Abstract
Drug-induced immune thrombocytopenia (DITP) is a relatively common and sometimes life-threatening condition caused by antibodies that bind avidly to platelets only when drug is present. How drug-dependent antibodies (DDAbs) are induced and how drugs promote their interaction with platelets are poorly understood, and methods for detecting DDAbs are suboptimal. A small animal model of DITP could provide a new tool for addressing these and other questions concerning pathogenesis and diagnosis. We examined whether the nonobese diabetic/severe combined immunodeficient (NOD/scid) mouse, which lacks xenoantibodies and therefore allows infused human platelets to circulate, can be used to study drug-dependent clearance of platelets by DDAbs in vivo. In this report, we show that the NOD/scid model is suitable for this purpose and describe studies to optimize its sensitivity for drug-dependent human antibody detection. We further show that the mouse can produce metabolites of acetaminophen and naproxen for which certain drug-dependent antibodies are specific in quantities sufficient to enable these antibodies to cause platelet destruction. The findings indicate that the NOD/scid mouse can provide a unique tool for studying DITP pathogenesis and may be particularly valuable for identifying metabolite-specific antibodies capable of causing immune thrombocytopenia or hemolytic anemia.
Introduction
Drug-induced immune thrombocytopenia (DITP) can be triggered by various medications through several distinct mechanisms.1,2 In a comprehensive survey of DITP cases reported since 1998, George and coworkers identified 17 drugs that were considered to be “probable” causes of DITP and 51 thought to be “definite” causes on the basis of having met, respectively, 3 or 4 well-defined clinical criteria.3,4 This analysis has helped greatly to define which drugs are capable of causing DITP but does not provide proof in an individual patient that a particular drug was responsible. Evidence supporting a cause-and-effect relationship between drug exposure and thrombocytopenia can be obtained by identifying a drug-dependent antibody (DDAb) that reacts with platelets only when the implicated drug is present.1,2,5 However, relatively few laboratories are experienced in DDAb detection, and it is not rare for antibody testing to be negative in a patient with a clinical history strongly suggestive of DITP.1,2 Moreover, there is not uniform agreement as to whether detection of a DDAb provides conclusive evidence that the antibody caused platelet destruction. Partly for these reasons, identification of a DDAb is not included among the George criteria. The most stringent of these criteria calls for thrombocytopenia to recur when a patient is exposed a second time to the implicated drug.3 While a rechallenge can provide convincing evidence that thrombocytopenia was drug-induced, it is often impractical and can be difficult to justify for reasons of patient safety.
A surrogate small animal model for direct demonstration of drug-dependent antibody-mediated platelet clearance could provide a useful alternative to a human challenge, a valuable tool with which to characterize the spectrum of drugs capable of causing DITP and a new approach to studying its pathogenesis. We recently found that human platelets transfused into nonobese diabetic/severe combined immunodeficient (NOD/scid) mice have a survival time only slightly less than that of murine platelets (approximately 3 days) and that circulating human platelets were rapidly destroyed after injection of a human, platelet-specific alloantibody.6 On the basis of these findings, we wondered whether the mouse model might provide a convenient way to document the pathogenicity of DDAbs and, possibly, a means of identifying some DDAbs not detected by conventional laboratory testing. In this report, we show that the model can, in fact, be used to show that a DDAb is capable of causing platelet destruction in an animal challenged with a drug for which the antibody is specific and that this approach may be particularly useful for the study of DDAbs induced by drug metabolites.
Methods
Antibodies and reagents
The quinine-dependent, platelet-reactive monoclonal antibody (mAb) 314.1 has been described previously.7 Human drug-dependent, platelet-reactive antibodies were from patients with DITP referred for study. Monoclonal antibody AP2 specific for the human GPIIb/IIIa complex8 was labeled with the fluorochrome Alexa-488 according to the manufacturer's guidelines (Invitrogen). Unless indicated, other reagents were obtained from Sigma-Aldrich.
Flow cytometry
Binding of monoclonal and human antibodies to human platelets was measured as previously described.7,9,10 In brief, 5 × 106 washed platelets suspended in phosphate-buffered saline containing 1% bovine serum albumin were incubated with antibody in a total volume of 50 μL for 60 minutes at room temperature and washed twice in bovine serum albumin–phosphate-buffered saline. Platelet-bound immunoglobulins (Igs) were detected with fluorescein isothiocyanate–labeled goat (Fab′)2 anti–mouse IgG(H+L) or fluorescein isothiocyanate goat (Fab′)2 anti–human IgG(H+L) from Jackson ImmunoResearch Laboratories. Drug-dependent antibodies were detected by carrying out the reaction in the presence and absence of the appropriate drug at a concentration of 0.4mM (0.12 mg/mL) for quinine and 4mM (1.0 mg/mL) for sulfamethoxazole or at other concentrations specified. Antibody titers were defined as the highest dilution of serum that produced a positive reaction (median fluorescence intensity twice the values obtained with drug alone and serum alone).
Measurement of human platelet survival in NOD/scid mice
The experimental procedure was as described previously6,11 with several modifications. All studies involving human subjects and animals were approved by the institutional review board of the BloodCenter of Wisconsin and the Medical College of Wisconsin's institutional animal care and use committee, respectively. Human blood from a group O donor was collected into acid-citrate-dextrose (ACD) anticoagulant containing 50 ng/mL prostaglandin E1 (PGE1) and centrifuged at 200g for 10 minutes. The supernatant platelet-rich plasma was allowed to stand for 5 minutes and was then centrifuged at 750g for 10 minutes. The resulting platelet button was gently resuspended in 0.2 mL of test plasma (anticoagulated with ACD) or serum containing 50 ng/mL PGE1. Sodium citrate (0.02M) was also added to serum samples. To remove non–drug-dependent antibodies present in some samples, test plasma and serum were preabsorbed with the same platelets that were to be infused into the mice, using 2 × 109 platelets/mL. The platelet suspension was allowed to stand at room temperature for 30 minutes and was then infused into the retro-orbital plexus of an anesthetized NOD/scid mouse aged 6-8 weeks (stock no. 001303; The Jackson Laboratory). After 1 hour, a tail blood sample was collected to establish the baseline (time 0) level of circulating human platelets. The 60-minute baseline time point was chosen because, in preliminary studies, it was found that levels of circulating human platelets sometimes fluctuate during the first 45 minutes after infusion. The test drug was then injected intraperitoneally immediately and after 5 hours. Drugs administered have T1/2 values in the circulation on the order of 2 to 13 hours.12-15 The second injection was given at 5 hours to assure reasonable levels of circulating drug throughout the 24-hour period of observation. Additional blood samples were collected 2, 5, and 24 hours after the baseline sample.
Circulating human platelets were quantified in 10- to 20-μL aliquots of tail vein blood collected into 1.0 mL of Tyrode-HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] buffer containing 0.4% sodium citrate and 50 ng/mL PGE1. The mixture was layered on 2 mL Fico/Lite medium (Atlanta Biologicals) and centrifuged for 15 minutes at 350g to pellet red cells and leukocytes. The supernatant platelet layer was aspirated, added to 3.0 mL Tyrode-HEPES buffer containing 67 ng/mL PGE1, centrifuged at 750g for 10 minutes, and resuspended in 0.10 mL of the same buffer containing Alexa-488-labeled mAb AP2 (0.5 μg), specific for the human GPIIb/IIIa complex. Samples were diluted with 0.3 mL of buffer and platelet-bound fluorochrome was analyzed by flow cytometry (LSRII, Becton Dickinson). The total platelet population (both mouse and human) was gated by size, and the peak containing labeled human platelets was identified by fluorescence. The fraction of human platelets in each sample was calculated as the ratio of AP2-labeled platelets to total platelets. The mouse component of the platelet distribution was unaffected by the human serum/plasma samples or drugs injected in these studies. For calculation of survival curves, human platelets present in the baseline sample were considered to represent 100%.
Results
The quinine-dependent mAb 314.1 causes drug-dependent clearance of human platelets
Monoclonal antibody (mAb) 314.1 is an IgG1 murine mAb that binds to an epitope in the beta propeller domain of glycoprotein IIb when quinine is present.7 In this and other respects, 314.1 closely mimics the behavior of antibodies that cause platelet destruction in patients with quinine-induced immune thrombocytopenia. To test the feasibility of using the NOD/scid mouse model to demonstrate drug-dependent, antibody-mediated platelet clearance, mice previously infused with human platelets were injected intraperitoneally with 50 μg mAb 314.1 followed by 74 μg quinine or buffer. As shown in Figure 1, platelets were cleared from the circulation within 2 hours of the time quinine was injected, whereas 314.1 alone and an irrelevant mAb (MOPC 21, Sigma-Aldrich) given with quinine were without effect. The amount of quinine administered was equivalent to 3.7 mg/kg body weight, comparable with a dose of 260 mg given to a 70-kg human. The findings show that after intraperitoneal injection, drug and mAb diffused into the animal's circulation in quantities sufficient to promote antibody-platelet interaction, leading to platelet destruction within a few hours.
Quinine-dependent mAb 314.1 promotes platelet destruction in NOD/scid mice given quinine. Human platelets were infused into NOD/scid mice followed by intraperitoneal injection of 50 μg of mAb 314.1 or control monoclonal. One hour later (time 0) and at 5 hours, buffer or quinine (74 μg), was injected intraperitoneally Platelet survival was markedly shortened in mice given mAb 314.1 and quinine (triangles) but not in mice given the mAb alone (squares) or quinine plus an irrelevant mAb (circles). Values shown are the average ± 1 SEM of triplicate experiments. ***P < .001 relative to controls.
Quinine-dependent mAb 314.1 promotes platelet destruction in NOD/scid mice given quinine. Human platelets were infused into NOD/scid mice followed by intraperitoneal injection of 50 μg of mAb 314.1 or control monoclonal. One hour later (time 0) and at 5 hours, buffer or quinine (74 μg), was injected intraperitoneally Platelet survival was markedly shortened in mice given mAb 314.1 and quinine (triangles) but not in mice given the mAb alone (squares) or quinine plus an irrelevant mAb (circles). Values shown are the average ± 1 SEM of triplicate experiments. ***P < .001 relative to controls.
Human platelets suspended in normal human plasma or serum survived “normally” for 24 hours in the mouse
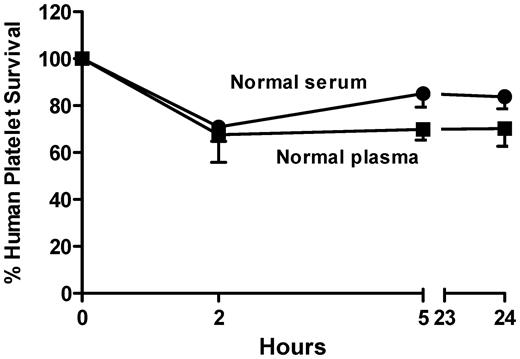
Prior studies of human platelet survival in the NOD/scid mouse involved infusion of platelets suspended in buffer followed by intraperitoneal injection of antibody.6,11 Antibodies from patients with DITP are usually available in limited quantities and sometimes become undetectable in in vitro assays when diluted more than a few fold.16 Therefore, in studying the ability of human DDAbs to cause platelet destruction in the mouse, it was desirable to maximize the concentration of antibody achieved in the animal's circulation by administering test plasma or serum intravenously, rather than by the intraperitoneal route. Accordingly, we determined whether the isolated platelet button could be suspended directly in plasma (anticoagulated with ACD) or serum to which sodium citrate (0.02M) had been added and could then be infused into the retro-orbital plexus without adversely affecting platelet survival. As shown in Figure 2, survival after 24 hours of platelets suspended in normal plasma or serum was 70% ± 15% and 84% ± 11% of the baseline values, respectively. Survival at 24 hours was unaffected by whether the platelets were suspended in freshly collected or frozen-stored plasma or serum (not shown).
Human platelets survive “normally” when infused into NOD/scid mice as a suspension in normal citrated plasma or serum containing 0.02M sodium citrate. Values shown are the average of triplicate experiments ± 1 SEM. Survival values at 24 hours of platelets suspended in normal plasma or serum was 70% ± 15% and 84% ± 11% of the baseline (time 0) values, respectively.
Human platelets survive “normally” when infused into NOD/scid mice as a suspension in normal citrated plasma or serum containing 0.02M sodium citrate. Values shown are the average of triplicate experiments ± 1 SEM. Survival values at 24 hours of platelets suspended in normal plasma or serum was 70% ± 15% and 84% ± 11% of the baseline (time 0) values, respectively.
Human platelets injected with DDAbs specific for quinine or sulfamethoxazole were rapidly cleared after injection of these drugs
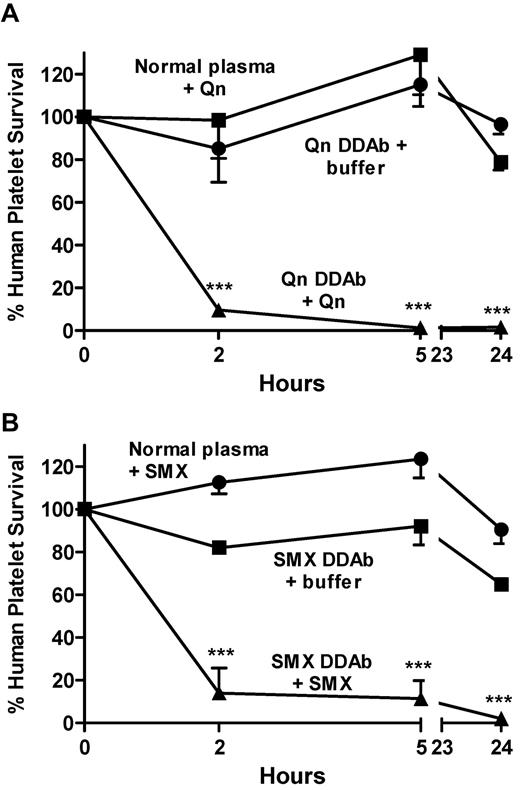
We then determined whether DDAbs from patients who experienced DITP would cause destruction of circulating human platelets when animals were challenged with the appropriate drug. Mice were infused with platelets suspended in normal plasma or in plasma from a patient with a potent quinine-specific antibody who developed severe thrombocytopenia after taking quinine. After a baseline platelet sample was obtained, the animals were injected intraperitoneally with 3.7 mg/kg quinine (74 μg). A second injection of drug was given at 5 hours. As shown in Figure 3A, more than 90% of the circulating human platelets were cleared within 2 hours after injection of quinine and none remained after 24 hours. Similar findings were obtained using a strong sulfamethoxazole-dependent antibody and intraperitoneal injection of 50 mg/kg sulfamethoxazole (approximately 4× a standard human dose; Figure 3B). The findings show that quinine and sulfamethoxazole, injected intraperitoneally, diffused into the circulation in quantities sufficient to cause the 2 DDAbs to bind to platelets and promote their clearance.
Human antibodies specific for quinine and sulfamethoxazole promote platelet destruction in NOD/scid mice given these drugs. Human platelets suspended in plasma containing DDAbs or normal plasma were infused into NOD/scid mice followed by intraperitoneal injection of quinine (3.7 mg/kg; A) or sulfamethoxazole (50 mg/kg; B) at 0 and 5 hours. Platelet survival was markedly shortened in mice given DDAb and the appropriate drug (triangles), but not in mice given DDAb alone (squares) or drug plus normal plasma (circles). Values shown are the average of triplicate experiments ± 1 SEM. ***P < .001 relative to controls.
Human antibodies specific for quinine and sulfamethoxazole promote platelet destruction in NOD/scid mice given these drugs. Human platelets suspended in plasma containing DDAbs or normal plasma were infused into NOD/scid mice followed by intraperitoneal injection of quinine (3.7 mg/kg; A) or sulfamethoxazole (50 mg/kg; B) at 0 and 5 hours. Platelet survival was markedly shortened in mice given DDAb and the appropriate drug (triangles), but not in mice given DDAb alone (squares) or drug plus normal plasma (circles). Values shown are the average of triplicate experiments ± 1 SEM. ***P < .001 relative to controls.
Sensitivity of the in vivo assay was enhanced by injecting drug at supratherapeutic concentrations
When studies like those illustrated in Figure 3A and B were done with other DDAbs, we found that drug-dependent platelet clearance was readily demonstrated with antibodies that gave strong signals in the flow cytometric assay but not with weaker ones (not shown). It seemed likely that this was a consequence of immediate dilution (approximately 5-10-fold) of the injected antibody in plasma of the recipient animals and a more gradual loss of antibody thereafter resulting from diffusion into the extravascular space. A general feature of DDAbs that cause thrombocytopenia in patients treated with drugs like quinine and sulfamethoxazole is that high concentrations of drug do not inhibit binding of the antibodies to their targets,9,10,17 a property that distinguishes them from classical “hapten-specific” antibodies.18-20 In fact, DDAbs bind to their targets best at the highest concentrations of drug that can be achieved in vitro.9,17,21 This is illustrated in Figure 4A and B, where it can be seen that the quantity of antibody bound to a platelet target steadily increases with drug concentration. In particular, it is clear that peak drug levels achieved after a standard therapeutic dose (10μM for quinine13 and 80μM for sulfamethoxazole12 ) are suboptimal for antibody binding.
Binding of quinine- and sulfamethoxazole-specific DDABs to platelets is enhanced by using drug at supratherapeutic concentrations. Reactions of quinine-specific (A) and sulfamethoxazole-specific (B) DDAbs used at dilutions of 1:5 and higher to normal platelets were enhanced by using the drugs at supratherapeutic concentrations. Binding of patient Ig to platelets was measured by flow cytometry. MFI indicates median platelet fluorescence; values on the abscissa indicate final concentration of drug in the reaction mixture. Vertical lines denote expected mean peak levels achieved after administration of a conventional dose of quinine (10μM) and sulfamethoxazole (80μM).12,13
Binding of quinine- and sulfamethoxazole-specific DDABs to platelets is enhanced by using drug at supratherapeutic concentrations. Reactions of quinine-specific (A) and sulfamethoxazole-specific (B) DDAbs used at dilutions of 1:5 and higher to normal platelets were enhanced by using the drugs at supratherapeutic concentrations. Binding of patient Ig to platelets was measured by flow cytometry. MFI indicates median platelet fluorescence; values on the abscissa indicate final concentration of drug in the reaction mixture. Vertical lines denote expected mean peak levels achieved after administration of a conventional dose of quinine (10μM) and sulfamethoxazole (80μM).12,13
We therefore tested whether sensitivity of the in vivo assay for detection of antibody-mediated platelet clearance could be enhanced by injecting drug in doses that were higher, but below the threshold for acute toxicity. For these studies, we used quinine- and sulfamethoxazole-dependent DDAbs that caused accelerated platelet clearance at dilutions of 1:5 and 1:2, respectively, when 3.7 mg/kg quinine or 50 mg/kg sulfamethoxazole were injected but failed to promote clearance when used at higher dilutions (not shown). As seen in Figure 5A-B, the same antibodies, used at dilutions of 1:15 and 1:45, promoted rapid platelet destruction when larger doses of quinine (50 mg/kg) or sulfamethoxazole (250 mg/kg) were injected. The highest dilutions of antibody that caused platelet destruction in these experiments (assuming an immediate 10-fold dilution after infusion) were comparable with the dilutions at which the 2 antibodies could be detected in vitro in a flow cytometric assay.
Drug-dependent clearance of human platelets in NOD/scid mice is enhanced by supra-therapeutic concentrations of drug. Human platelets suspended in plasma containing DDAbs at the indicated dilutions were infused into NOD/scid mice followed by intraperitoneal injection of quinine (50 mg/kg; A) or sulfamethoxazole (250 mg/kg; B) at 0 and 5 hours. Platelet survival was markedly shortened in mice given DDAbs diluted 1:15 (squares) and 1:45 (triangles) followed by injection of the appropriate drug. Values shown are the average of triplicate experiments ± 1 SEM. **P < .01, ***P < .001 relative to controls.
Drug-dependent clearance of human platelets in NOD/scid mice is enhanced by supra-therapeutic concentrations of drug. Human platelets suspended in plasma containing DDAbs at the indicated dilutions were infused into NOD/scid mice followed by intraperitoneal injection of quinine (50 mg/kg; A) or sulfamethoxazole (250 mg/kg; B) at 0 and 5 hours. Platelet survival was markedly shortened in mice given DDAbs diluted 1:15 (squares) and 1:45 (triangles) followed by injection of the appropriate drug. Values shown are the average of triplicate experiments ± 1 SEM. **P < .01, ***P < .001 relative to controls.
Drug metabolites produced by mice in vivo promote platelet clearance by metabolite-specific antibodies
Drug-dependent antibodies are sometimes not detected in patients with a clinical picture strongly suggestive of DITP.1,2 One important reason for this is that a metabolite, rather than the primary drug, can be the sensitizing agent.1,22-24 It is sometimes possible to identify a metabolite-specific antibody by using the appropriate metabolite or a urinary extract obtained from a person taking the drug of interest for in vitro testing.24 However, most drugs have multiple metabolites that are not readily available,25 and urinary extracts are complicated to prepare and may lack sensitivity.24 Although drug metabolism can be species-specific, mice and humans share various metabolic pathways.25-27 In particular, conjugation of drugs to glucuronic acid by ester or ether linkage through the action of uridine 5′-diphosphate-glucuronyltranferases is similar in the 2 species.26 We previously described patients with DITP whose antibodies were specific for glucuronide conjugates of the non-steroidal anti-inflammatory drug (NSAID) naproxen24 and the anti-pyretic acetaminophen,28 and failed to react with platelets in the presence of the unmodified primary drugs. Using serum from these patients, we tested whether the NOD/scid mouse, when challenged with naproxen or acetaminophen, would produce a sufficient amount of the glucuronide conjugate to promote platelet clearance by the metabolite-specific antibodies. As shown in Figure 6A, injection of 50 mg/kg acetaminophen into mice previously infused with platelets and an antibody specific for acetaminophen glucuronide28 led to clearance of approximately 80% of the transfused cells after 24 hours. In contrast, acetaminophen alone and patient serum alone had no effect. Figure 6B shows the results of similar experiments in which 75 mg/kg naproxen was given to animals previously infused with platelets and an antibody specific for naproxen glucuronide.24 As in the study with acetaminophen, nearly all platelets were cleared within 24 hours when the combination of drug and patient serum was administered, whereas platelets survived normally when patient serum alone or drug alone was given.
Drug metabolites produced in vivo promote accelerated clearance of platelets by metabolite-specific DDAbs. Human platelets suspended in serum containing DDAbs specific for acetaminophen glucuronide (A) or naproxen glucuronide (B) were infused into NOD/scid mice. Acetaminophen (50 mg/kg) or naproxen (75 mg/kg) were injected intraperitoneally at 0 and 5 hours. Platelet survival was significantly shortened in mice given DDAb and the unmodified primary drug (triangles) but not in mice given DDAb (squares) or drug with normal serum (circles). Values shown are the average of triplicate experiments ± 1 SEM. *P < .05, **P < .01, ***P < .001 relative to controls.
Drug metabolites produced in vivo promote accelerated clearance of platelets by metabolite-specific DDAbs. Human platelets suspended in serum containing DDAbs specific for acetaminophen glucuronide (A) or naproxen glucuronide (B) were infused into NOD/scid mice. Acetaminophen (50 mg/kg) or naproxen (75 mg/kg) were injected intraperitoneally at 0 and 5 hours. Platelet survival was significantly shortened in mice given DDAb and the unmodified primary drug (triangles) but not in mice given DDAb (squares) or drug with normal serum (circles). Values shown are the average of triplicate experiments ± 1 SEM. *P < .05, **P < .01, ***P < .001 relative to controls.
Discussion
Platelet-reactive antibodies induced by drugs like cinchona alkaloids, antibiotics, NSAIDs, and other medications are remarkable in being innocuous in the absence of the sensitizing drug but in binding avidly to epitopes on platelet membrane glycoproteins [usually alphIIb/beta3 integrin (GPIIb/IIIa)], when soluble drug is present.1,2 How drugs induce such antibodies and how, once they are formed, the sensitizing drug causes them to bind to their targets, is poorly understood.1,9 An animal model could be extremely useful for studying these and other questions concerning the pathophysiology of DITP but its development has been impeded by the fact that DDAbs found in patients react only with platelets from higher primates29 and human platelets transfused to lower species are normally cleared by xenoantibodies within minutes of being infused. Our findings suggest that the immunodeficient NOD/scid mouse, which lacks xenoantibodies and allows infused human platelets to circulate, can be used to circumvent these obstacles.
When drug and antibody were both injected by the intraperitoneal route, we found that rapid drug-dependent platelet clearance could be demonstrated with the quinine-specific mAb 314.1 (Figure 1). However, preliminary studies with human antibodies showed that only the most potent DDAbs produced a similar effect, presumably because of antibody dilution during diffusion from the peritoneum to the circulation. To obtain higher levels of circulating antibody, we determined whether antibody and platelets could be infused together into the retro-orbital plexus followed by intraperitoneal injection of drug at a later time. Preliminary studies showed that platelets suspended in normal citrated plasma or in citrated serum survived well for the first 24 hours and established baseline values (Figure 2). Using this approach with human DDAbs, it was possible to demonstrate rapid platelet clearance by stronger antibodies specific for quinine and sulfamethoxazole after intraperitoneal injection of these drugs at doses comparable with those used in treatment of a human patient (Figure 3). However, the assay was less sensitive than flow cytometry for antibody detection. On the basis of studies showing that DDAb-platelet interaction is enhanced by higher concentrations of drug (Figure 4), we tested whether DDAb-induced platelet clearance could be enhanced by injecting larger doses of drug. When quinine and sulfamethoxazole were used at supra-therapeutic doses, it was found that antibodies specific for quinine and sulfamethoxazole caused a significant shortening of platelet lifespan even when used at a dilution of 1:45, roughly the same dilution at which they could be detected in the in vitro flow cytometric assay (Figure 5).
As noted, sensitivity to drug metabolites can cause DITP22-24,28 but the responsible antibodies can be very difficult to detect.1,2 For this reason, the extent to which drug metabolites induce immune cytopenia and the range of metabolites that can cause this condition is unknown. A major reason for examining the NOD/scid model was to determine whether it could be used to detect metabolite-dependent platelet antibodies. Studies illustrated in Figure 6A and B provide evidence that mice injected intraperitoneally with acetaminophen and naproxen produce metabolites that accumulate in the circulation at levels sufficient to cause platelet destruction by antibodies specific for the glucuronide conjugates of these drugs.24,28 Many drugs become linked to glucuronide in vivo, a form in which urinary excretion is facilitated,25,26 and glucuronides appear to be especially prone to induce DDAbs specific for platelets.24,28 Findings described here suggest that the mouse model may provide an efficient way to screen suspect patient samples for glucuronide-specific antibodies.
Glucuronides can also induce antibodies capable of causing life-threatening immune hemolytic anemia,30,31 and there is no apparent reason why the model cannot be used to study this condition as well. Metabolites other than glucuronides can also induce sensitization leading to immune cytopenia. We previously described a patient with life-threatening immune hemolytic anemia triggered by the NSAID diclofenac whose antibody was specific for this drug modified by both Phase I (4′ oxidation) and Phase II (glucuronidation) metabolic pathways.31 The extent to which the mouse can be used to detect DDAbs specific for more complex metabolites of this type requires further study. However, murine and human cytochrome P450 enzymes involved in Phase I drug metabolism are similar in many respects.27 It may therefore be possible to use the mouse for this purpose.
Many drugs described as likely triggers for DITP on clinical grounds1,3,4 are highly insoluble in water and are very difficult to use in assays for antibody detection, which necessarily require suspension of target platelets in an aqueous medium. This could explain why DDAbs specific for many drugs poorly soluble in water have not been described even though clinical observations suggest they can cause DITP. It will be of interest to determine whether the in vivo assay is capable of detecting platelet-reactive antibodies induced by such drugs.
In summary, our findings suggest that the NOD/scid mouse can provide a useful tool for studying in vivo platelet destruction mediated by drug-dependent antibodies and for confirming the pathogenicity of antibodies detected in the laboratory. Further studies are needed to define the sensitivity of the model for detection of DDAbs relative to in vitro assays. However studies described here suggest that, for detection of quinine- and sulfamethoxazole-specific antibodies, the sensitivities are comparable, provided antibody is injected intravenously and drug is used at supra-therapeutic concentrations. A particularly attractive possibility is that the model will facilitate studies to define more fully the extent to which immune cytopenia is caused by sensitivity to drug metabolites.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grant HL-13 629 from the National Heart, Lung, and Blood Institute and by the Blood Research Foundation of the BloodCenter of Wisconsin.
National Institutes of Health
Authorship
Contribution: D.W.B and R.H.A. designed the study, provided oversight of laboratory work, and prepared the manuscript; P.J.N. provided intellectual insight in the design of the study; and D.N. and B.B. performed laboratory studies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel Bougie, PO Box 2178, Milwaukee, WI 53201; e-mail: Dan.bougie@bcw.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal