related to lymphocyte subsets, may reveal potential recipient mechanisms that either reduce TRALI symptoms or, perhaps more importantly, identify those recipients that may be more susceptible to TRALI reactions.
Methods
Mice
Male BALB/c (H-2d, BALB/cAnNCrl) mice and CB.17 (H-2d, CB17/Icr-Prkdcscid/IcrCrl) severe combined immunodeficient (SCID) mice, 6 to 12 weeks of age, were obtained from Charles River Laboratories. All animal studies were approved by the St Michael's Hospital Animal Care Committee (protocol no. 108). SCID mice were bled before the experiments, and sera were screened for the presence of murine IgG by an enzyme-linked immunosorbent assay (ELISA) as previously described23 ; any mouse with a serum IgG concentration greater than 50 μg/mL was deemed “leaky” and killed.
MHC I mAb (34-1-2s) production and purification
The hybridoma 34-1-2s produces a mAb (IgG2a, κ) against H-2Kd and H-2Dd MHC class I molecules and was purchased from the ATCC. The mAb recognizes a monomorphic region of the MHC class I α3 domain (independently of MHC/β2 microglobulin association) and also cross-reacts with MHC class I molecules from the b, q, s, and r strains of inbred mice.24-26 The 34-1-2s antibody induces TRALI-like symptoms independently of complement as it causes TRALI in C5-deficient DBA mice (E.R.S., J.W.S., unpublished data, July 2009). The hybridoma was grown in protein-free hybridoma medium, PFHM II (Invitrogen) in CELLine flasks (BD Biosciences) and incubated at 37°C and 5% CO2. Hybridoma supernatant containing the mAb was collected and filtered through a 0.2-μm filter. The mAb solution was then purified by protein A Sepharose affinity chromatography, concentrated, and dialyzed in phosphate-buffered saline (PBS; pH 7.4). The final concentration of the high purity mAb was adjusted to 5 mg/mL as determined by Bio-Rad protein assay. Flow cytometric studies confirmed that active binding of 34-1-2s remained intact during the purification.
TRALI induction
BALB/c or SCID mice were weighed and then challenged intravenously via tail vein injection with the indicated titrations (0.45, 2.3, 4.5, 9.1, and 22.7 mg/kg) of 34-1-2s mAb or PBS. Various physiologic measurements were then performed at the indicated time points.
Temperature measurements
Rectal temperatures were measured as an indication of systemic shock at 30-minute intervals up to 120 minutes after mAb infusion using a digital, blunt-tipped stem thermometer (VWR International).
Wet-to-dry lung weight ratio
As a measure of pulmonary edema, wet-to-dry lung weight ratios were determined. Mice were killed by cervical dislocation at the indicated times after 34-1-2s mAb infusion, and the lungs were removed, weighed (wet weight), and then dried in an oven at 60°C for at least 48 hours and then weighed for dry weight. The wet-to-dry weight ratio was calculated as: net wet weight/net dry weight.
MIP-2 measurements
Blood was collected from the indicated mice, and sera was generated on ice and tested for the presence of the CXCL2 chemokine Macrophage Inflammatory Protein 2 (MIP-2) using an ultra-sensitive commercial solid-phase ELISA kit (Mouse CXCL2/MIP-2 Quantikine ELISA Kit; R&D Systems, Cedarlane Laboratories). The MIP-2 assay had a sensitivity of more than 1.5 pg/mL.
Acute lung injury measurements
To characterize the magnitude of lung injury, oxygenation monitoring, lung function measurements, and intravital microscopy were preformed. Mice were anesthetized with ketamine (200 mg/kg intraperitoneally, Bimeda MTC Animal Health) and xylazine (10 mg/kg intraperitoneally; Bayer) and placed in the supine position on a homeothermic blanket (Harvard Apparatus). Body temperature was maintained at 37°C with a feedback coupled rectal thermoprobe. After tracheotomy, mice were intubated with a polyethylene tube (Portex FineBore Polythene Tubing, 0.58 mm ID/0.96 mm OD; Smiths Medical International) and ventilated with room air at 100 breaths/minute (tidal volume of 10 mL/kg at a positive end-expiratory pressure of 2 cmH2O, flexiVent; Scireq). Arterial oxygenation was monitored continuously by pulse oximetry (MouseOx; Starr Life Sciences). Respiratory mechanics were assessed with the flexivent system by use of the forced oscillation technique27 and subsequent fitting of the acquired impedance data to the constant phase model,28 allowing for direct measurements of inspiratory capacity, pressure-volume relationships, Newtonian resistance of the central airways (Rn), quasi-static compliance (Cst), and tissue damping (G; ie, energy dissipation of lung tissue [tissue resistance]) in the ventilated mouse. After baseline recordings, 150 μg of 34-1-2s Ab was infused via a catheter (Portex FineBore Polythene Tubing, 0.28 mm ID/0.61 mm OD; Smiths Medical International) positioned in the right external jugular vein. Measurements of arterial oxygenation and respiratory mechanics were repeated at the indicated time points after mAb infusion. Lung intravital microscopy was performed in a separate set of experiments as previously described.29,30 Briefly, mice were anesthetized with medetomidine (0.5 mg/kg intraperitoneally, Domitor; Dr E. Graeub AG), fentanyl (0.05 mg/kg intraperitoneally; Janssen Cilag), and midazolam (5 mg/kg intraperitoneally, Dormicum; Roche), tracheotomized, intubated, and ventilated with room air at 100 breaths/minute with end-inspiratory and end-expiratory pressures of 11 and 1 cmH2O, respectively (Animal Respirator Compact 4600 Series; TSE Systems). Visual access to the surface of the upper right lung lobe was obtained by preparation of a thoracic window. The window was resealed with a transparent membrane, and intrathoracic air was removed via a transdiaphragmally placed intrapleural catheter. Mice were positioned under an upright microscope (Axiotechvario 100HD; Carl Zeiss), and subpleural alveoli were visualized during the expiratory plateau phase using dark-field illumination (Maxxvison), imaged by a silicone-intensified tube camera (CF 8/4 FMC; Kappa), and digitally recorded (DVCAM, DSR-25; Sony Deutschland). For measurement of interstitial thickness, areas of interest (AOI) encompassing 5 to 7 alveoli were defined by anatomic landmarks. Areas of individual alveoli within each AOI were planimetrically measured (ImageJ software, Version 1.36b; National Institutes of Health, Bethesda, MD), and the fraction of interstitial space reflecting the amount of interstitial thickening was calculated as area of the AOI/(area of the AOI − sum of the areas of the individual alveoli within the AOI). Intravital microscopic images were obtained at baseline and 30 minutes after iv infusion of 150 μg of 34-1-2s Ab.
Pulmonary neutrophil accumulation
Mice were anaesthetized using avertin (2% final in PBS), and the chest cavity was exposed, the lung lobes excised, homogenized, and filtered through a 40-μm cell strainer (BD Biosciences). The cell suspension was then washed with PBS (Ca++/Mg++-free; Invitrogen) and centrifuged at 280g for 7 minutes at 4°C. Red blood cells in the preparation were removed by ammonium chloride/potassium lysis solution (0.15M NH4Cl, 10mM KHCO3, Na2-ethylenediaminetetraacetic acid, pH 7.2-7.4). The cell preparation was then washed twice with cold PBS, and cells were mounted on microscope slides using a Shandon Cytospin 4 (Thermo Electron) and stained with a hematoxylin and eosin kit (Harleco-Hemacolor; EMD Chemicals). Polymorphonuclear leukocytes (PMNs) were enumerated and calculated as a percentage of total nucleated cells.
Lung histology
For lung histology, lungs were removed and fixed in 10% formalin, paraffin embedded, and sectioned by a microtome. The sections were stained with hematoxylin and eosin (Harleco/Surgipath) and mounted on microscope slides by the Pathology Diagnostic Laboratories at St Michael's Hospital. The sections were examined under Permount using a Nikon Eclipse E800 equipped with a 40×/0.75 oil objective lens and images were taken with a Nikon DXM1200 digital camera and acquired with Nikon ACT-1 (v2.70) software.
Lymphocyte production and adoptive transfers
BALB/c splenocytes were produced for adoptive transfer of lymphocyte populations into SCID mouse recipients. Briefly, spleens were removed from BALB/c mice, homogenized into a cell suspension, layered over a Percoll (GE Healthcare) density gradient (52%, 69%, and 78%), and centrifuged at 1660g for 30 minutes. Mononuclear lymphocytes were harvested from the 69%/78% interface, and the lymphocytes were washed twice. To deplete the indicated lymphocyte populations, the mononuclear lymphocytes were applied to the EasySep Magnetic cell sorting kit (Stem Cell Technologies) to extract CD4+CD8+ and/or CD19+ lymphocytes. The depletion efficiencies were determined to be more than 95% by flow cytometry. SCID mice were reconstituted by injecting 20 × 106 cells isolated cell populations intravenously, via the tail vein immediately followed by a 2.3-mg/kg dose 34-1-2s in the opposite tail vein.
Statistical analysis
Significance between means was determined by Student t test.
Results
34-1-2s caused significant hypothermia in SCID mice
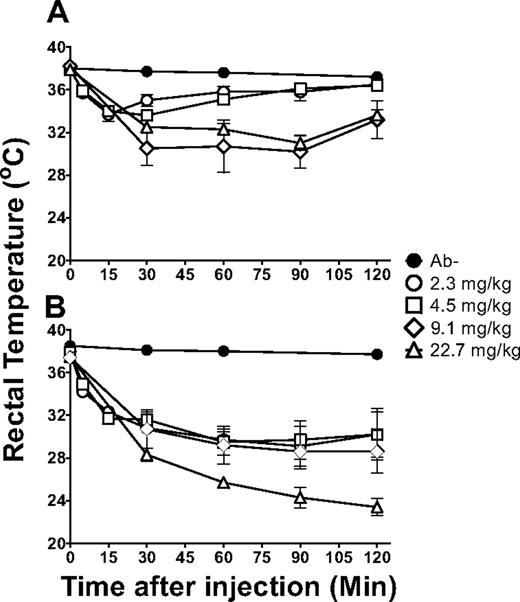
Rectal temperatures were monitored as a measure of systemic shock induced by the 34-1-2s infusions. When BALB/c mice were administered titrations of 34-1-2s, rectal temperatures decreased within 5 minutes after infusion and reached nadirs within 30 minutes in a dose-dependent fashion (Figure 1A). Maximal reductions were observed by 90 minutes after infusion, but within 2 hours, however, the rectal temperatures began to recover (Figure 1A). In contrast, compared with BALB/c mice, when SCID mice were infused with titrations of 34-1-2s, a significant reduction in rectal temperatures was observed (Figure 1B). The temperature reduction in the infused SCID mice was maximal by 90 minutes after infusion but did not recover during the experimental time points (Figure 1B).
34-1-2s induced severe hypothermia in SCID mice. Rectal temperatures over time in (A) BALB/c mice and (B) SCID mice either not injected (naive, ●) or injected with 2.3 mg/kg (○), 4.5 mg/kg (□), 9.1 mg/kg (◊), or 22.7 mg/kg (▵) of 34-1-2s mAb. There were 5 to 25 mice per group. Data are mean ± SD temperatures.
34-1-2s induced severe hypothermia in SCID mice. Rectal temperatures over time in (A) BALB/c mice and (B) SCID mice either not injected (naive, ●) or injected with 2.3 mg/kg (○), 4.5 mg/kg (□), 9.1 mg/kg (◊), or 22.7 mg/kg (▵) of 34-1-2s mAb. There were 5 to 25 mice per group. Data are mean ± SD temperatures.
34-1-2s caused mortality in SCID mice but not in BALB/c mice
When BALB/c mice were injected with 34-1-2s, there was no mortality observed. In contrast, when SCID mice were infused with the antibody, a dose-dependent mortality was noted; within 60 minutes after mAb infusion, high doses (22.7 mg/kg) of the antibody induced 40% mortality (Figure 2).
34-1-2s caused mortality only in SCID mice. Kaplan-Meier survival plot of SCID mice (n > 5 for each dose group) injected with the indicated does of 34-1-2s mAb. No mortality occurred in BALB/c mice (N = 21). Results are presented for SCID mice injected with 2.3 mg/kg (○), 4.5 mg/kg (□), 9.1 mg/kg (◊), or 22.7 mg/kg (▵) of 34-1-2s mAb.
34-1-2s caused mortality only in SCID mice. Kaplan-Meier survival plot of SCID mice (n > 5 for each dose group) injected with the indicated does of 34-1-2s mAb. No mortality occurred in BALB/c mice (N = 21). Results are presented for SCID mice injected with 2.3 mg/kg (○), 4.5 mg/kg (□), 9.1 mg/kg (◊), or 22.7 mg/kg (▵) of 34-1-2s mAb.
34-1-2s induced quicker pulmonary neutrophil accumulation and serum levels of MIP-2 in SCID mice
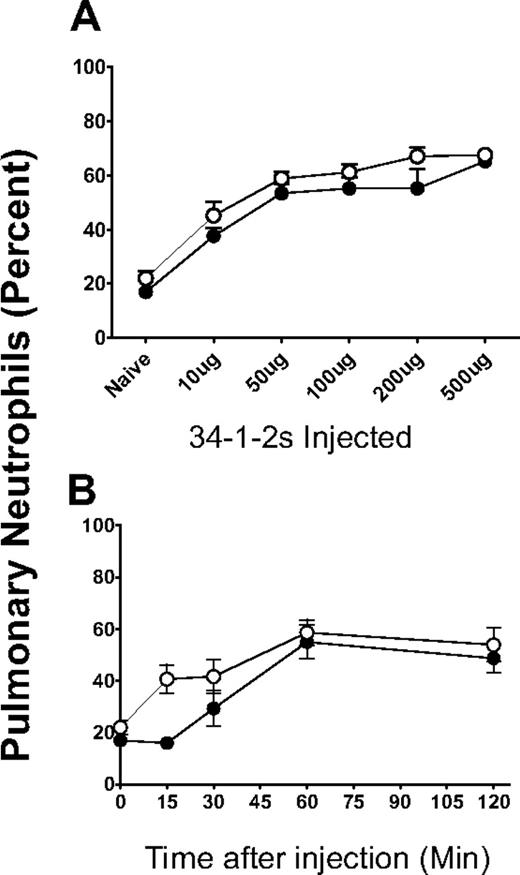
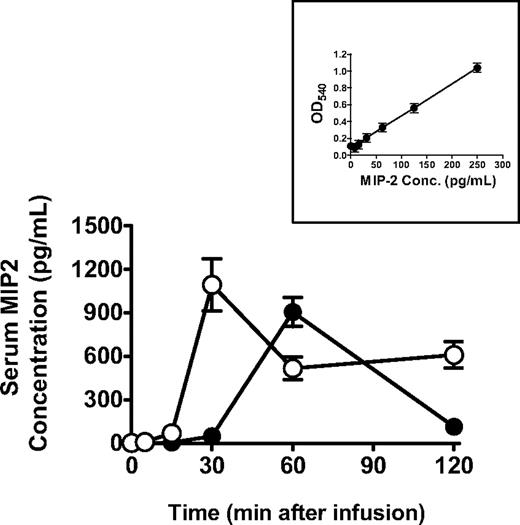
Pulmonary neutrophil accumulation induced by 34-1-2s was measured and showed that, although both BALB/c and SCID mice had significant increases in neutrophils within the lungs after mAb infusion (Figure 3A), the accumulation within SCID mouse lungs began within 5 minutes compared with BALB/c mice (Figure 3B). Correlated with these findings were the observation that levels of serum MIP-2, the murine equivalent to human interleukin-8, occurred more quickly compared with BALB/c mice and the levels remained high throughout the protocol (Figure 4).
34-1-2s induced a concentration-dependent pulmonary neutrophil accumulation. (A) BALB/c mice (●, N > 5) or SCID mice (○, N > 5) were injected with the indicated does of 34-1-2s mAb; and at 2 hours after injection, the mice were killed and their lungs were homogenized and the cells were loaded on to microslides by a cytospin and stained with hematoxylin and eosin. (B) BALB/c mice (●, N > 5) or SCID mice (○, N > 5) were injected with a 2.3-mg/kg dose of 34-1-2s mAb (determined from panel A; 50 μg); and at the indicated times, the mice were killed and their lungs were homogenized and the cells were loaded on to microslides by a cytospin and stained with hematoxylin and eosin. Data in both panels are percentage neutrophils counted within total nucleated cells.
34-1-2s induced a concentration-dependent pulmonary neutrophil accumulation. (A) BALB/c mice (●, N > 5) or SCID mice (○, N > 5) were injected with the indicated does of 34-1-2s mAb; and at 2 hours after injection, the mice were killed and their lungs were homogenized and the cells were loaded on to microslides by a cytospin and stained with hematoxylin and eosin. (B) BALB/c mice (●, N > 5) or SCID mice (○, N > 5) were injected with a 2.3-mg/kg dose of 34-1-2s mAb (determined from panel A; 50 μg); and at the indicated times, the mice were killed and their lungs were homogenized and the cells were loaded on to microslides by a cytospin and stained with hematoxylin and eosin. Data in both panels are percentage neutrophils counted within total nucleated cells.
34-1-2s induces a quicker serum MIP-2 response in SCID mice. BALB/c mice (●, N = 5) or SCID mice (○, N = 5) were injected with 2.3 mg/kg 34-1-2s mAb, and MIP-2 levels were measured in the sera of the mice at the indicated times by commercial ELISA. Data are mean ± SEM MIP-2 concentration from 5 mice in each group. (Inset) MIP-2 standard curve.
34-1-2s induces a quicker serum MIP-2 response in SCID mice. BALB/c mice (●, N = 5) or SCID mice (○, N = 5) were injected with 2.3 mg/kg 34-1-2s mAb, and MIP-2 levels were measured in the sera of the mice at the indicated times by commercial ELISA. Data are mean ± SEM MIP-2 concentration from 5 mice in each group. (Inset) MIP-2 standard curve.
34-1-2s caused significant lung injury in SCID mice but not BALB/c mice
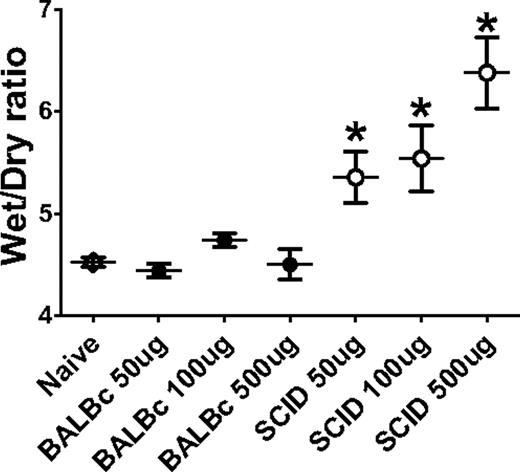
Postmortem measurement of wet-to-dry lung weight ratios were used to determine extravascular pulmonary fluid, an indicator of pulmonary edema. The wet-to-dry lung weight ratios of BALB/c mice infused with titrations of 34-1-2s did not significantly change over the 2 hours after mAb infusion (Figure 5). In contrast, however, significant increases in wet-to-dry ratios were observed in SCID mice infused with titrations of 34-1-2s (Figure 5).
34-1-2s induces a concentration-dependent lung edema in SCID mice. BALB/c mice (●, N > 5) or SCID mice (○, N > 5) were injected with the indicated does of 34-1-2s mAb; and at 2 hours after injection, the mice were killed and lung wet-to-dry ratios were measured. Data are the wet-to-dry ratio at 2 hours after mAb infusion.
34-1-2s induces a concentration-dependent lung edema in SCID mice. BALB/c mice (●, N > 5) or SCID mice (○, N > 5) were injected with the indicated does of 34-1-2s mAb; and at 2 hours after injection, the mice were killed and lung wet-to-dry ratios were measured. Data are the wet-to-dry ratio at 2 hours after mAb infusion.
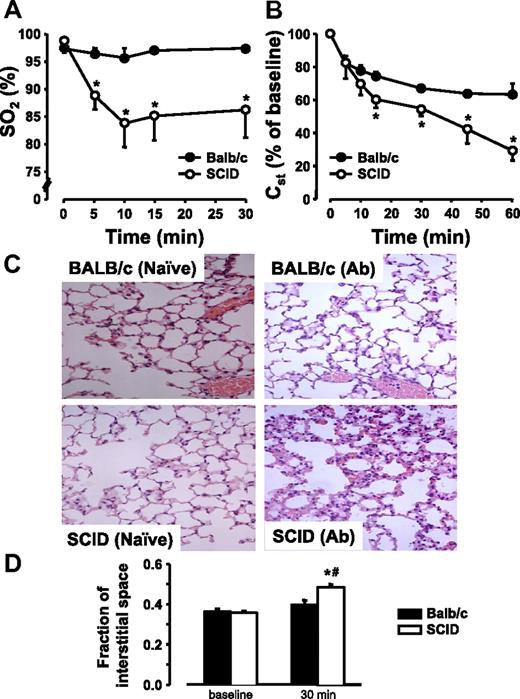
To visualize the lung damage induced by 34-1-2s antibody infusions, BALB/c mice and SCID mice were paired and prepared for pulmonary oxygenation measurements and intravital microscopy. BALB/c mice infused with 34-1-2s did not have any change in saturated oxygen (SO2) levels over time (Figure 6A). In contrast, however, SCID mice infused with antibody displayed significant reductions in SO2 levels within 5 minutes after antibody administration (Figure 6A). Similarly, although Cst was reduced in BALB/c mice after antibody infusion (Figure 6B), SCID mice had significantly more pronounced reductions in pulmonary compliance within 10 minutes after antibody infusion (Figure 6B). Likewise, inspiratory capacity and the total volume inspired during an increase in airway pressure from 2 to 30 mmHg (V) were much more reduced by 34-1-2s in SCID compared with BALB/c mice, whereas tissue resistance (G) was increased (Table 1). Central airway resistance (Rn) was higher in SCID compared with BALB/c at baseline but did not decrease markedly in response to 34-1-2s in either strain (Table 1). Histologic analyses revealed moderate interstitial leukocytosis in antibody-infused BALB/c mice. In contrast, lungs of 34-1-2s–treated SCID mice showed significant interstitial accumulations of leukocytes and concomitant septal thickening indicative of interstitial edema (Figure 6C). In line with this notion, the analysis of intravital microscopic recordings (supplemental Figure 2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) revealed that the fraction of pulmonary interstitial space was significantly increased in the antibody-infused SCID mice compared with BALB/c mice by 30 minutes after administration of 34-1-2s (Figure 6D).
34-1-2s causes significant lung damage in SCID mice. BALB/c mice (●, N = 5) or SCID mice (○, N = 5) were injected with 2.3 mg/kg 34-1-2s mAb and (A) SO2 levels, and (B) Cst were determined at the indicated time points. Histologic micrographs (C; original magnification ×40, see “Lung histology”) reveal moderate leukocyte infiltration in BALB/c mice but marked cellular infiltration and septal thickening in SCID mice 2 hours after infusion of 34-1-2s mAb (500 μg). Interstitial space fraction (D) as a quantitative measure of septal thickening was determined intravital microscopic recordings in BALB/C and SCID mice at baseline and 30 minutes after infusion of 34-1-2s mAb (50 μg). Representative dark-field images are given in supplemental Figure 2.
34-1-2s causes significant lung damage in SCID mice. BALB/c mice (●, N = 5) or SCID mice (○, N = 5) were injected with 2.3 mg/kg 34-1-2s mAb and (A) SO2 levels, and (B) Cst were determined at the indicated time points. Histologic micrographs (C; original magnification ×40, see “Lung histology”) reveal moderate leukocyte infiltration in BALB/c mice but marked cellular infiltration and septal thickening in SCID mice 2 hours after infusion of 34-1-2s mAb (500 μg). Interstitial space fraction (D) as a quantitative measure of septal thickening was determined intravital microscopic recordings in BALB/C and SCID mice at baseline and 30 minutes after infusion of 34-1-2s mAb (50 μg). Representative dark-field images are given in supplemental Figure 2.
Respiratory mechanics in 34-1-2s-treated BALB/c and SCID mice
| . | BALB/c . | SCID . | ||
|---|---|---|---|---|
| 0 minutes . | 60 minutes . | 0 minutes . | 60 minutes . | |
| IC/bw, mL/kg | 42.8 ± 1.15 | 40.0 ± 2.25 | 39.1 ± 4.35 | 26.8 ± 5.4*† |
| V, μL | 949 ± 555 | 876 ± 75* | 793 ± 12† | 469 ± 12*† |
| G, cmH2O/mL | 4.30 ± 0.47 | 4.19 ± 0.28 | 5.65 ± 1.43 | 9.03 ± 2.7*† |
| Rn, cmH2O · s/mL | 0.45 ± 0.07 | 0.40 ± 0.02 | 50.64 ± 0.07† | 0.59 ± 0.14† |
| . | BALB/c . | SCID . | ||
|---|---|---|---|---|
| 0 minutes . | 60 minutes . | 0 minutes . | 60 minutes . | |
| IC/bw, mL/kg | 42.8 ± 1.15 | 40.0 ± 2.25 | 39.1 ± 4.35 | 26.8 ± 5.4*† |
| V, μL | 949 ± 555 | 876 ± 75* | 793 ± 12† | 469 ± 12*† |
| G, cmH2O/mL | 4.30 ± 0.47 | 4.19 ± 0.28 | 5.65 ± 1.43 | 9.03 ± 2.7*† |
| Rn, cmH2O · s/mL | 0.45 ± 0.07 | 0.40 ± 0.02 | 50.64 ± 0.07† | 0.59 ± 0.14† |
Respiratory mechanics in BALB/c and SCID mice as analyzed by the flexivent technique at baseline (0 minutes) and 60 minutes after induction of TRALI by infusion of 34-1-2s (150 μg). Data from n = 5 each.
P < .05 vs BALB/c.
P < .05 vs baseline.
SCID mice reconstituted with purified T lymphocytes populations were protected from severe TRALI
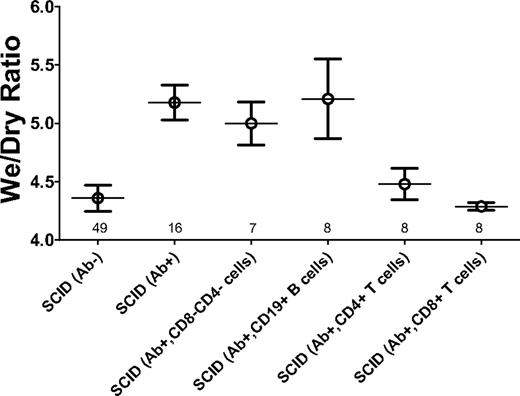
We analyzed whether the absence of T and/or B cells in the SCID mice was responsible for the severe pulmonary damage observed. CD19+ B cells, CD4+ T cells, or CD8+ T cells were purified from BALB/c spleens and infused into SCID mice before administration of 34-1-2s. Transfer of CD19+ B cells did not significantly alter the induction of hypothermia (Figure 7) in the SCID mice, nor did they change the lung damage as measured by lung wet-to-dry ratios (Figure 8). In contrast, transfer of CD8+ T cells significantly reduced the ability of 34-1-2s to induce hypothermia in the SCID mice (Figure 7). In addition, CD8+ T cells and, to a lesser extent, purified CD4+ T cells, significantly reduced the ability of 34-1-2s to induce lung damage as measured by wet-to-dry weight ratios (Figure 8).
CD8+ T cells relieve 34-1-2 mAb-induced hypothermia in SCID mice. Rectal temperatures over time (minutes) in control mice receiving nothing (●, N = 10) or SCID mice injected with 2.3 mg/kg 34-1-2s alone (○, N = 20) or SCID mice receiving antibody and a transfer of 2 × 107 purified CD8+ T cells (▵, N = 6), CD4+ T cells (▿, N = 7), purified CD19+ B cells (◊, N = 6), or splenocytes depleted of both CD4+ and CD8+ T cells (▴, N = 6). Temperatures in BALB/c mice receiving only antibody (□, N = 21) are also shown.
CD8+ T cells relieve 34-1-2 mAb-induced hypothermia in SCID mice. Rectal temperatures over time (minutes) in control mice receiving nothing (●, N = 10) or SCID mice injected with 2.3 mg/kg 34-1-2s alone (○, N = 20) or SCID mice receiving antibody and a transfer of 2 × 107 purified CD8+ T cells (▵, N = 6), CD4+ T cells (▿, N = 7), purified CD19+ B cells (◊, N = 6), or splenocytes depleted of both CD4+ and CD8+ T cells (▴, N = 6). Temperatures in BALB/c mice receiving only antibody (□, N = 21) are also shown.
Purified CD4+ and CD8+ T cells rescue 34-1-2 mAb-induced lung damage. Wet-to-dry ratios of the lungs from control SCID mice receiving nothing (Ab−) or in SCID mice 2 hours after being injected with 2.3 mg/kg 34-1-2s alone (Ab+) or in SCID mice receiving antibody (Ab+) and a transfer of either 2 × 107 splenocytes depleted of both CD4+ and CD8+ T cells (CD4−CD8−) or purified CD19+ B cells, CD4+ T cells, or CD8+ T cells. Numbers above the x-axis refer to the number of mice in each group studied.
Purified CD4+ and CD8+ T cells rescue 34-1-2 mAb-induced lung damage. Wet-to-dry ratios of the lungs from control SCID mice receiving nothing (Ab−) or in SCID mice 2 hours after being injected with 2.3 mg/kg 34-1-2s alone (Ab+) or in SCID mice receiving antibody (Ab+) and a transfer of either 2 × 107 splenocytes depleted of both CD4+ and CD8+ T cells (CD4−CD8−) or purified CD19+ B cells, CD4+ T cells, or CD8+ T cells. Numbers above the x-axis refer to the number of mice in each group studied.
Discussion
A previous report of 34-1-2s–induced TRALI in a murine model demonstrated that the antibody caused TRALI reactions in male BALB/c mice characterized by hypothermia, increased pulmonary permeability, pulmonary pathology, and mortality.31 It was also shown that recipient granulocytes and leukotrienes significantly contributed to the lung pathology.31 Subsequently, Looney et al22 demonstrated the central role played by neutrophil Fcγ receptors (R) in mediating this mAb lung injury. The results of the present study additionally reveal that recipient CD8+ and CD4+ T cells significantly reduce severe TRALI reactions.
In contrast to Looney et al,22 our results with BALB/c mice showed that, whereas 34-1-2s mAb induced significant neutrophil accumulation within the lungs, even high concentrations (22.7 mg/kg) of the mAb did not cause significant lung damage or mortality (Figures 2, 4). The reasons for this are unclear but may relate to the environmental conditions in which the mice are raised. Looney et al32 have recently shown that, when mice are moved from standard vivarium conditions into germ-free housing, the ability of 34-1-2s to induce TRALI reactions was lost. Our results do not appear to be an isolated case as it has also been shown by others that 34-1-2s induces only mild TRALI reactions in male BALB/c mice.33 Thus, the possible reasons for differences in observations between the various in vivo murine models of TRALI using 34-1-2s may relate to environmental conditions (eg, murine gut flora).34 Nonetheless, it is clear that 34-1-2s can induce a spectrum of TRALI reactions in male BALB/c mice from mild to severe depending on several factors, including mAb dose and environmental factors.
The absence of any significant lung damage in BALB/c mice in our hands led us to study whether recipient factors, such as lymphocytes, might influence the severity of TRALI reactions. Because neutrophil activation is known to be a key effector cell response in the pathogenesis of TRALI and SCID mice contain normal levels of neutrophils compared with BALB/c mice, we expected to observe TRALI in both mouse strains.11,12 Instead, we observed that, compared with BALB/c mice, infusion of the 34-1-2s antibody induced significantly more severe hypothermia (Figure 1), mortality (Figure 2), granulocyte accumulation (Figure 3), and lung injury (Figures 4, 6). Because 34-1-2s induced similar amounts of PMN accumulation in both BALB/c and SCID mice, we hypothesized that the absence of lymphocytes in SCID mice made them more susceptible to antibody-mediated TRALI. This was confirmed by demonstrating that, when the SCID mice were reconstituted with purified lymphocyte populations and then challenged with 34-1-2s, there was a differential ability of the different lymphocyte populations to modulate TRALI reactions. The adoptive transfer of purified CD19+ B cells did not significantly affect the mAb's ability to induce TRALI reactions. In contrast, only purified CD8+ T cells or CD4+ T cells were able to protect SCID mice against 34-1-2s–mediated lung damage (Figures 7, 8). The mechanisms responsible for the ability of T cells to modulate TRALI reactions are unclear, but they did not relate to simple adsorption of the antibody onto the lymphocytes making them act as an antibody sink. This was confirmed by demonstrating that splenocytes, depleted of both CD4+ and CD8+ T-cell populations, had significantly higher binding reactivity with 34-1-2s (supplemental Figure 1) but nonetheless failed to reduce the severe TRALI reactions (Figure 8).
There is evidence that recipient T cells have a role to play in other inflammatory lung injury models, although earlier reports demonstrated an apparent controversy whether these cells are involved. For example, horses treated with antithymocyte globulin exhibited an acute respiratory distress syndrome,35 whereas lethal fibroinflammatory lung injury induced in mice by bleomycin treatment was markedly reduced by antagonizing CD3+ T cells in vivo.36 In contrast, however, Morris et al37 showed that neutrophil accumulation in lipopolysaccharide-treated nude mice was not different from that of BALB/c mice, and they suggested that T cells may not have a role in the resolution of endotoxin-induced acute lung injury. On the other hand, Majeski et al38 showed that the murine acute respiratory distress syndrome mediated by reovirus was critically dependent on T cells and their ability to secret interferon-γ. They speculated that removing T cells from the mice would reduce the lung damage induced by the infectious insult.
More recently, however, several reports have suggested a significant role for T cells in lung injury models. CD4+ regulatory T cells have been shown to be important in controlling PMN recruitment in a murine model of chronic lung injury,39 whereas others have demonstrated that αβ T cells could mediate cecal puncture-induced inflammatory lung injury.40 Furthermore, Sekine et al41 have recently demonstrated that interleukin-18 secreted by Th1 cells plays a significant role in modulating the lung injury associated with acute graft-versus-host disease. The results of our study further implicate recipient T cells in playing an important role in reducing the pathogenesis of antibody-mediated TRALI. It is possible that T cells mediate their suppressive effects by direct interactions with neutrophils where mAb-armed neutrophils bind to T cells via an antibody bridge and the T cells inactivate the neutrophils in a reverse cytolytic pathway, the so-called veto effect.42 On the other hand, there is increasing evidence of the colocalization of neutrophils and T cells at sites of persistent infections, chronic inflammation, or sites of tumors.43 For example, it has been demonstrated that both CD4+ and CD8+ T cells can contribute to the delayed cell death of neutrophils via the production of cytokines, particularly IFN-γ, which is known to extend the life span of neutrophils.44 Our current data may be the first to suggest that T cells may actively suppress neutrophil activation in antibody-mediated TRALI. We are currently studying this.
In conclusion, we have demonstrated that the presence of recipient CD4+ and CD8+ T cells reduces the severity of antibody-mediated TRALI by actively inhibiting neutrophil-mediated lung damage. These results suggest that understanding how lymphocytes may modulate neutrophil activity may identify recipient factors that may be predictive of whether a severe TRALI response will take place.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Alan H. Lazarus (St Michael's Hospital) for his helpful discussions and critical review of the manuscript.
This work was supported by a Canadian Institutes of Health Research/Canadian Blood Services Priority Research Grant.
Authorship
Contribution: Y.L.F. designed research, performed and supervised all experiments, collected, analyzed, and interpreted data, performed statistical analysis, and wrote the first draft of the manuscript; M.K., A.T., R.A., E.R.S., and L.C. performed experiments and collected, analyzed, and interpreted data; W.M.K. and J.F. designed research, edited the manuscript, and contributed animals; and J.W.S. provided financial resources, designed research, analyzed and interpreted data, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John W. Semple, St Michael's Hospital, 30 Bond St, Toronto, ON, Canada, M5B 1W8; e-mail: semplej@smh.toronto.on.ca.