Abstract
Regulated vascular endothelial growth factor (VEGF) signaling is required for proper angiogenesis, and excess VEGF signaling results in aberrantly formed vessels that do not function properly. Tumor endothelial cells have excess centrosomes and are aneuploid, properties that probably contribute to the morphologic and functional abnormalities of tumor vessels. We hypothesized that endothelial cell centrosome number is regulated by signaling via angiogenic factors, such as VEGF. We found that endothelial cells in developing vessels exposed to elevated VEGF signaling display centrosome overduplication. Signaling from VEGF, through either MEK/ERK or AKT to cyclin E/Cdk2, is amplified in association with centrosome overduplication, and blockade of relevant pathway components rescued the centrosome overduplication defect. Endothelial cells exposed to elevated FGF also had excess centrosomes, suggesting that multiple angiogenic factors regulate centrosome number. Endothelial cells with excess centrosomes survived and formed aberrant spindles at mitosis. Developing vessels exposed to elevated VEGF signaling also exhibited increased aneuploidy of endothelial cells, which is associated with cellular dysfunction. These results provide the first link between VEGF signaling and regulation of the centrosome duplication cycle, and suggest that endothelial cell centrosome overduplication contributes to aberrant angiogenesis in developing vessel networks exposed to excess angiogenic factors.
Introduction
Blood vessels supply both normal and diseased tissues with the oxygen and nutrients necessary for growth and survival. Thus, proper blood vessel formation and expansion are critical for normal development and for the progression of diseases, such as cancer.1,2 Blood vessel networks expand via angiogenesis, a process whereby vessels form by sprouting migration from preexisting vessels. Angiogenic expansion requires regulated endothelial cell division. Endothelial cell division in developing vessels, as in other cells, is a tightly regulated process ensuring that DNA goes through only one round of replication per cell cycle. The centrosome that composes the microtubule organizing center during interphase also replicates only once per cell cycle, to provide 2 centrosomes that facilitate mitotic spindle assembly during mitosis.3 Cell-cycle regulation is well characterized in terms of timing, checkpoints, and regulation of DNA replication. However, regulation of centrosome duplication is less well understood in general, and even less is known about how this critical cellular process is regulated in endothelial cells. Centrosome overduplication is associated with elevated cyclin E/Cdk2 activity in other cell types; loss of p53, which can inhibit cyclin E accumulation, also promotes centrosome overduplication.4 Tumor endothelial cells have excess centrosomes and are aneuploid, but the signaling pathways responsible for this phenotype are unknown.5,6
Endothelial cell proliferation and migration are normally tightly regulated to form proper vessels, and angiogenic factors, such as vascular endothelial growth factor-A (VEGF) signaling, have a central role in these processes.7,8 Developing vessels express several VEGF receptors, including Flk-1 (VEGFR-2) and Flt-1 (VEGFR-1). Genetic loss of VEGF pathway components leads to vessel perturbations and embryonic lethality, but the phenotypes differ. Homozygous loss of function for flk-1 or heterozygosity for Vegfa results in dramatically reduced blood vessel formation because VEGF binding to Flk-1 positively activates downstream signaling that promotes endothelial proliferation, migration, and survival.9-13 In contrast, loss of flt-1 leads to vessel overgrowth and dysmorphogenesis that results from both increased endothelial cell proliferation and decreased vessel branching.14-16 We and others have shown that Flt-1 functions developmentally as a VEGF sink to negatively modulate VEGF-mediated signaling through Flk-1, and the flt-1−/− mutation thus behaves like a gain-of-function perturbation in VEGF signaling.17-19 Tumor endothelial cells are exposed to elevated VEGF levels produced by tumor cells, and tumor vessels also express low levels of Flt-1 compared with normal vessels, suggesting that multiple inputs promote excess VEGF signaling in tumor endothelial cells.20,21
We hypothesized that signaling of angiogenic factors, such as VEGF, regulates centrosome number in endothelial cells, and that elevated VEGF misregulates endothelial cell centrosome duplication. Our hypothesis predicts that endothelial cells with aberrant centrosome numbers are not restricted to tumor vessels, but are found more generally in any environment with elevated endothelial VEGF signaling. Here, we show that elevated VEGF or FGF signaling leads to excess centrosomes in endothelial cells. This mis-regulation uses both MEK/ERK and AKT signaling pathways downstream of VEGF-A to enhance cyclin E/Cdk2 activity. Furthermore, endothelial cells with excess centrosomes survive and form aberrant spindles during cell division, and vessels exposed to high VEGF signaling have elevated levels of endothelial cell aneuploidy. These are the first data to link regulation of endothelial cell centrosome duplication to upstream VEGF signaling, and they highlight a novel mechanism that probably contributes to the dysfunction of vessels exposed to elevated angiogenic factor signaling.
Methods
Cells, yolk sacs, and VEGF manipulations
Wild-type (WT) and flt-1−/− mouse embryonic stem (ES) cells were differentiated for 8 days as described previously.22,23 For analysis of differentiated and dissociated ES cell cultures, WT and flt-1−/− ES cell lines expressing H2B-eGFP downstream of the PECAM-1 promoter/enhancer were used.24 Cultures were dissociated for 20 minutes in trypsin-ethylenediaminetetraacetic acid (Invitrogen), strained to eliminate clumps (70-μm Nylon strainer, BD Biosciences 352350), and plated on 0.1% gelatin-coated dishes for 18 hours before fixation.
Human umbilical vein endothelial cells (HUVECs, Lonza Group) were cultured in endothelial growth medium-2 (EGM-2), as suggested by Lonza Group Ltd (cc-3162), or EGM-2 + 200 ng/mL VEGF-A or FGF2, with or without inhibitor because basal medium alone did not support endothelial cell proliferation. Supplemented growth factors included VEGF165 and FGF-2 (PeproTech 100-20 and 100-18B). Inhibitors included U0126 (MEK inhibitor), AKT inhibitor, SB 203580 (p38 MAPK inhibitor), and bisindolylmaleimide I (protein kinase C [PKC] inhibitor) (Calbiochem 662005, 124005, 559398, and 203290), and were applied at 5μM, 10mM, 10μM, and 10μM, respectively. Medium was replaced daily for 4 or 10 days, and cells were maintained at 30% to 70% confluence.
For HUVEC division rate analysis, HUVECs were plated at 103 cells/dish and cultured overnight in normal medium. The next day (day 0), cells were treated with low VEGF or high VEGF for 4 days, with medium replaced daily. Cell number was scored daily for 4 days. For signaling pathway analysis, HUVECs were serum-starved for 12 hours in endothelial basal medium-2 (Lonza Group) supplemented with 0.1% fetal bovine serum (Invitrogen, 35-010-CV), and then treated with low VEGF, high VEGF, or high VEGF + inhibitor for 5 minutes, 1 hour, or 12 hours. Before inhibitor treatments, cells were incubated in serum starvation media plus inhibitor for 1 hour. For apoptosis experiments, HUVECs were cultured in high VEGF for 4 days with or without the addition of 20μM 4-hydroxynonenal (HNE, Cayman Chemical) for the final 24 hours of the 4-day treatment, to induce apoptosis as described previously.25
For flt-1−/− embryonic yolk sac analysis, flt-1+/− mice were intercrossed and embryos were harvested on embryonic day 9.5 (E9.5). WT and flt-1−/− littermate yolk sacs were used for analysis after embryos were genotyped as described previously.15 Animal experiments were approved by the Institutional Animal Care and Use Committee at the University of North Carolina.
Immunofluorescence and microscopy
Differentiated ES cell cultures, cells dissociated from differentiated ES cell cultures, or HUVECs were fixed in ice cold 50% methanol/50% acetone for 5 minutes, and stained as described previously.22,23 Yolk sacs were fixed and stained as described previously.26 Primary antibodies were raised against human protein sequences unless otherwise indicated and included rabbit anti-γ-tubulin (1:1000, Sigma-Aldrich T3559), mouse antipericentrin (1:1000, Abcam), rabbit antinucleophosmin-1 (NPM; 1:500, Santa Cruz Biotechnology, sc-6013-R), rat antimouse PECAM-1 (1:1000, BD Biosciences PharMingen, 553370), rabbit antiactive caspase 3 (1:500, Abcam catalog no. ab2302), rabbit anticaspase 3 (1:500, Abcam 44976), and rabbit anti-α-tubulin (1:500, Abcam 15246). Cy3-conjugated anti-γ-tubulin antibody was used for yolk sac labeling (1:250, Sigma-Aldrich, C7604). Secondary antibodies were used at 1:250 and include goat antirat, donkey antirabbit, donkey antigoat, or goat antimouse Alexa 488 (Invitrogen, A11006, A21206, A11055, and A11029) and donkey antirabbit Alexa 594 (Invitrogen, 21207). Cells were stained with the DNA dye DRAQ5 for 30 minutes at room temperature (1:1000, Biostatus Limited, DR50050). Confocal images were acquired using a Zeiss LSM 5 Pascal microscope. For flow cytometry analysis, HUVECs were treated with low or high VEGF for 4 days, fixed and stained with propidium iodide, and fluorescence-activated cell sorting (FACS) was performed as described.27
Western blots and Cdk2 activity assay
Western blot analysis was performed as previously described, with slight modifications.28 Briefly, HUVEC lysates were collected using Mammalian Cell Lysis Buffer (Fermentas, K0301) per product instructions, and proteins were separated on a 10% sodium dodecyl sulfate–polyacrylamide gel and transferred to a polyvinylidene fluoride membrane (GE Healthcare, RPN303F). Primary antibodies raised against human protein sequences were used and included rabbit anticyclin E (1:500, Santa Cruz Biotechnology, sc-481), goat antiactin (1:500, Santa Cruz Biotechnology, sc-1615), rabbit anti-phospho-NPM (Thr199; 1:1000, Abcam, ab59353), rabbit anti-NPM (1:1000, Abcam, ab15440), rabbit anti-phospho-ERK1/2 (Thr202/Tyr204) (1:1000, Cell Signaling Technology, 9101S), mouse anti-ERK 2 (1:1000, Santa Cruz Biotechnology, sc-154), rabbit anti-phospho-AKT (Ser473; 1:1000, Cell Signaling Technology, 4060S), and rabbit anti-AKT (1:1000, Cell Signaling Technology, 9272). Signal was detected with horseradish peroxidase (HRP) antirabbit (GE Healthcare, NA934V), HRP antimouse (GE Healthcare, NA931V), or HRP antigoat (Santa Cruz Biotechnology, sc2020), and imaged via enhanced chemiluminescence (GE Healthcare, RPN2132). For the Cdk2 activity assay, HUVECs were serum-starved and treated with low or high VEGF for 12 hours. The Cdk2 activity assay was performed as described previously.27 Briefly, 12-hour lysates were subjected to immunoprecipitation with anti-Cdk2 (Santa Cruz Biotechnology, sc-163). Immune-complex kinase reactions were carried out in 25-μL kinase buffer containing 5 μg of histone H1 (Sigma-Aldrich, H4524), 1μM adenosine triphosphate, and 5 μCi of [γ-32P]adenosine triphosphate (PerkinElmer Life and Analytical Sciences, BLU002A250UC), and incubated at 30°C for 30 minutes. After the reaction was stopped, proteins were separated on a 10% sodium dodecyl sulfate–polyacrylamide gel, and gels were dried and autoradiographed.
Lentivirus infection
Human cyclin E–targeted shRNA vectors were obtained from the Open Biosystems TRC1 shRNA pLKO1 vector library (Open Biosystems). Targeted sequences were TRCN0000045298, TRCN0000045299, TRCN0000045300, TRCN0000045301, and TRCN0000045302. Virus was produced in 293T cells and collected at 36 to 60 hours after transfection at a minimum of 1 × 106 IU/mL. Cells were infected with lentivirus for 6 hours at 37°C and then treated with low or high VEGF medium for 4 days. Virus lacking a target sequence (empty vector) was used as a control.
Chromosome number analysis
WT and flt-1−/− ES cells were differentiated for 8 days, dissociated for 30 to 45 minutes in 2 mg/mL collagenase (Worthington 46S9287), and endothelial cells were isolated via magnetic bead isolation per product instructions (Invitrogen sheep antirat Dynabeads, 110.35). Rat antimouse PECAM-1 antibody was conjugated to magnetic beads for endothelial cell isolation (BD Biosciences PharMingen, 553370). After isolation, endothelial cells were cultured overnight and then treated with 0.1 μg/mL colcemid (Invitrogen, 15210) for 12 hours to halt cells in metaphase. The cells were trypsinized, fixed in 1:3 MeOH/acetic acid, and analyzed for chromosome number. Chromosome analysis was performed by KaryoLogic, according to published protocols.29
Statistical analysis
The 2-tailed Fisher exact test was used to determine statistical significance in all cases. Error bars represent SD between experiments.
Results
High VEGF signaling increases the frequency of endothelial cells with excess centrosomes in developing vessels
Centrosome duplication is tightly regulated by cell-cycle cues, and excess centrosomes can promote errors in chromosome segregation during mitosis, leading to the production of aneuploid daughter cells and aberrant cellular behaviors.30,31 During early G1, cells contain a single centrosome, composed of a mother-daughter centriole pair surrounded by pericentriolar material. By the G1/S transition, the 2 centrioles have separated, and each nucleates the growth of a new centriole. Centriole growth continues through S phase and early G2. At the onset of mitosis, centrosomes move to opposite ends of the cell and initiate the formation of a bipolar spindle. After cytokinesis, each daughter cell contains one centrosome. Thus, cells containing more than 1 centrosome in G1 or more than 2 centrosomes thereafter have excess centrosomes (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).3
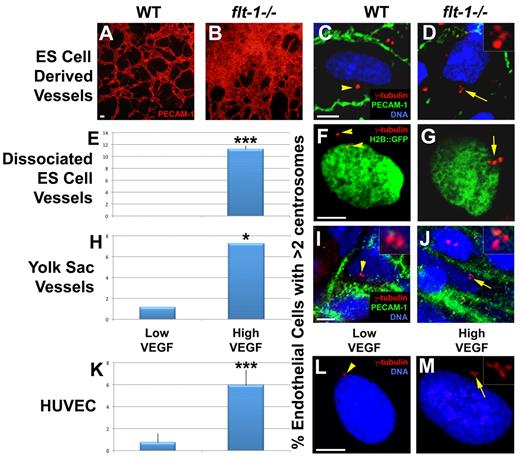
Murine endothelial cells isolated from xenograft tumors have an increased frequency of excess centrosomes, but the reason for this is unclear.6 Because tumor vessels are often exposed to high levels of angiogenic factors, such as VEGF secreted from tumor cells, we hypothesized that the presence of excess centrosomes in tumor endothelial cells is not unique to tumor endothelial cells but is a general consequence of elevated VEGF signaling. Thus, we asked whether loss of the VEGF receptor flt-1 led to excess centrosomes in endothelial cells of developing vessels because flt-1−/− vessels experience increased VEGF signaling.17-19 We first counted centrosome numbers in WT and flt-1−/− mutant vessels that form during mouse ES cell differentiation. Pluripotent ES cells induced to differentiate give rise to a variety of cell types, including 3-dimensional, lumenized vessels in a VEGF signaling context that mimics developmental scenarios.23,32 Differentiated flt-1−/− ES cell cultures displayed dramatic vascular overgrowth compared with WT ES cell cultures, and we observed endothelial cells with excess centrosomes in flt-1−/− ES cell–derived vessels (Figure 1A-D). To rigorously score centrosome numbers in ES cell–derived endothelial cells, we dissociated differentiated ES cell cultures carrying an H2B::eGFP transgene linked to a PECAM-1 enhancer-promoter that is expressed in endothelial cells.24 After a short attachment period, centrosomes were labeled with anti-γ-tubulin, and centrosome numbers in H2B::eGFP-expressing cells were quantified. Anti-γ-tubulin antibody colocalized with several distinct centrosomal markers in endothelial cells, confirming the specificity of the staining (supplemental Figure 1B-J). We found that endothelial cells from flt-1−/− ES cell–derived vessels had a significantly increased frequency of excess centrosomes compared with endothelial cells from WT vessels (Figure 1E-G).
High VEGF signaling leads to an increased frequency of excess centrosomes in endothelial cells of developing vessels. (A-B) Day 8 ES cell–derived WT and flt-1−/− mutant vessels were stained for PECAM-1 (red); note the vessel overgrowth and dysmorphogenesis in panel B. (C-J) Endothelial cells of WT and flt-1−/− mutant vessels were analyzed for centrosome numbers. (C-D) Day 8 ES cell cultures were fixed and stained for γ-tubulin (red), PECAM-1 (green), and DRAQ5 nuclear dye (blue). (F-G) Day 8 ES cell cultures that were WT or flt-1−/− and carried a PECAM-H2B::GFP transgene (green) were dissociated and attached to tissue culture dishes before fixation and staining for γ-tubulin (red). (E) Percentage of PECAM-H2B::GFP-positive cells with more than 2 centrosomes (WT, n = 159; flt-1−/−, n = 336). (I-J) WT and flt-1−/− embryos were harvested at E9.5, and yolk sacs were stained for γ-tubulin (red), PECAM-1 (green), and DRAQ5 nuclear dye (blue). (H) Percentage of PECAM-positive yolk sac endothelial cells with more than 2 centrosomes (WT, n = 88; flt-1−/−, n = 180). (L-M) HUVECs were incubated for 96 hours in low or high VEGF and then stained for γ-tubulin (red) and DRAQ5 nuclear dye (blue). (K) Percentage of HUVECs with more than 2 centrosomes (low VEGF, n = 2393; high VEGF, n = 3011). Arrows point to areas of cells with more than 2 centrosomes, and arrowheads point to areas of cells with 1 or 2 centrosomes. (D,I-J,M) Insets: Centrosomes at higher magnification. (A-B) Scale bar represents 50 μm. (C-M) Scale bar represents 5 μm. All experiments were performed at least 3 times. *P < .05, low VEGF vs high VEGF. ***P < .0001, low VEGF vs high VEGF. (A-B) Panels imaged with Olympus IX50 and 10×/0.25 NA CPlan RT objective; samples were in Aqua/Polymount (Polysciences); images acquired with Olympus DP71 camera and DP Controller Version 3.1.1.267 software. (C-M) Panels imaged with Zeiss LSM 5 Pascal and 63×/1.4 NA oil objective; samples were in Aqua/Polymount; images acquired with PASCAL Release Version 4.2 SP1 software. All images managed in Abobe Photoshop CS 2 9.0.
High VEGF signaling leads to an increased frequency of excess centrosomes in endothelial cells of developing vessels. (A-B) Day 8 ES cell–derived WT and flt-1−/− mutant vessels were stained for PECAM-1 (red); note the vessel overgrowth and dysmorphogenesis in panel B. (C-J) Endothelial cells of WT and flt-1−/− mutant vessels were analyzed for centrosome numbers. (C-D) Day 8 ES cell cultures were fixed and stained for γ-tubulin (red), PECAM-1 (green), and DRAQ5 nuclear dye (blue). (F-G) Day 8 ES cell cultures that were WT or flt-1−/− and carried a PECAM-H2B::GFP transgene (green) were dissociated and attached to tissue culture dishes before fixation and staining for γ-tubulin (red). (E) Percentage of PECAM-H2B::GFP-positive cells with more than 2 centrosomes (WT, n = 159; flt-1−/−, n = 336). (I-J) WT and flt-1−/− embryos were harvested at E9.5, and yolk sacs were stained for γ-tubulin (red), PECAM-1 (green), and DRAQ5 nuclear dye (blue). (H) Percentage of PECAM-positive yolk sac endothelial cells with more than 2 centrosomes (WT, n = 88; flt-1−/−, n = 180). (L-M) HUVECs were incubated for 96 hours in low or high VEGF and then stained for γ-tubulin (red) and DRAQ5 nuclear dye (blue). (K) Percentage of HUVECs with more than 2 centrosomes (low VEGF, n = 2393; high VEGF, n = 3011). Arrows point to areas of cells with more than 2 centrosomes, and arrowheads point to areas of cells with 1 or 2 centrosomes. (D,I-J,M) Insets: Centrosomes at higher magnification. (A-B) Scale bar represents 50 μm. (C-M) Scale bar represents 5 μm. All experiments were performed at least 3 times. *P < .05, low VEGF vs high VEGF. ***P < .0001, low VEGF vs high VEGF. (A-B) Panels imaged with Olympus IX50 and 10×/0.25 NA CPlan RT objective; samples were in Aqua/Polymount (Polysciences); images acquired with Olympus DP71 camera and DP Controller Version 3.1.1.267 software. (C-M) Panels imaged with Zeiss LSM 5 Pascal and 63×/1.4 NA oil objective; samples were in Aqua/Polymount; images acquired with PASCAL Release Version 4.2 SP1 software. All images managed in Abobe Photoshop CS 2 9.0.
We next sought to determine whether flt-1−/− vessels had excess centrosomes in vivo. We counted centrosomes in endothelial cells of WT and flt-1−/− yolk sac vessels at E9.5. Like flt-1−/− mutant ES cell–derived vessels, flt-1−/− yolk sac vessels display dysmorphogenesis and a vascular overgrowth phenotype.14 Consistent with our findings in ES cell–derived vessels, in vivo flt-1−/− mutant yolk sac vessels had significantly more endothelial cells with excess centrosomes than WT vessels (Figure 1H-J). Together, these data show that loss of flt-1 results in an increased frequency of endothelial cells with excess centrosomes.
Because flt-1−/− endothelial cells experience abnormally high VEGF signaling, we hypothesized that elevated VEGF signaling promotes mis-regulation of centrosome duplication in endothelial cells. We directly assessed the effects of elevated VEGF signaling on endothelial cells by culturing HUVECs in EGM-2 medium (low VEGF), or EGM-2 supplemented with 200 ng/mL VEGF165 (high VEGF) for 4 days, followed by centrosome number analysis. We found that treatment of HUVECs with high VEGF resulted in a significant increase in the frequency of cells with greater than 2 centrosomes compared with low VEGF-treated controls (Figure 1K-M). These data are consistent with a model whereby elevated VEGF signaling resulting from either a loss of flt-1 or exposure to excess VEGF leads to excess centrosomes in proliferating endothelial cells.
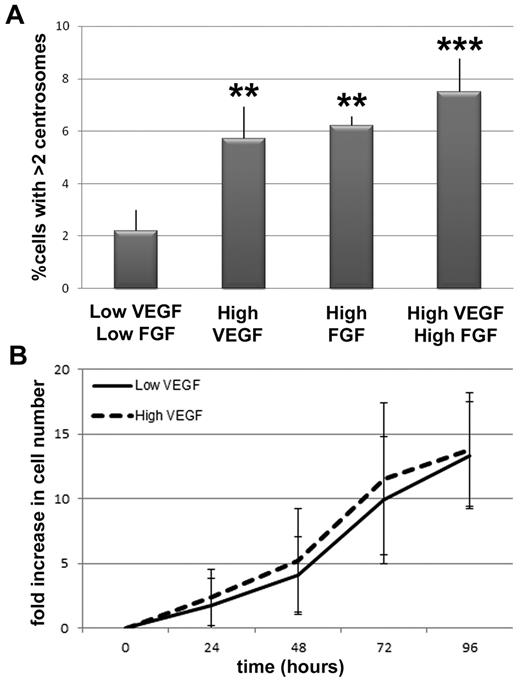
To determine whether the observed centrosome phenotype was unique to elevated VEGF signaling, or a more general feature of elevated angiogenic factor signaling, we assessed centrosome duplication in the presence of elevated fibroblast growth factor-2 (FGF-2). HUVECs incubated in high FGF-2 had a significant increase in the frequency of cells with excess centrosomes that was similar to the frequency seen with high VEGF treatment (Figure 2A). However, incubation in both high VEGF and high FGF-2 did not lead to a further increase in the frequency of endothelial cells with excess centrosomes, suggesting that the growth factor effects are not additive. Because both growth factors activate similar signaling pathways in endothelial cells, this result indicates that these pathways are probably activated at maximum capacity with the addition of one growth factor.
VEGF and FGF increase the frequency of excess centrosomes in endothelial cells independent of proliferative changes. (A) Percentage of HUVECs with more than 2 centrosomes in indicated conditions (low VEGF/low FGF, n = 1594; high VEGF, n = 1717; high FGF, n = 1563; high VEGF/high FGF, n = 866). **P < .001 vs low VEGF/low FGF. ***P < .0001 vs low VEGF/low FGF. (B) HUVEC growth curves in low and high VEGF, expressed as fold increase in cell number relative to t = 0. Solid line indicates low VEGF conditions; and dashed line, high VEGF conditions. All experiments were performed at least 3 times.
VEGF and FGF increase the frequency of excess centrosomes in endothelial cells independent of proliferative changes. (A) Percentage of HUVECs with more than 2 centrosomes in indicated conditions (low VEGF/low FGF, n = 1594; high VEGF, n = 1717; high FGF, n = 1563; high VEGF/high FGF, n = 866). **P < .001 vs low VEGF/low FGF. ***P < .0001 vs low VEGF/low FGF. (B) HUVEC growth curves in low and high VEGF, expressed as fold increase in cell number relative to t = 0. Solid line indicates low VEGF conditions; and dashed line, high VEGF conditions. All experiments were performed at least 3 times.
The VEGF-induced excess centrosome phenotype in endothelial cells is not downstream of elevated proliferation or cytokinesis defects
To begin to determine the mechanism(s) responsible for the VEGF-induced centrosome overduplication phenotype, we first determined whether HUVECs exposed to high VEGF proliferated more during the time course than controls because more cell-cycle transits might produce centrosome defects in a nonspecific manner. The number of cell doublings was independent of VEGF levels over the time course. Cells divided every 26.5 hours on average in low VEGF and every 26.2 hours on average in high VEGF, suggesting that the number of centrosome duplication cycles that occurred during the time course was equivalent in the 2 treatments (Figure 2B). These results show that the excess centrosome defect observed in high VEGF-treated endothelial cells is not the result of increased cell proliferation, and they suggest that the elevated VEGF levels in “high VEGF” treatment selectively affect centrosome numbers.
We reasoned that excess centrosomes could result from direct misregulation of pathways that regulate centrosome duplication, or they could be downstream of a cytokinesis defect. Failure to complete cytokinesis during cell division leads to daughter cells with 2 centrosomes that duplicate in the next S phase to produce cells with 4 centrosomes. To test whether high VEGF signaling promotes incomplete cytokinesis in endothelial cells, we analyzed DNA content in HUVECs incubated in low or high VEGF conditions because polyploidy is an expected consequence of incomplete cytokinesis. FACS sorting for DNA content showed that high VEGF-treated cells had similar levels of polyploid cells compared with low VEGF-treated cells (supplemental Figure 2A-B). We also analyzed the distribution of centrosome number in endothelial cells with greater than 2 centrosomes, as incomplete cytokinesis is predicted to result in a preponderance of aberrant cells with 4 centrosomes. We found that centrosome number distribution in high VEGF-treated endothelial cells with more than 2 centrosomes peaked at 3 centrosomes and was not skewed to 4 centrosomes (supplemental Figure 2C). Taken together, our findings suggest that high VEGF-treated endothelial cells do not acquire excess centrosomes as a result of incomplete cytokinesis.
High VEGF signaling promotes endothelial centrosome overduplication via hyperactivation of cyclin E/Cdk2
To test the hypothesis that abnormally high VEGF signaling directly promotes mis-regulation of centrosome duplication in endothelial cells, we investigated signaling downstream of VEGF in HUVECs. Cyclin E/Cdk2 activity initiates centrosome duplication beginning at the G1/S cell-cycle transition, and abnormally high levels are associated with centrosome overduplication in other cell types.33-37 Cyclin E/Cdk2 phosphorylates NPM at the centrosome, and this phosphorylation is required for the initiation of centrosome duplication.38 However, upstream signaling factors that feed into this pathway have not been elucidated. To determine whether increased cyclin E/Cdk2 activity in endothelial cells is responsible for VEGF-induced centrosome overduplication, we first examined cyclin E levels. HUVECs were serum-starved to synchronize the cells in a G1-like arrest, then exposed to low or high VEGF conditions for 12 hours because at that time the synchronized cells are at the G1/S transition that signals the onset of centrosome duplication (data not shown). We found increased levels of cyclin E protein in high VEGF-treated endothelial cells relative to low VEGF-treated cells (Figure 3A). To determine whether the increase in cyclin E levels was accompanied by an increase in cyclin E/Cdk2 activity, we analyzed Cdk2 activity. Cdk2 was isolated by immunoprecipitation from the same lysates used to quantify cyclin E levels, and its ability to phosphorylate histone H1 in an immune-complex kinase assay was assessed. Cdk2 activity was elevated in high VEGF-treated endothelial cells relative to controls, at the 12-hour time point and at earlier and later time points as well (Figure 3B; and data not shown). These data show that abnormally high VEGF signaling leads to elevated cyclin E/Cdk2 activity in endothelial cells.
VEGF signals through cyclin E/Cdk2 to promote mis-regulation of centrosome duplication in endothelial cells. (A-C) HUVECs were serum starved and then treated for 12 hours with low or high VEGF. (A) Western blot of lysates hybridized with anticyclin E, normalized to actin. (B) Autoradiogram of Cdk2 immune-complex kinase assay normalized to total Cdk2 immunoprecipitation (IP). (C) Western blot of lysates hybridized with anti-P-NPM, normalized to total NPM. (D-E) HUVECs were infected with empty vector (EV) or cyclin E shRNA (CycE KD) lentivirus for 6 hours and then incubated in low or high VEGF for 96 hours. (D) Western blot of lysates hybridized to anticyclin E and normalized to actin. (E) After lentiviral infection and VEGF treatment, cells were fixed, stained for γ-tubulin and DRAQ5, and centrosome numbers were counted (low VEGF, n = 656; high VEGF, n = 750; high VEGF + EV, n = 717; high VEGF + cyclin E knockdown [CycE KD], n = 696). All experiments were performed at least 3 times. ***P < .0001 vs low VEGF. #P < .05 vs high VEGF.
VEGF signals through cyclin E/Cdk2 to promote mis-regulation of centrosome duplication in endothelial cells. (A-C) HUVECs were serum starved and then treated for 12 hours with low or high VEGF. (A) Western blot of lysates hybridized with anticyclin E, normalized to actin. (B) Autoradiogram of Cdk2 immune-complex kinase assay normalized to total Cdk2 immunoprecipitation (IP). (C) Western blot of lysates hybridized with anti-P-NPM, normalized to total NPM. (D-E) HUVECs were infected with empty vector (EV) or cyclin E shRNA (CycE KD) lentivirus for 6 hours and then incubated in low or high VEGF for 96 hours. (D) Western blot of lysates hybridized to anticyclin E and normalized to actin. (E) After lentiviral infection and VEGF treatment, cells were fixed, stained for γ-tubulin and DRAQ5, and centrosome numbers were counted (low VEGF, n = 656; high VEGF, n = 750; high VEGF + EV, n = 717; high VEGF + cyclin E knockdown [CycE KD], n = 696). All experiments were performed at least 3 times. ***P < .0001 vs low VEGF. #P < .05 vs high VEGF.
To determine whether the increase in cyclin E levels induced by elevated VEGF signaling is required for misregulation of centrosome duplication in endothelial cells, we reduced cyclin E levels in high VEGF-incubated HUVECs using lentivirus-delivered shRNA. Cells were cultured in high VEGF for 96 hours after infection with either cyclin E shRNA virus or empty vector control. High VEGF incubation for 96 hours increased cyclin E protein levels, similar to the 12-hour analysis, and cyclin E shRNA infection reduced cyclin E protein levels in high VEGF-treated HUVECs to approximately the levels seen in low VEGF-treated cells (Figure 3D). HUVECs incubated in high VEGF with reduced cyclin E levels had a reduced frequency of endothelial cells with excess centrosomes relative to incubation with high VEGF and elevated cyclin E levels (Figure 3E). These data show that elevated cyclin E levels downstream of abnormally high VEGF signaling contribute substantially to centrosome overduplication in endothelial cells, and they suggest that elevated cyclin E/Cdk2 activity is responsible for the VEGF-induced misregulation of endothelial centrosome duplication.
Increased cyclin E/Cdk2 activity results in increased NPM phosphorylation at serine-199 in other cell types. This modification initiates centrosome duplication by promoting translocation of NPM from the centrosome, where it inhibits centrosome duplication, to the nucleus.38 Analysis of NPM phosphorylation in HUVECs after serum starvation and incubation in low or high VEGF for 12 hours showed that P-NPM (serine-199) levels were elevated in high VEGF-treated cells relative to controls (Figure 3C). Taken together, these results are consistent with a model whereby abnormally high VEGF signaling leads to increased cyclin E levels and cyclin E/Cdk2 hyperactivation, which in turn leads to increased P-NPM and centrosome overduplication.
MEK/ERK and AKT promote cyclin E accumulation and centrosome overduplication downstream of elevated VEGF signaling
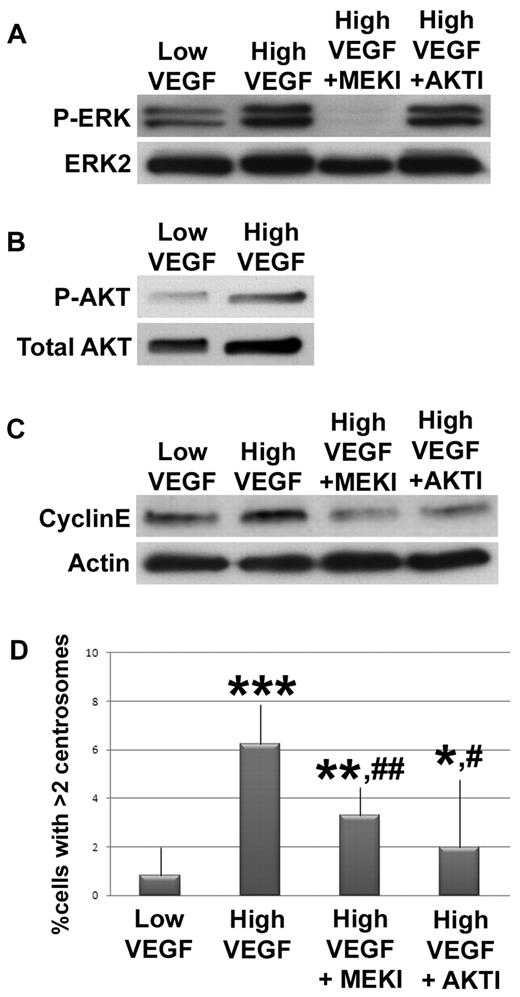
We next investigated signaling between the initial VEGF signal and the cyclin E/Cdk2 hyperactivity that promotes centrosome overduplication in endothelial cells. VEGF activates a MEK/ERK signaling cascade, and MEK/ERK signaling promotes cyclin E accumulation in other cell types.39,40 Thus, we hypothesized that elevated VEGF signals through MEK/ERK to elevate cyclin E levels and promote centrosome overduplication in endothelial cells. We assessed ERK1/2 phosphorylation in HUVECs as a proxy for ERK activation, after serum starvation and exposure to low or high VEGF for 5 minutes, and found that P-ERK levels were increased in high VEGF-treated cells relative to controls (Figure 4A). Next, we asked whether MEK inhibition rescued centrosome overduplication. HUVECs incubated in high VEGF with a MEK inhibitor (U0126) had reduced P-ERK activation, reduced cyclin E levels, and significantly rescued centrosome numbers relative to HUVECs incubated in high VEGF without inhibitor (Figure 4A-C). Thus, MEK inhibition rescued pathway hyperactivation induced by high VEGF and also rescued the centrosome overduplication defect, indicating that MEK/ERK signaling downstream of VEGF activation contributes to misregulation of centrosome duplication in endothelial cells. PKC also acts downstream of VEGF to promote ERK activation, and treatment with a PKC inhibitor also partially rescued high VEGF-induced centrosome overduplication (supplemental Figure 3). High FGF treatment also led to elevated ERK activation and cyclin E levels, suggesting that FGF also promotes centrosome overduplication via signaling through MEK/ERK to cyclin E (supplemental Figure 4).
VEGF signals through MEK/ERK and AKT to mis-regulate centrosome duplication in endothelial cells. HUVECs were serum starved, then incubated with low or high VEGF. (A) HUVECs treated for 5 minutes without inhibitor or with U0126 (MEKI) or 1L-6-hydroxymethyl-chiro-inositol 2-(R)-O-methyl-3-O-oxtadecylcarbonate (AKTI) were lysed, hybridized to anti-P-ERK, and normalized to total ERK2. (B) HUVECs treated with low or high VEGF for 1 hour were lysed, hybridized to anti-P-AKT, and normalized to total AKT. (C) HUVECs treated with indicated levels of VEGF for 12 hours without inhibitors or with MEKI or AKTI were lysed, hybridized to anticyclin E, and normalized to actin. (D) HUVECs treated with indicated levels of VEGF for 96 hours without inhibitors or with MEKI or AKTI were fixed, stained for γ-tubulin and DRAQ5, and centrosome numbers were counted (low VEGF, n = 1036; high VEGF, n = 1369; high VEGF + MEKI, n = 1152; high VEGF + AKTI, n = 586). All experiments were performed at least 3 times. *P < .05 vs low VEGF. **P < .001 vs low VEGF. ***P < .0001 vs low VEGF. #P < .05 vs high VEGF. ##P < .001 vs high VEGF.
VEGF signals through MEK/ERK and AKT to mis-regulate centrosome duplication in endothelial cells. HUVECs were serum starved, then incubated with low or high VEGF. (A) HUVECs treated for 5 minutes without inhibitor or with U0126 (MEKI) or 1L-6-hydroxymethyl-chiro-inositol 2-(R)-O-methyl-3-O-oxtadecylcarbonate (AKTI) were lysed, hybridized to anti-P-ERK, and normalized to total ERK2. (B) HUVECs treated with low or high VEGF for 1 hour were lysed, hybridized to anti-P-AKT, and normalized to total AKT. (C) HUVECs treated with indicated levels of VEGF for 12 hours without inhibitors or with MEKI or AKTI were lysed, hybridized to anticyclin E, and normalized to actin. (D) HUVECs treated with indicated levels of VEGF for 96 hours without inhibitors or with MEKI or AKTI were fixed, stained for γ-tubulin and DRAQ5, and centrosome numbers were counted (low VEGF, n = 1036; high VEGF, n = 1369; high VEGF + MEKI, n = 1152; high VEGF + AKTI, n = 586). All experiments were performed at least 3 times. *P < .05 vs low VEGF. **P < .001 vs low VEGF. ***P < .0001 vs low VEGF. #P < .05 vs high VEGF. ##P < .001 vs high VEGF.
AKT is a second signaling arm downstream of VEGF that also affects cyclin E levels.40-42 To determine whether elevated AKT activity downstream of VEGF signaling in endothelial cells also contributes to centrosome overduplication, HUVECs were serum-starved and treated with low or high VEGF for 1 hour; then AKT phosphorylation was assessed. We found elevated P-AKT in high VEGF-treated HUVECs (Figure 4B). In addi-tion, HUVECs were incubated in high VEGF with an AKT inhibitor (1L-6-hydroxymethyl-chiro-inositol 2-(R)-O-methyl-3-O-oxtadecylcarbonate). AKT inhibitor treatment significantly reduced cyclin E levels and the percentage of cells with excess centrosomes relative to high VEGF-treated HUVECs without inhibitor (Figure 4C-D). As predicted, activated ERK levels induced by high VEGF treatment were not affected by treatment with AKT inhibitor (Figure 4A), suggesting that VEGF signaling through AKT to cyclin E represents an additional mechanism whereby VEGF modulates centrosome duplication in endothelial cells. We also asked whether VEGF signals through p38 MAPK to affect centrosome number and found that 96-hour incubation in high VEGF plus p38 MAPK inhibitor did not rescue centrosome number, suggesting that VEGF does not signal through p38 MAPK to affect centrosome duplication (supplemental Figure 4). Taken together, these data suggest that VEGF signals through both MEK/ERK and AKT to promote cyclin E/Cdk2 hyperactivity and centrosome overduplication in endothelial cells.
Endothelial cells containing excess centrosomes survive and undergo mitosis
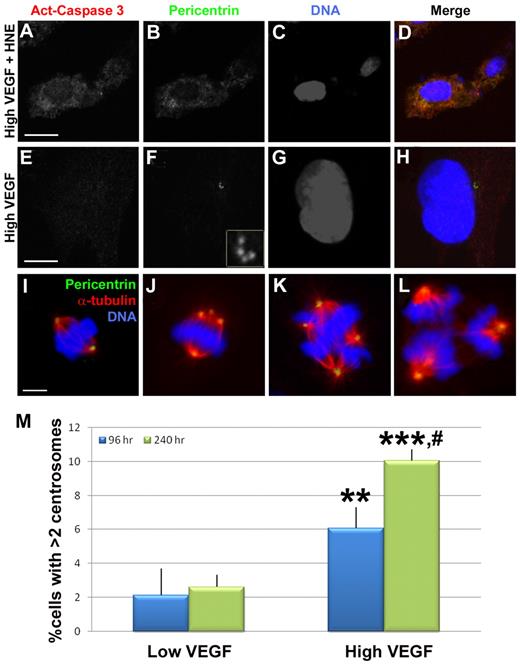
To begin to assess the biologic consequences of centrosome overduplication, we examined whether endothelial cells containing excess centrosomes survived or underwent programmed cell death. High VEGF-treated HUVECs were stained for centrosomes and activated caspase 3, a marker of apoptotic cells. Although control cells with HNE-induced apoptosis were positive for activated caspase 3, there was no detectable activated caspase 3 reactivity in HUVECs with excess centrosomes (0 of 15 cells with excess centrosomes were activated caspase-3 positive) (Figure 5A-H). We also analyzed the frequency of endothelial cells with excess centrosomes as a function of exposure time to high VEGF, reasoning that significant “drop-out” would result in a constant percentage over time, whereas survival of endothelial cells with excess centrosomes would lead to increased percentages over time. A significantly higher percentage of endothelial cells had excess centrosomes when treated for 240 hours in high VEGF compared with the normal 96-hour treatment (Figure 5M). These data indicate that endothelial cells with excess centrosomes do not undergo apoptosis at significant frequencies.
Mis-regulation of centrosome duplication does not lead to apoptosis in endothelial cells. (A-H) HUVECs were stained with antiactivated caspase 3 (red), pericentrin (green), and DRAQ5 nuclear dye (blue) after treatment with high VEGF for 96 hours, with (A-D) or without (E-H) the apoptosis-promoting drug HNE for the final 48 hours. (F) Inset: Centrosomes at higher magnification. HNE-treated cells were positive for activated caspase 3, but high VEGF-treated HUVECs containing excess centrosomes did not stain for activated caspase 3. (I-L) HUVECs were stained with antipericentrin (green), anti-α-tubulin (red), and DRAQ5 to visualize mitotic figures. Bipolar spindles containing 2 centrosomes (I) or more than 2 centrosomes (J) were observed in addition to multipolar spindles (K-L) containing more than 2 centrosomes. (M) HUVECs were incubated with low or high VEGF for the indicated times, then fixed and stained for γ-tubulin and DRAQ5 for centrosome counts (96-hour low VEGF, n = 890; 240-hour low VEGF, n = 976; 96-hour high VEGF, n = 1023; 240-hour high VEGF, n = 897). Experiments were performed in triplicate. Scale bar represents 5 μm. **P < .001 vs 96-hour low VEGF. ***P < .0001 vs 96-hour low VEGF. #P < .05 vs 96-hour high VEGF. (A-L) Panels imaged with Zeiss LSM 5 Pascal and 63×/1.4 NA oil objective; samples were in Aqua/Polymount; images acquired with PASCAL Release Version 4.2 SP1 software and managed in Abobe Photoshop CS 2 9.0.
Mis-regulation of centrosome duplication does not lead to apoptosis in endothelial cells. (A-H) HUVECs were stained with antiactivated caspase 3 (red), pericentrin (green), and DRAQ5 nuclear dye (blue) after treatment with high VEGF for 96 hours, with (A-D) or without (E-H) the apoptosis-promoting drug HNE for the final 48 hours. (F) Inset: Centrosomes at higher magnification. HNE-treated cells were positive for activated caspase 3, but high VEGF-treated HUVECs containing excess centrosomes did not stain for activated caspase 3. (I-L) HUVECs were stained with antipericentrin (green), anti-α-tubulin (red), and DRAQ5 to visualize mitotic figures. Bipolar spindles containing 2 centrosomes (I) or more than 2 centrosomes (J) were observed in addition to multipolar spindles (K-L) containing more than 2 centrosomes. (M) HUVECs were incubated with low or high VEGF for the indicated times, then fixed and stained for γ-tubulin and DRAQ5 for centrosome counts (96-hour low VEGF, n = 890; 240-hour low VEGF, n = 976; 96-hour high VEGF, n = 1023; 240-hour high VEGF, n = 897). Experiments were performed in triplicate. Scale bar represents 5 μm. **P < .001 vs 96-hour low VEGF. ***P < .0001 vs 96-hour low VEGF. #P < .05 vs 96-hour high VEGF. (A-L) Panels imaged with Zeiss LSM 5 Pascal and 63×/1.4 NA oil objective; samples were in Aqua/Polymount; images acquired with PASCAL Release Version 4.2 SP1 software and managed in Abobe Photoshop CS 2 9.0.
We next asked whether endothelial cells with excess centrosomes were capable of cell division, because mitosis in the presence of excess centrosomes can lead to aneuploidy.30,31,43 Examination of high VEGF-treated HUVECs revealed examples of bipolar spindle formation (Figure 5I-J). Although cells with 2 centrosomes had normal spindles (Figure 5I), cells with excess centrosomes displayed aberrant spindles (Figure 5J). The cells with excess centrosomes are predicted to complete mitosis but have an increased probability of aneuploidy resulting from uneven pulling forces from the spindles.31,43 We also observed mitotic endothelial cells with multipolar spindles (Figure 5K-L). These cells are also predicted to complete mitosis with high frequency but with gross aneuploidy as a result of having more than 2 spindles.
Endothelial cells of developing vessels exposed to elevated VEGF signaling have increased aneuploidy
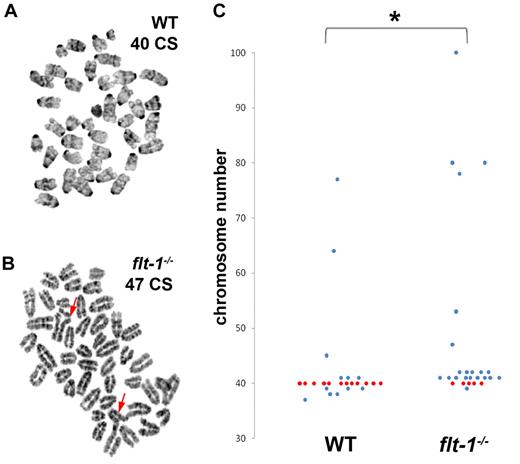
Because endothelial cells with excess centrosomes survive and divide, we hypothesized that developing vessels containing endothelial cells with excess centrosomes would also exhibit increased levels of aneuploidy. Thus, we assayed endothelial cells isolated from ES cell–derived vessels for chromosome number. As predicted, flt-1−/− endothelial cells isolated from ES cell–derived vessels, which had excess centrosomes, also displayed increased aneuploidy (average 48 chromosomes/cell, normal = 40) compared with endothelial cells isolated from WT vessels (average 42 chromosomes/cell; Figure 6). Flt-1−/− endothelial cells also had chromosomal aberrations, including chromosome breaks and triradials, that were not detected in WT endothelial cells (Figure 6A-B). These results suggest that vessels containing endothelial cells with excess centrosomes accumulate aneuploid cells that contribute to vessel dysfunction.
Flt-1−/− mutant endothelial cells from developing vessels display aneuploidy and chromosome aberrations. Endothelial cells from WT and flt-1−/− ES cell–derived vessels were isolated via magnetic bead isolation and analyzed for chromosome number and abnormalities. (A-B) Giemsa-stained WT (A) and flt-1−/− (B) endothelial cell metaphase spreads with 40 and 47 chromosomes (CS), respectively (Mus musculus 2n = 40). Arrows in panel B point to abnormal triradial chromosome configurations. (C) Scatter plot showing chromosome number in WT versus flt-1−/− endothelial cells from developing vessels. Each dot represents one cell; red dots, cells with 40 CS; and blue dots, aneuploid cells. WT, n = 25; flt-1−/−, n = 25. *P ≤ .05.
Flt-1−/− mutant endothelial cells from developing vessels display aneuploidy and chromosome aberrations. Endothelial cells from WT and flt-1−/− ES cell–derived vessels were isolated via magnetic bead isolation and analyzed for chromosome number and abnormalities. (A-B) Giemsa-stained WT (A) and flt-1−/− (B) endothelial cell metaphase spreads with 40 and 47 chromosomes (CS), respectively (Mus musculus 2n = 40). Arrows in panel B point to abnormal triradial chromosome configurations. (C) Scatter plot showing chromosome number in WT versus flt-1−/− endothelial cells from developing vessels. Each dot represents one cell; red dots, cells with 40 CS; and blue dots, aneuploid cells. WT, n = 25; flt-1−/−, n = 25. *P ≤ .05.
Discussion
Our work demonstrates that elevated angiogenic factor signaling promotes centrosome overduplication in the endothelial cells of developing vessels, and it provides a mechanistic understanding of this phenotype. We also show that endothelial cells with excess centrosomes are not restricted to tumor vessels, but are a hallmark of vessels exposed to elevated VEGF signaling in several contexts and are associated with aberrant cell divisions and aneuploidy. Thus, misregulation of centrosome duplication is a novel aspect of deregulated angiogenic factor signaling that impacts the phenotype and potentially the function of endothelial cells in blood vessels.
Developing vessels lacking flt-1 function have an elevated frequency of endothelial cells with aberrant centrosome numbers in both ES cell-derived vessels and in vivo, in the developing vessels of the embryonic yolk sac. These vessels also have an elevated mitotic index, suggesting that flt-1 mutant endothelial cells have a shorter cell-cycle progression time relative to WT endothelial cells.15 It is possible that a low “normal” frequency of centrosome overduplication is amplified in the flt-1 mutant background because of this acceleration of the cell cycle. However, the difference in mitotic indices between WT and flt-1−/− endothelial cells is small (1.4% [WT] vs 2.8% [flt-1−/−]) for yolk sac endothelial cells15 relative to the difference in the frequency of endothelial cells with excess centrosomes (0% [WT] vs 11% [flt-1−/−], this study), suggesting that centrosome overduplication in endothelial cells of flt-1−/− developing vessels is not solely a consequence of an accelerated cell cycle. Moreover, we demonstrate a significant increase in centrosome overduplication in HUVECs exposed to elevated VEGF signaling, that is not accompanied by an overall increase in doubling time. These findings indicate that misregulation of centrosome duplication is a primary effect of abnormally high VEGF signaling.
Elucidation of signaling downstream of VEGF that promotes centrosome overduplication in endothelial cells provides a mechanistic basis for the phenotype, and it also supports a model whereby aberrant centrosome numbers are a direct effect of perturbed VEGF signaling. We show that downstream of elevated VEGF, both MEK/ERK signaling, and AKT signaling are elevated, as expected from previous studies of VEGF signaling in endothelial cells.40,41 Both MEK/ERK and AKT feed into regulation of the cell-cycle regulator cyclin E/Cdk2 activity in other cell types, and cyclin E/Cdk2 is implicated in regulation of centrosome duplication.33-37 Thus, we investigated cyclin E/Cdk2 in VEGF-stimulated endothelial cells and found that both cyclin E levels and Cdk2 activity were increased, consistent with a model whereby VEGF signaling exerts a direct effect on cyclin E/Cdk2. Moreover, blockade of any component of these signaling axes downstream of VEGF significantly reduced the VEGF-induced centrosome overduplication defect, showing that these activities are required for the phenotype. Both MEK/ERK and AKT signaling have numerous effects on endothelial cells, but the finding that attenuated signaling through either cassette also reduced the high VEGF-induced elevated levels of cyclin E suggests that direct effects from VEGF through MEK/ERK and AKT to cyclin E/Cdk2 regulate centrosome duplication in endothelial cells (Figure 7). Blockade of PKC, but not p38 MAPK, also partially rescued centrosome number, suggesting that several, but not all, signaling cassettes downstream of VEGF affect centrosome overduplication in endothelial cells. Phosphorylation of the centrosome-associated protein NPM downstream of cyclin E/Cdk2 occurs in high VEGF-stimulated endothelial cells, and this phosphorylation can affect centrosome duplication.38 The microtubule-binding protein ninein has a complex localization pattern in endothelial cells that is regulated by phosphorylation downstream of VEGF,44 so it may also be a downstream effector of VEGF effects on centrosomes. FAK mutant endothelial cells or endothelial cells expressing Ser-732-mutated FAK protein were reported to have excess centrosomes,45 suggesting that both proper adhesion to substrates and VEGF signaling are important for regulation of centrosome numbers in endothelial cells.
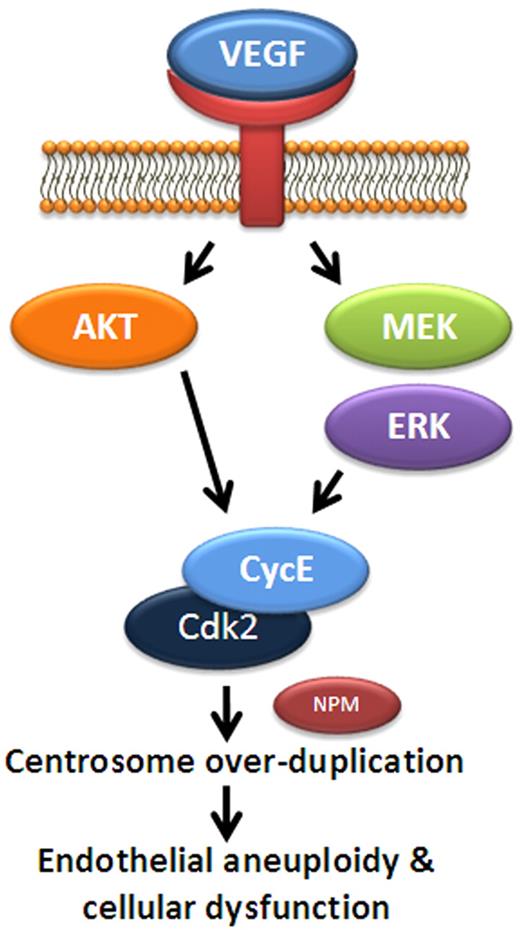
Model for VEGF regulation of endothelial centrosome duplication. A schematic of proposed signaling to regulate centrosome duplication in endothelial cells. In this model, VEGF promotes MEK/ERK and AKT signaling, leading to increased levels of cyclin E. Cyclin E binds Cdk2 to activate cyclin E/Cdk2 activity and promotes centrosome duplication, perhaps via phosphorylation of NPM. Elevated VEGF levels lead to abnormally high Cdk2/cyclin E activity and centrosome overduplication.
Model for VEGF regulation of endothelial centrosome duplication. A schematic of proposed signaling to regulate centrosome duplication in endothelial cells. In this model, VEGF promotes MEK/ERK and AKT signaling, leading to increased levels of cyclin E. Cyclin E binds Cdk2 to activate cyclin E/Cdk2 activity and promotes centrosome duplication, perhaps via phosphorylation of NPM. Elevated VEGF levels lead to abnormally high Cdk2/cyclin E activity and centrosome overduplication.
What are the consequences of aberrant centrosome numbers in endothelial cells of developing vessels? Endothelial cells with excess centrosomes as a result of elevated VEGF signaling do not undergo apoptosis, but rather they appear to survive and accumulate. Moreover, we demonstrate that endothelial cells with excess centrosomes can form aberrant spindles during mitosis, an abnormality that is predicted to lead to endothelial aneuploidy. Consistent with this hypothesis, endothelial cells isolated from flt-1−/− mutant developing vessels, which experienced elevated VEGF signaling, had an increased frequency of aneuploidy, as assayed by abnormal chromosome number, and they also had chromosome breaks and triradials, which result from asymmetric sister chromatid exchange. The centrosome clustering and multipolar spindle formation that result from excess centrosomes are predicted to induce chromosome gain and/or loss as well as chromosome breaks at mitosis because of unequal pulling forces on the chromosomes, so the cytogenetic abnormalities seen in endothelial cells from flt-1−/− mutant vessels probably result from the increased frequency of excess centrosomes. Our data also provide a mechanism for the finding that isolated tumor endothelial cells, which presumably experience elevated VEGF signaling, have an increased frequency of excess centrosomes and elevated levels of aneuploidy.5,6 We predict that aneuploid endothelial cells probably contribute to the aberrant vascular phenotypes associated with elevated VEGF signaling, via gain or loss of chromosomes that encode genes involved in proliferation, migration, and cell-cell adhesion.
Our work has implications for regulation of angiogenesis in nondevelopmental contexts. Elevated VEGF signaling is associated with aberrant angiogenesis in several pathologies, such as diabetes and cancer. Moreover, tumors express numerous growth factors, and elevated FGF also leads to overduplication of centrosomes in endothelial cells. Tumor vessels differ substantially from normal vessels: they are tortuous in phenotype and leaky in function and thus poor at oxygen delivery.46-48 In addition, endothelial cells isolated from some tumors appear to be more “progenitor-like” and capable of differentiating into other mesodermal lineages.49 It is tempting to speculate that the introduction of aneuploidy downstream of centrosome duplication defects contributes to these changes. In any case, our demonstration of a clear link between VEGF signaling and centrosome duplication in endothelial cells of developing vessels suggests that this cellular phenotype contributes to the aberrant angiogenesis that accompanies elevated VEGF signaling.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Rebecca Rapoport and Shana McAlexander for technical support, Gefei Zeng for help with GFP expression in ES cells, Mark Peifer for critical comments on the manuscript, and the University of North Carolina Lenti-shRNA Core Facility and the UNC Flow Cytometry Core Facility for help with cyclin E knockdown and FACS analysis, respectively.
This work was supported by the National Institutes of Health grants HL43174 and HL86564 (V.L.B.), predoctoral fellowship support grant T32HD46369 (S.M.T.), AHA grant 0715187U (S.M.T.), grant K01-CA094907 (J.G.C.), and predoctoral fellowship support grant T32ES07017 (K.R.N.).
National Institutes of Health
Authorship
Contribution: S.M.T. designed and performed experiments, analyzed data, and wrote the manuscript; K.R.N. and H.L.P. performed experiments and analyzed data; G.C.R., S.L.R., and J.G.C. contributed substantial intellectual input; and V.L.B. designed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Victoria L. Bautch, Department of Biology, CB #3280, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599; e-mail: bautch@med.unc.edu.

![Figure 3. VEGF signals through cyclin E/Cdk2 to promote mis-regulation of centrosome duplication in endothelial cells. (A-C) HUVECs were serum starved and then treated for 12 hours with low or high VEGF. (A) Western blot of lysates hybridized with anticyclin E, normalized to actin. (B) Autoradiogram of Cdk2 immune-complex kinase assay normalized to total Cdk2 immunoprecipitation (IP). (C) Western blot of lysates hybridized with anti-P-NPM, normalized to total NPM. (D-E) HUVECs were infected with empty vector (EV) or cyclin E shRNA (CycE KD) lentivirus for 6 hours and then incubated in low or high VEGF for 96 hours. (D) Western blot of lysates hybridized to anticyclin E and normalized to actin. (E) After lentiviral infection and VEGF treatment, cells were fixed, stained for γ-tubulin and DRAQ5, and centrosome numbers were counted (low VEGF, n = 656; high VEGF, n = 750; high VEGF + EV, n = 717; high VEGF + cyclin E knockdown [CycE KD], n = 696). All experiments were performed at least 3 times. ***P < .0001 vs low VEGF. #P < .05 vs high VEGF.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/16/10.1182_blood-2010-01-266197/4/m_zh89991059760003.jpeg?Expires=1765915469&Signature=qMU8TeYRZMGMbOMzz7OjNNCU6FmngiW64~H6pVYmDlhTxnfzoYwvQTJrdaCPJ52DW8WPP-3vFuoqAY99szXT0W72~KTA9QkTJCHqVKz8TS95xLeVsP4~ESU2GT82Y8dhFKIrpmjJIsu0tdluGV8T3B98R53aTKUfBGpiSoTHEiceSOVlmOhpUHLv5pawTNAzWlNJstchUBU8Qh8Uu7-tF-sf4kfADLGopCxxR8REm3a2LPo~l8VTB-XbhZc3XqFQl707OkCq0Ixh-LxLWlvxmsLTq6D-YfV4VrYFYH9rBR7fltICySCKEwC8PvCK3p4euQLx3yJRNq1biVdxiq~KNA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal