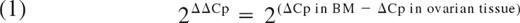Abstract
Ovarian tissue cryopreservation is currently proposed to young cancer patients to preserve their fertility before radiochemotherapy. The potential risk is that the tissue might harbor malignant cells that could induce disease recurrence. We therefore decided to evaluate the presence of leukemic cells in cryopreserved ovarian tissue from 18 leukemic patients: 6 with chronic myeloid leukemia (CML) and 12 with acute lymphoblastic leukemia (ALL). In each case, histology, quantitative reverse-transcribed polymerase chain reaction (RT-PCR) and long-term (6 months) xenografting to immunodeficient mice were used. Histology did not identify any malignant cells in the ovarian tissue. By quantitative RT-PCR, 2 of 6 CML patients were positive for BCR-ABL in their ovarian tissue. Among the 12 ALL patients, 7 of the 10 with available molecular markers showed positive leukemic markers in their ovarian tissue (translocations or rearrangement genes). Four mice grafted with ovarian tissue from ALL patients developed intraperitoneal leukemic masses. In conclusion, this study demonstrates, by quantitative RT-PCR, ovarian contamination by malignant cells in acute as well as chronic leukemia, whereas histology fails to do so. Moreover, chemotherapy before ovarian cryopreservation does not exclude malignant contamination. Finally, reimplantation of cryopreserved ovarian tissue from ALL and CML patients puts them at risk of disease recurrence.
Introduction
Advances in the diagnosis and treatment of childhood, adolescent, and adult cancer have greatly increased the life expectancy of young women with cancer. Unfortunately, aggressive chemotherapy and radiotherapy generally result in the loss of both endocrine and reproductive functions, leading to premature menopause and infertility (for review, see Donnez et al1 ). Before such potentially sterilizing treatments, it is our duty to discuss fertility preservation options with these young women.
Three nonexclusive methods can be proposed to preserve female fertility: in vitro fertilization with embryo cryopreservation, oocyte cryopreservation, and ovarian tissue cryopreservation. In vitro fertilizations with embryo cryopreservation or mature oocyte cryopreservation are applicable options if chemotherapy can be delayed (time required for ovarian stimulation). Immature oocyte retrieval in the luteal phase, followed by in vitro maturation, has been proposed to cancer patients if there is not sufficient time for conventional follicular phase oocyte retrieval in a stimulated cycle before chemotherapy.2 So far, however, no pregnancies have been reported with this technique in cancer patients. For women who cannot delay the start of chemotherapy and for prepubertal patients, cryopreservation of ovarian tissue is the main option available to preserve their fertility before cancer treatment.
Hematologic malignancies are the most frequent indication for ovarian tissue cryopreservation in our department and represent 44% of all malignant indications. Among them, Hodgkin lymphoma accounts for 22% of cases, followed by leukemia (11.3%), and non-Hodgkin lymphoma (11%).
The prospect of reversing treatment-related premature ovarian failure using autotransplantation of frozen-thawed ovarian tissue harvested before chemo-radiotherapy is becoming increasingly real, with 11 live births already reported with this technique.3-9 It is expected that, in the near future, more and more cancer patients cured of their disease will request reimplantation of cryopreserved ovarian tissue.1 However, very little has been published on the safety of ovarian tissue transplantation in cancer patients.10
One major concern raised by the use of ovarian cortical fragments in cancer patients is the potential risk that the frozen-thawed ovarian tissue might harbor malignant cells that could induce a recurrence of the disease after reimplantation. In the case of leukemia, malignant cells may be present in the bloodstream and at risk of being transferred.
We therefore decided to conduct a study to evaluate the presence of leukemic cells in cryopreserved human ovarian tissue from patients with chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL). Detection of minimal residual disease in ovarian tissue was carried out by quantitative reverse-transcribed polymerase chain reaction (RT-PCR) and long-term xenografting (6 months) to immunodeficient mice.
Methods
Patients
Use of human tissue for this study was approved by the Institutional Review Board of the Université Catholique de Louvain. Frozen-thawed ovarian tissue was issued from 18 leukemic patients: 6 with CML and 12 with ALL. Patients were between 2 and 31 years of age when their ovarian tissue was cryopreserved (1999-2008). The mean age of patients with ALL was 14.5 years and with CML 24.7 years.
For each patient, the specific cytogenetic abnormality or gene rearrangement present in the blood or bone marrow at diagnosis was tested in the frozen-thawed ovarian tissue by PCR.
For this study, one cryovial of ovarian tissue per patient was thawed after obtaining informed consent. The amount of tissue thawed represented less than 10% of the total amount of cryobanked ovarian cortical tissue, and minimal residual disease testing of ovarian tissue from leukemic patients was considered a mandatory safety measure before any future transplantation could be contemplated. Part of the thawed tissue was assigned to PCR analysis and the rest to xenotransplantation, as detailed in “Transplantation to SCID mice.”
All the CML patients (n = 6) were carriers of the BCR-ABL1 gene (with a major breakpoint [M-BCR], which produces the p210 gene product). CML is characterized by the Philadelphia chromosome, which is the result of reciprocal translocation t(9;22)(q34;q11.2) that unites the ABL1 gene from chromosome 9 with the BCR gene from chromosome 22, creating a fusion gene known as BCR-ABL1. Tumor-specific breakpoint cluster region/proto-oncogene tyrosine protein kinase ABL1 (BCR-ABL1) transcripts can be detected using quantitative RT-PCR.10,11
ALL patients (n = 12) were carriers of various mutations, reciprocal chromosomal translocations, hyperdiploidy or hypodiploidy. Two patients showed specific translocations, one t(9;22)(q34;q11.2) and one t(1;19)(q23;p13.3), that could be assessed by quantitative RT-PCR. The patient with t(9;22) had a minor breakpoint (m-BCR), which produces the p190 gene product.11 The t(1;19) translocation leads to a chimeric gene as a result of fusion of the PBX1 and E2A genes from chromosomes 1 and 19, respectively.12 In 8 cases, immunoglobulins and/or T-cell receptor (TCR-γ) rearrangement genes were tested in the ovarian tissue by molecular genetic analysis.13 In the 2 remaining ALL patients, no molecular markers of disease were found and no molecular genetic analysis could be performed on their ovarian tissue.
Freezing and thawing procedures
Freezing of ovarian tissue was undertaken according to the protocol described by Gosden et al.14 Biopsy samples were immediately transferred to the laboratory in Leibovitz L-15 medium supplemented with Glutamax (Invitrogen); in the laboratory, the remaining medullar tissue was gently removed. The cortical samples were cut into small strips (± 4 × 1-2 mm). For each patient, one piece was fixed in 4% formaldehyde for histologic processing. The other fragments were suspended in cryoprotective medium and then placed into precooled 2-mL cryogenic vials (Simport) filled with Leibovitz (L-15) medium supplemented with 4 mg/mL of human serum albumin (Red Cross) and 1.5M of dimethyl sulfoxide (Sigma-Aldrich). The cryotubes were cooled in a programmable freezer (Kryo 10, Series III; Planer) with the following program: (1) cooled from 0°C to −8°C at −2°C/min; (2) seeded manually by touching the cryotubes with forceps prechilled in liquid nitrogen; (3) cooled to −40°C at −0.3°C/min; (4) cooled to −150°C at −30°C/min; and (5) transferred to liquid nitrogen (−196°C) immediately for storage.
The thawing procedure was as follows: the cryogenic vials were thawed at room temperature (between 21°C and 23°C) for 2 minutes and immersed in a water bath at 37°C for another 2 minutes. Ovarian tissue was immediately transferred from the vials to tissue-culture dishes (BD Biosciences) in Leibovitz (L-15) medium and subsequently washed 3 times (5 minutes) at room temperature with fresh medium to remove cryoprotectant before further processing.
Histologic evaluation
A piece of fresh ovarian tissue was fixed in 4% formaldehyde, embedded in paraffin, and serially sectioned (5-μm-thick sections) at the time of cryopreservation. Every third slide was stained with hematoxylin and eosin (Merck) for histologic evaluation to detect the presence of malignant cells and follicle density.
Image acquisition
Slides were analyzed using an Axioskop 50 microscope (Carl Zeiss) and pictures were taken with a Leica DFC320 (Leica Microsystems Ltd). Leica software was used to acquire images digitally and manipulate images (Leica IM50, Version 4.0 Release 132; (Leica Microsystems Imaging Solutions Ltd). Magnifications are specified in the figure legends for each micrograph and numeric aperture of the objective lens used was 0.25 for all.
PCR
Determination of E2A-PBX1 and BCR-ABL fusion gene transcripts.
For PCR analysis of blood and bone marrow at the time of diagnosis, leukocytes were obtained after erythrocyte lysis by centrifugation and counted in a Z1 Particle Counter (Coulter Corporation). Total RNA was extracted from 107 leukocytes with Trizol Reagent (Invitrogen) or TriPure Isolation Reagent (Roche Diagnostics) according to the manufacturer's recommendations. Ovarian tissue was immediately placed in Trizol Reagent or TriPure Isolation Reagent, then ground before RNA extraction. RNA concentrations were measured by fluorometry. The cDNA synthesis reaction was performed with 1 μg of RNA in a volume of 20 μL, containing 19.95μM random hexamers, 10mM dithiothreitol (Invitrogen), 0.5mM deoxynucleotide triphosphates (Roche Diagnostics), 0.025 U/μL Superscript Reverse Transcriptase (Invitrogen), 2 U/μL Ribonuclease Inhibitor Recombinant (RNase out; Invitrogen), and 5 times first-stand buffer (final dilution, 1×; Invitrogen). Reverse transcription was carried out for 60 minutes at 37°C. We diluted 20 μL of the total reverse transcription volume in 80 μL of nuclease-free water (Promega) for E2A-PBX1 detection and in 30 μL of nuclease-free water (Promega) for BCR-ABL detection.
Quantitative RT-PCR amplification reactions were performed with primers and probes described by Gabert et al.11 Each quantitative RT-PCR was performed in a final volume of 25 μL, containing 5 μL of the diluted cDNA (50 ng of RNA equivalent for E2A-PBX1 detection and 100 ng of RNA equivalent for BCR-ABL detection). Thermal cycling was started with an initial denaturation step at 95°C for 10 minutes, followed by 41 cycles at 95°C for 15 seconds, and 60°C for 20 seconds (or 41 cycles at 95°C for 3 seconds and 65°C for 10 seconds for BCR-ABL detection). Patient samples, and positive and negative controls, were amplified in simplicate. Positive controls were commercial plasmid DNA calibrators containing target gene sequences.11 For negative controls, PCR was carried out without cDNA. Results were expressed as the difference in expression levels between the control gene (Abelson: ABL) and the relevant fusion transcript gene: 2ΔCp = 2(Cp control gene − Cp relevant gene).14-17 The difference in expression levels of a fusion gene between 2 samples was calculated using the following equation:
Detection of clonal immunoglobulin and TCR gene recombinations.
For blood and bone marrow, after erythrocyte lysis, leukocytes were obtained by centrifugation and counted in a Z1 Particle Counter (Coulter Corporation). Total DNA was extracted from 107 leukocytes with the QIAGEN DNA Blood Mini-Kit (QIAGEN) according to the manufacturer's recommendations. Ovarian tissue was immediately placed in phosphate-buffered saline before DNA extraction with the DNeasy Blood and Tissue Kit (QIAGEN) according to the manufacturer's recommendations. Tissue DNA concentrations were measured by fluorometry. PCR was performed as recommended by BIOMED-2.13 PCR products obtained from immunoglobulins (Ig) and TCR gene rearrangements were analyzed by gene scanning, as recommended by Van Dongen et al.13 This allows discrimination between monoclonal (with identical junctional regions) and polyclonal (with highly diverse junctional regions) lymphoid cells.
Transplantation to SCID mice
The guidelines for animal welfare were approved by the Committee on Animal Research of the Université Catholique de Louvain.
Eighteen severe combined immunodeficient (SCID) 5- to 11-week-old female mice (Charles River Laboratories) were operated on. They were bred as previously described.18
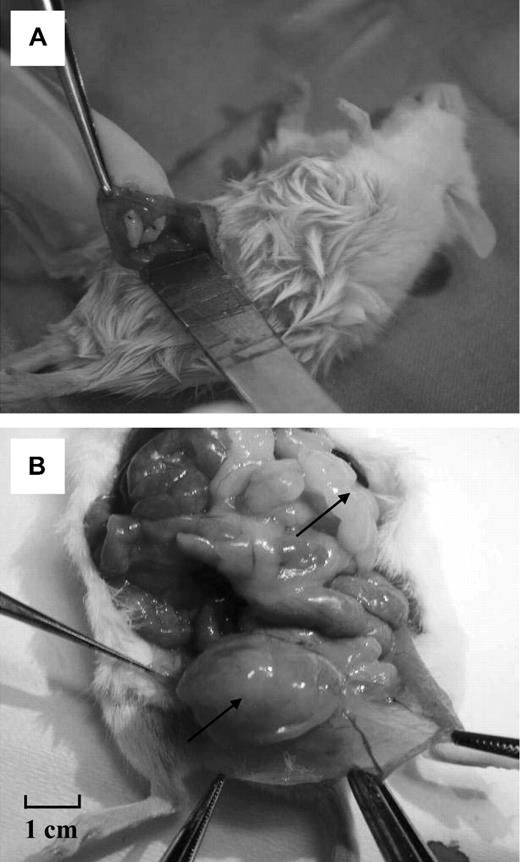
The mice were given an intraperitoneal injection of ketamine (75 mg/kg; Anesketin, Eurovet) and medetomidine (1 mg/kg; Domitor, Pfizer) for anesthesia, and buprenorphine (0.1 mg/kg; Temgesic, Schering Plough) for analgesia. A small median slit was made in the abdomen and peritoneum of the animals, and human ovarian tissue strips were fixed to the inner side of the peritoneum with 1 or 2 stitches of 6-0 Prolene (Figure 1A). The abdominal wall was then closed with 6-0 Prolene and the skin with 4/0 Surgilon. After surgery, anesthesia was reversed by injection of atipamezole (1 mg/kg; Antisedan, Pfizer). The mice were kept for 6 months in sterile conditions.
Macroscopic view of the surgical procedure and a diseased mouse after long-term grafting (external and internal views). (A) Two human ovarian fragments from a patient with leukemia are grafted intraperitoneally to a female SCID mouse. (B) The same mouse after death. Multiple large, hard masses were found in the abdomen. One is shown in a parasplenic location and another developing from the ovarian graft.
Macroscopic view of the surgical procedure and a diseased mouse after long-term grafting (external and internal views). (A) Two human ovarian fragments from a patient with leukemia are grafted intraperitoneally to a female SCID mouse. (B) The same mouse after death. Multiple large, hard masses were found in the abdomen. One is shown in a parasplenic location and another developing from the ovarian graft.
Mice were subsequently killed by cervical dislocation. Ovarian grafts were recovered and assigned to PCR analysis (Trizol Reagent or phosphate-buffered saline) or histologic analysis (formol). Murine liver, spleen, and para-aortic nodes were also recovered, fixed in 4% formaldehyde for 24 hours, and embedded in paraffin for histologic evaluation.
Results
Ovarian tissue histology
For each patient, ovarian cortical strips (4 × 1 × 1 mm) were sent for histologic analysis at the time of ovarian tissue cryopreservation. The samples were evaluated by 2 experienced pathologists. By light microscopy, no malignant cells were found in the ovarian tissue of any patients (CML or ALL). Moreover, ovarian follicles were present in all the samples.
PCR analysis
Of 18 patients, 16 could be analyzed for leukemic cells by PCR, but 2 showed no molecular markers (ALL patients 12 and 17; Table 1).
Patient and pathology characteristics, PCR markers tested on frozen-thawed ovarian tissue, and xenografting experiment results
| Patient no. . | Age at OTC, y . | Pathology . | Gonadotoxic chemotherapy before OTC* . | Molecular markers . | Ovarian tissue PCR . | Macroscopic evaluation of grafts . | Microscopic evaluation of grafts . | Microscopic evaluation of mouse livers . |
|---|---|---|---|---|---|---|---|---|
| 1 | 31 | CML | 0 | BCR-ABL | Positive | Normal | Normal | Normal |
| 2 | 17 | CML | 0 | BCR-ABL | Negative | Normal | Normal | Normal |
| 3 | 30 | CML | 0 | BCR-ABL | Positive | Normal | Normal | Normal |
| 4 | 19 | CML | 0 | BCR-ABL | Negative | Normal | Normal | Normal |
| 5 | 32 | CML | 0 | BCR-ABL | Negative | Normal | Normal | Normal |
| 6 | 19 | CML | 0 | BCR-ABL | Negative | Normal | Normal | Normal |
| 7 | 15 | ALL | 1, 8 | BCR-ABL | Positive | Normal | Normal | Normal |
| 8 | 21 | ALL | 0 | E2A-PBX1 | Positive | 2 leukemic ovarian masses | Leukemic invasion | Normal |
| 9 | 27 | ALL | (2, 3, 4, 5, 6) ×2 | Ig and TCR-γ | Negative | Normal | Normal | Normal |
| 10 | 20 | ALL | 1, 2, 3, 4, 7, 8 | Ig and TCR-γ | Positive | Normal | Normal | Normal |
| 11 | 12 | ALL | 0 | Ig | Positive | Normal | Normal | Normal |
| 12 | 15 | ALL | 1, 2 | None | NA | Diffuse leukemic masses | Leukemic invasion | Positive |
| 13 | 15 | ALL | 0 | Ig | Positive | 1 leukemic ovarian mass | Leukemic invasion | Normal |
| 14 | 14 | ALL | 0 | Ig | Positive | Diffuse leukemic masses | Leukemic invasion | Positive |
| 15 | 16 | ALL | 1, 2 | Ig | Negative | Normal | Normal | Normal |
| 16 | 5 | ALL | 1, 2 | Ig and TCR-γ | Negative | Normal | Normal | Normal |
| 17 | 3 | ALL | 1, 2 | None | NA | Normal | Normal | Normal |
| 18 | 11 | ALL | 1, 2 | TCR-γ | Positive | Normal | Leukemic invasion | Normal |
| Patient no. . | Age at OTC, y . | Pathology . | Gonadotoxic chemotherapy before OTC* . | Molecular markers . | Ovarian tissue PCR . | Macroscopic evaluation of grafts . | Microscopic evaluation of grafts . | Microscopic evaluation of mouse livers . |
|---|---|---|---|---|---|---|---|---|
| 1 | 31 | CML | 0 | BCR-ABL | Positive | Normal | Normal | Normal |
| 2 | 17 | CML | 0 | BCR-ABL | Negative | Normal | Normal | Normal |
| 3 | 30 | CML | 0 | BCR-ABL | Positive | Normal | Normal | Normal |
| 4 | 19 | CML | 0 | BCR-ABL | Negative | Normal | Normal | Normal |
| 5 | 32 | CML | 0 | BCR-ABL | Negative | Normal | Normal | Normal |
| 6 | 19 | CML | 0 | BCR-ABL | Negative | Normal | Normal | Normal |
| 7 | 15 | ALL | 1, 8 | BCR-ABL | Positive | Normal | Normal | Normal |
| 8 | 21 | ALL | 0 | E2A-PBX1 | Positive | 2 leukemic ovarian masses | Leukemic invasion | Normal |
| 9 | 27 | ALL | (2, 3, 4, 5, 6) ×2 | Ig and TCR-γ | Negative | Normal | Normal | Normal |
| 10 | 20 | ALL | 1, 2, 3, 4, 7, 8 | Ig and TCR-γ | Positive | Normal | Normal | Normal |
| 11 | 12 | ALL | 0 | Ig | Positive | Normal | Normal | Normal |
| 12 | 15 | ALL | 1, 2 | None | NA | Diffuse leukemic masses | Leukemic invasion | Positive |
| 13 | 15 | ALL | 0 | Ig | Positive | 1 leukemic ovarian mass | Leukemic invasion | Normal |
| 14 | 14 | ALL | 0 | Ig | Positive | Diffuse leukemic masses | Leukemic invasion | Positive |
| 15 | 16 | ALL | 1, 2 | Ig | Negative | Normal | Normal | Normal |
| 16 | 5 | ALL | 1, 2 | Ig and TCR-γ | Negative | Normal | Normal | Normal |
| 17 | 3 | ALL | 1, 2 | None | NA | Normal | Normal | Normal |
| 18 | 11 | ALL | 1, 2 | TCR-γ | Positive | Normal | Leukemic invasion | Normal |
Data indicate the number of CML and ALL patients, their age at the time of OTC, and the type of gonadotoxic chemotherapy received before OTC. Molecular markers present in blood or bone marrow at the time of diagnosis were tested by PCR on frozen-thawed ovarian tissue. Results of the grafting experiments, showing the macroscopic aspect of the grafts at mouse death, as well as the histology of ovarian xenografts and mouse livers, are also presented. CML patients received hydroxycarbamide with or without imatinib.
OTC indicates ovarian tissue cryopreservation; Ig, immunoglobulin rearrangement genes; TCR, T-cell receptor rearrangement genes; and NA, not appropriate.
1 indicates methotrexate, intrathecally; 2, cortisone, intravenously; 3, cyclophosphamide; 4, vincristine; 5, doxorubicin, 6, cytosine arabinoside; 7, daunorubicin; and 8, asparaginase.
Among the CML patients, 2 of 6 were found to be positive for the BCR-ABL leukemic marker in their ovarian tissue (Table 1). All CML patients received hydroxycarbamide with or without imatinib and were referred for ovarian tissue cryopreservation before gonadotoxic treatment before bone marrow transplantation. Amplification curve results for the BCR-ABL1 gene (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) showed a positive signal in the bone marrow of diseased patients at diagnosis and also in their frozen-thawed ovarian tissue (patients 1 and 3). Quantitative RT-PCR allowed quantification and comparison of expression levels between bone marrow and ovarian tissue. Table 2 shows expression of the BCR-ABL fusion gene to be 100 times lower in ovarian tissue than in bone marrow in patient 1 and 280 times lower in patient 3.
Expression levels of the fusion gene by quantitative RT-PCR in bone marrow and ovarian tissue
| Patient no. . | Pathology . | Molecular markers . | 2ΔCp in BM . | 2ΔCp in OT . | 2ΔΔCp . |
|---|---|---|---|---|---|
| 1 | CML | t(9;22)(q34;q11.2); BCR-ABL | 0.3 | 0.003 | 100 |
| 3 | CML | t(9;22)(q34;q11.2); BCR-ABL | 0.84 | 0.003 | 280 |
| 7 | ALL | t(9;22)(q34;q11.2); BCR-ABL | 0.17 | 0.55 | 0.3 |
| 8 | ALL | t(1;19)(q23;p13.3); E2A-PBX1 | 20.82 | 0.32 | 65 |
| Patient no. . | Pathology . | Molecular markers . | 2ΔCp in BM . | 2ΔCp in OT . | 2ΔΔCp . |
|---|---|---|---|---|---|
| 1 | CML | t(9;22)(q34;q11.2); BCR-ABL | 0.3 | 0.003 | 100 |
| 3 | CML | t(9;22)(q34;q11.2); BCR-ABL | 0.84 | 0.003 | 280 |
| 7 | ALL | t(9;22)(q34;q11.2); BCR-ABL | 0.17 | 0.55 | 0.3 |
| 8 | ALL | t(1;19)(q23;p13.3); E2A-PBX1 | 20.82 | 0.32 | 65 |
In each tissue, results are expressed as the difference in expression levels between the control gene and the relevant fusion transcript gene (2ΔCp = 2 (Cpcontrol gene − Cprelevant gene).The difference in expression levels of a fusion gene between bone marrow and ovarian tissue was calculated using 2ΔΔCp = 2(ΔCp in BM − ΔCp in ovarian tissue). BM was not available for PCR analysis on the day of OTC.
BM indicates bone marrow; and OT, ovarian tissue.
Among the ALL patients (n = 12), 2 were excluded from PCR analysis because no molecular markers were available. Of the remaining 10, 7 showed positive molecular markers in their cryopreserved ovarian tissue. One was positive for the BCR-ABL fusion gene, the second for t(1;19)(q23;p23.3), and the other 5 for Igs and/or TCR-γ rearrangement genes.
The 3 patients in whom Ig and/or TCR-γ rearrangement genes were negative in ovarian tissue had already received chemotherapy before ovarian tissue cryopreservation (patients 9, 15, and 16). Indeed, 2 patients had already received one regimen of intrathecal injection of methotrexate associated with cortisone, and 1 patient had undergone 2 regimens of cortisone, cyclophosphamide, vincristine, doxorubicin, and cytosine arabinoside therapy.
Among the 7 patients who were positive for ALL markers in their ovarian tissue, 4 had not received any chemotherapy before ovarian tissue cryopreservation (patients 8, 11, 13, and 14) and 3 had already undergone one regimen of chemotherapy (patients 7, 10, and 18). Indeed, these last 3 patients had received intrathecal methotrexate associated with cortisone, and patient 10 also received cyclophosphamide, vincristine, daunorubicin, and asparaginase.
Quantification of the fusion gene in ALL patients (Table 2) showed its expression to be 3 times higher in ovarian tissue than in bone marrow in patient 7 and 65 times lower in ovarian tissue than in bone marrow in patient 8.
Xenografting experiment
Eighteen SCID mice were grafted with ovarian tissue from CML patients (n = 6) or ALL patients (n = 12). None of the mice died during the 6-month grafting period.
Macroscopic analysis
After 6 months of grafting, all the mice were killed, and grafted human ovarian tissue as well as murine spleen, liver, and lymph nodes were recovered.
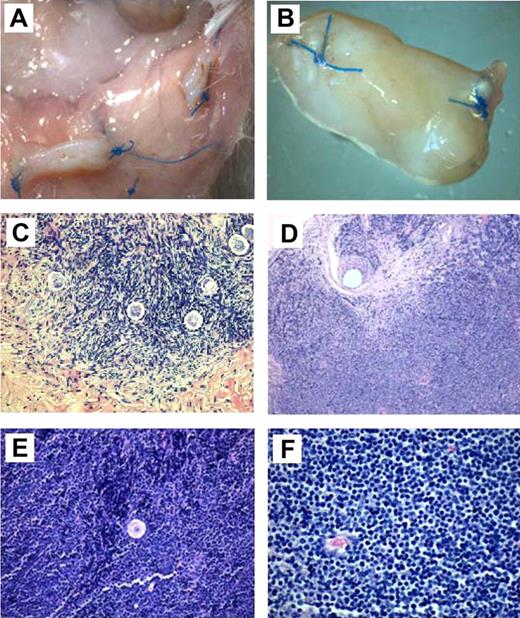
In mice grafted with ovarian tissue from CML patients, no enlarged abdominal nodes or hepatosplenomegaly was found during dissection. At macroscopic evaluation, the normal ovarian grafts (Figure 2A) were found to have shrunk in size by approximately 40%. Nevertheless, antral preovulatory follicles and corpora lutea were found in the xenografted ovarian tissue. Human ovarian grafts were assigned to histology and PCR.
Macroscopic and histologic analysis of ovarian fragments recovered from the mice after 6 months' grafting. (A) Macroscopic view of normal frozen-thawed ovarian grafts. (B) Macroscopic view of an enlarged ovarian graft. Note the stitches at both ends. (C) Histologic aspect of a normal ovarian-xenografted fragment. Five ovarian follicles can be recognized in a cellular stroma surrounded by a fibrotic area. Original magnification ×100. (D) Human ovarian graft from a patient with ALL with massive cellular invasion. Note the 6-0 Prolene stitch in the graft (arrow). Original magnification ×50. (E) Human follicle encircled by a large number of lymphocytes. Normal ovarian stroma is no longer present. Original magnification ×100. (F) Massive lymphocytic invasion of the ovarian graft. The histologic abnormalities observed in these lymphocytes were identified as malignant in nature and attributed to leukemic invasion. Original magnification ×200.
Macroscopic and histologic analysis of ovarian fragments recovered from the mice after 6 months' grafting. (A) Macroscopic view of normal frozen-thawed ovarian grafts. (B) Macroscopic view of an enlarged ovarian graft. Note the stitches at both ends. (C) Histologic aspect of a normal ovarian-xenografted fragment. Five ovarian follicles can be recognized in a cellular stroma surrounded by a fibrotic area. Original magnification ×100. (D) Human ovarian graft from a patient with ALL with massive cellular invasion. Note the 6-0 Prolene stitch in the graft (arrow). Original magnification ×50. (E) Human follicle encircled by a large number of lymphocytes. Normal ovarian stroma is no longer present. Original magnification ×100. (F) Massive lymphocytic invasion of the ovarian graft. The histologic abnormalities observed in these lymphocytes were identified as malignant in nature and attributed to leukemic invasion. Original magnification ×200.
After grafting of ovarian tissue from ALL patients, 4 mice (cases 8, 12, 13, and 14) showed enlarged ovarian tissue grafts that appeared as hard, white masses at the grafting site (Figures 1B,2B). These masses were macroscopically identified as the grafts because stitches used to fix the ovarian fragments were still visible (Figure 2B,D). Two of these 4 mice (cases 12 and 14) also presented with large, hard, white burgeoning masses (Figure 1B), which corresponded to massive peritoneal invasion. Indeed, these masses were present at different sites (hepatic, splenic, perirenal, and para-aortic) and were visible and palpable at macroscopic evaluation before mouse death.
Three of the 4 diseased mice (cases 8, 13, and 14) were grafted with ovarian tissue from patients who had not received any chemotherapy before ovarian tissue cryopreservation. The fourth diseased mouse was grafted with ovarian tissue for which no molecular markers were available, from a patient who had already received one injection of intrathecal methotrexate associated with cortisone.
It is noteworthy that the ovarian grafts presenting as tumoral masses never showed follicular development on their surface.
Microscopic analysis
Ovarian xenografts.
All the ovarian grafts were recovered from all the mice (Table 1).
In mice grafted with ovarian tissue from CML patients, serial sections of grafts did not show the presence of malignant cells, and human ovarian xenografts had a normal histologic appearance (Figure 2C).
For grafts issuing from ALL patients, 5 of 12 showed obvious invasion by lymphoblasts. Histology confirmed disease in 4 mice presenting with macroscopic signs of malignancy, plus case 18, which exhibited leukemic invasion at microscopic analysis (Figure 2D-F). Figure 2E shows one of these 5 xenografts (case 8), with massive proliferation of malignant lymphocytes, in the middle of which a secondary-stage follicle is clearly visible.
It is important to note that none of the patients with PCR-negative tissue was associated with leukemic progression in mice.
Mouse liver.
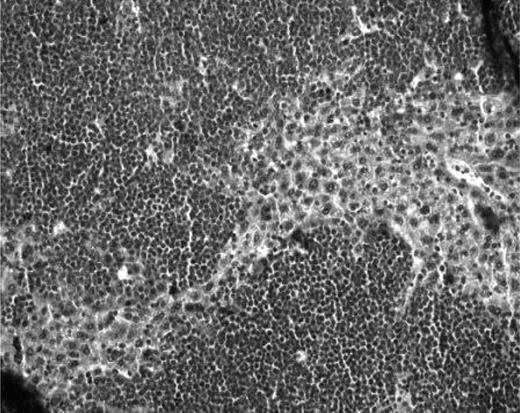
Mouse livers were free of disease, except in 2 ALL cases (cases 12 and 14; Figure 3), which also showed diffuse leukemic abdominal masses.
Histologic analysis of murine livers. Histologic aspect of a liver from a mouse developing abdominal masses, with large lymphocytic invasion of the hepatic tissue (case 12). Original magnification ×200.
Histologic analysis of murine livers. Histologic aspect of a liver from a mouse developing abdominal masses, with large lymphocytic invasion of the hepatic tissue (case 12). Original magnification ×200.
PCR analysis
Insufficient quantity of samples after RNA or DNA extraction did not allow valid interpretation of the results, except in cases 13 and 14, where material was abundant. PCR showed positive leukemic markers in grafted human ovarian tissue, as well as the spleen and abdominal masses (supplemental Figure 2).
Discussion
Shaw et al19 previously reported that ovarian tissue collected from mice with lymphoma could transfer the disease to healthy recipient animals. More recent studies tested the safety of grafting cryopreserved human ovarian tissue from Hodgkin disease patients and suggested that ovarian tissue transplantation may be considered safe in this case.20-22 Recent successful autotransplantation of frozen-thawed ovarian tissue from Hodgkin patients failed to demonstrate any signs of disease recurrence.3,23
However, in leukemia, malignant cells may be present in the bloodstream, running the risk of transferring diseased cells. A study by Jahnukainen et al24 showed that transplantation of testicular cells from leukemic donor rats transmits acute leukemia to healthy recipients. For ovarian tissue from hematologic cancer patients, it is therefore of primordial importance to identify minimal residual disease before ovarian transplantation.10,25
In vitro studies: histology and PCR
In the present study, we used molecular biology to evaluate the presence of leukemic cells in cryopreserved ovarian tissue from patients with CML and ALL, the 2 types of leukemia that are the most frequent indications for cryopreservation of ovarian tissue.
Meirow et al10 recently identified CML cells in frozen-thawed ovarian tissue from a patient with the disease by quantitative RT-PCR, which proved to be positive for BCR-ABL transcripts. This avoided transplantation of the stored ovarian tissue. A recent retrospective analysis of 5571 autopsy findings of females younger than 40 years of age in Japan found leukemic involvement of the ovaries in 8.4% of leukemic patients.26 Very little has been published on the incidence of ovarian metastasis in CML. In the case of ALL, ovarian metastases have been found in up to 30% of patients at autopsy but are rarely found clinically.27
In our study, histology failed to detect malignant cells in fresh or frozen ovarian tissue from either CML or ALL patients. However, using disease-specific PCR techniques, we found contamination of ovarian tissue in 33% and 70% of CML and ALL patients, respectively.
Concerning CML, as the presence of the BCR-ABL gene is characteristic of the disease, molecular detection of leukemic cells in ovarian tissue can always be carried out. On the contrary, for ALL, genetic markers are not always detected.28 Most published series of patients with ALL indicate that more than 85% have an abnormal clone identified by conventional cytogenetic studies. However, major chromosomal translocations are reported in approximately 48% and 49% of pediatric and adult ALL cases, respectively.29 In this form of the disease, specific immunoglobulin and TCR gene rearrangements are thus often tested when specific fusion transcripts are not detectable by PCR in blood or bone marrow.28 Nevertheless, some ALL cases do not display any markers at all and, so far, there are no sensitive molecular methods to evaluate the risk of contamination by malignant cells.
Ovarian tissue cryopreservation should preferably be performed before the initiation of gonadotoxic chemotherapy to cryopreserve undamaged ovarian follicles.30-32 In the case of CML, hematologists and oncologists have time to send their patients to the gynecologist for fertility preservation options, such as ovarian tissue cryopreservation, if bone marrow transplantation is indicated. In our series, all CML patients underwent ovarian tissue cryopreservation before sterilizing chemotherapy. However, with ALL, gonadotoxic chemotherapy is often started before fertility preservation options can be proposed because of the urgency of treatment. It is thus important to establish whether previous chemotherapy can help to eliminate malignant cells from the ovarian tissue.
All CML patients received hydroxycarbamide, with or without imatinib, before bone marrow transplantation. Nevertheless, PCR results showed that, even after a minimum of 6 months of this treatment, ovarian tissue was still positive (patients 1 and 3).
Among the 10 ALL patients with available molecular markers, 4 patients who underwent ovarian tissue cryopreservation before receiving any chemotherapy had positive PCR results for the presence of leukemic markers in their ovarian tissue (patients 8, 11, 13, and 14). Our experiments thus prove that CML and ALL cells are present in frozen-thawed ovarian tissue or its vascular network. The remaining 6 ALL patients with available molecular markers had already received some chemotherapy before ovarian tissue cryopreservation, but 3 of them still showed positive ovarian tissue (patients 7, 10, and 18). Moreover, 1 of these patients (patient 10) had already received a full cycle of 6 chemotherapeutic agents (cortisone, methotrexate, cyclophosphamide, vincristine, daunorubicin, and asparaginase). One cycle of chemotherapy may therefore be insufficient to completely eliminate all ALL and CML cells, and prior chemotherapy aiming to purge the ovary of malignant cells before ovarian tissue cryopreservation may be inadequate.
On the other hand, even one cycle of chemotherapy may be deleterious for oocyte quality.30-32 It is thus obvious that ovarian tissue cryopreservation should ideally be performed before the initiation of chemotherapy to freeze ovarian tissue with an intact follicular pool and high-quality oocytes.
In vivo study
The present study reports, for the first time, in vivo investigation of residual disease in frozen human ovarian tissue from ALL and CML patients using a xenografting model. For each patient, frozen-thawed ovarian tissue was xenografted to an SCID mouse for 6 months. No mice grafted with ovarian tissue from CML patients became ill. On the contrary, mice grafted with human ovarian tissue from ALL patients developed peritoneal leukemic masses from the ovarian grafts. In 3 of 4 mice developing leukemic masses, the frozen-thawed ovarian tissue used for grafting was positive by PCR for leukemic markers, and in the fourth mouse there were no molecular markers. These grafted ovarian fragments came from patients who had not received any chemotherapy before ovarian tissue cryopreservation. Our xenografting experiments demonstrate the viability and malignant potential of ALL cells present in frozen ovarian tissue. Other forms of leukemia and lymphoma may well behave very differently. However, in clinical practice, even if molecular tests on ovarian tissue are negative, there is still a potential risk of disease with reimplantation, knowing that patients in remission can present with negative molecular results in blood and/or bone marrow and still relapse.
In addition, leukemia-invaded ovarian fragments showed almost no signs of follicular development in our study, indicating destruction of residual healthy follicles in ovarian tissue, which can no longer be functional.
This study provides clear evidence of ovarian contamination by leukemic cells in acute (ALL) as well as chronic (CML) leukemia and proves that human ovarian tissue positive for ALL markers by PCR can transmit the disease to mice. Nevertheless, all mice grafted with ovarian fragments that were PCR-negative for ALL markers, or fragments (PCR-positive or -negative) from CML patients, remained disease-free. Further studies should investigate the safety of the transplantation procedure in these 2 subgroups because the number of experiments conducted was not sufficient to allow any conclusive statements to be made.
When appropriate, however, leukemic patients should benefit from fertility preservation techniques. Ovarian tissue cryopreservation is often the only option we can offer these patients because of the lack of time for ovarian stimulation or because of their prepubertal age. Ultimately, our objective is to offer young patients at risk of treatment-induced premature ovarian failure realistic and safe fertility preservation options. For these patients, follicle culture with in vitro maturation may be a solution.33-38 Another option could be grafting of isolated follicles, enzymatically purified from frozen-thawed ovarian tissue.39,40 Research in this exciting new field needs to continue to develop safe options for fertility preservation.
In conclusion, this study demonstrates, by quantitative RT-PCR, ovarian contamination by malignant cells in acute (ALL) as well as chronic (CML) leukemia, whereas histology fails to do so. Moreover, chemotherapy before ovarian cryopreservation does not exclude malignant contamination. These murine xenografting results clearly show that reimplantation of cryopreserved ovarian tissue from patients with ALL puts them at risk of disease recurrence.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof A. Ferrant (Department of Hematology, Université Catholique de Louvain [UCL]), Prof J. M. Scheiff (Department of Clinical Biology), and Prof E. Marbaix and Dr I. Théate (Department of Pathology, UCL) for their valuable scientific advice and for reading the histologic slides of the grafts, and Dolores Gonzalez and Dr Kamil Zalewski for their technical assistance.
This work was supported by Fonds National de la Recherche Scientifique de Belgique (grant 3.4590.08 F and 7.4562.08), the Fonds Spéciaux de Recherche, the Fondation Saint Luc, the Foundation Against Cancer (nonprofit organization), the Center du Cancer, and donations from A. Frère, Ph de Spoelberch, and P. Ferrero.
Authorship
Contribution: M.-M.D. was the principal investigator, conducted the study, and wrote the manuscript; C.M. performed laboratory investigation, including grafting procedures, and data collection; P.S. developed markers for detection and performed PCR, evaluated the results, and participated in writing the manuscript; A.V.L. evaluated results and reviewed the manuscript; C.A. performed laboratory investigation and interpreted data; and J.D. was the principal investigator, treated patients, and provided critical review.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jacques Donnez, Department of Gynecology, Université Catholique de Louvain, Cliniques Universitaires St Luc, Ave Hippocrate 10, 1200 Brussels, Belgium; e-mail: jacques.donnez@uclouvain.be.