Abstract
Alternatively activated macrophages (AAM) accumulate in tissues during Th2-associated immune responses like helminth infections and allergic disorders. These cells differentiate in response to interleukin 4 (IL-4)/IL-13–mediated activation of Stat6 and possess potent inhibitory activity against T cells. The molecular mechanism that leads to T-cell suppression remains unclear and could involve soluble factors or inhibitory ligands. Microarray analysis revealed that the inhibitory ligand, programmed death ligand 2 (PD-L2) was strongly induced by IL-4 in macrophages from wild-type but not Stat6-deficient mice. PD-L2 expression correlated with other established markers for AAM-like Relm-α/Fizz1, arginase1, or Ym1 and thereby serves as useful surface marker to identify and isolate AAM from tissues. Antibodies against PD-L2 blocked the inhibitory activity of AAM and retroviral expression of PD-L2 in macrophages from Stat6−/− mice was sufficient to inhibit T-cell proliferation, which demonstrates that PD-L2 mediates potent and nonredundant inhibition of T cells independently of other Stat6-regulated genes. Infection of conditional IL-4/IL-13–deficient mice with the helminth Nippostrongylus brasiliensis further showed that PD-L2 expression was dependent on IL-4/IL-13 from Th2 cells. In vivo blockade of PD-L2 during N brasiliensis infection caused an enhanced Th2 response in the lung, indicating that AAM inhibit Th2 cells by expression of PD-L2.
Introduction
The effector phase of Th2-biased immunity, which develops in response to allergens or infection with parasitic nematodes, is mainly regulated by the transcription factor Stat6 that mediates signaling through the receptors for interleukin 4 (IL-4) and IL-13. Stat6 is recruited to phosphorylated tyrosine motifs in the cytoplasmic tail of the IL-4Rα chain, gets phosphorylated by Jak kinases, and finally translocates as a homodimer to the nucleus where it regulates expression of numerous target genes.1 IL-4Rα can also trigger Stat6-independent signaling pathways leading to activation of the mitogen-activated protein kinase (MAPK) or phosphoinositide-3 kinase (PI3K) pathways that can enhance cell growth and survival.2 Th2 cells require Stat6 to maintain their differentiation state, although initial commitment is Stat6-independent.3,4 Stat6 regulates class switch recombination to IgE in B cells and expression of chemokines like Ccl11, Ccl17, Ccl22, and Ccl24, which mediate recruitment of eosinophils and other effector cells to target tissues.1 Stat6 further induces goblet cell hyperplasia, collagen production by fibroblasts, activates smooth muscle cells, and is required for expulsion of gastrointestinal helminths.
However, Stat6 is also associated with immunosuppression and poor immunosurveillance. Studies with tumor models have shown that Stat6-deficient mice develop enhanced tumor immunity compared with wild-type mice.5,6 Furthermore, it has been shown that infection of BALB/c mice with Leishmania major results in progressive inflammation mainly due to IL-4Rα/Stat6-mediated signals.7 IL-4 or IL-13 inhibit the differentiation of classically activated macrophages (CAM), which are required for parasite clearance, and rather induce the Stat6-mediated differentiation of alternatively activated macrophages (AAM). These cells are generally associated with chronic Th2-biased inflammatory conditions or tissue injury and produce characteristic markers like resistin-like molecule α (Relm-α/Fizz1), the chitinase-like protein Ym1, and arginase 1 (Arg1).8 One mechanism by which AAM can inhibit T-cell proliferation is metabolic starvation. T cells require arginine for proliferation, and AAM deplete arginine by expression of Arg1.9,10 Indeed, Arg1 from AAM has been shown to protect Schistosoma mansoni–infected mice from IL-4/IL-13–mediated lethal inflammation.10,11 Relm-α/Fizz1 is another secreted factor that has been reported to suppress activation of Th2 cells.12,13 In addition, suppression could also be mediated by direct cell contacts between AAM and T cells, as it has been described for AAM isolated from the peritoneum of Brugia malayi–infected mice.14 Potential inhibitory ligands on AAM include E-cadherin, which binds to killer cell lectin-like receptor G1 (KLRG-1)15 and members of the B7-family like CD80 and CD86 which bind cytotoxic T-lymphocyte antigen 4 (CTLA-4) or programmed death ligand 1 (PD-L1) and PD-L2, which bind the receptor PD-1.16 The potent inhibitory activity of AAM is comparable with that of myeloid-derived suppressor cells (MDSC) or Foxp3-expressing regulatory T cells (Treg).17,18 However, the suppressive activity of AAM is unique because it depends on IL-4/IL-13–mediated signals, although it remains unclear whether Stat6 or activation of the MAPK or PI3K pathway are involved.
Numerous unsolved questions remain to be addressed with regard to Stat6-controlled development, recruitment, turnover, and effector function(s) of AAM. Here, we phenotypically and functionally compared macrophages from wild-type and Stat6-deficient mice after in vitro exposure to IL-4 or infection with the helminth Nippostrongylus brasiliensis. Stat6-mediated signaling in macrophages was required for rapid PD-L2 expression and cell contact-dependent inhibition of T-cell activation. PD-L2 expression correlated with other established markers for AAM. Antibody-mediated blockade of PD-L2 restored T-cell proliferation, and retroviral transduction of PD-L2 into Stat6-deficient macrophages was sufficient to inhibit T-cell proliferation. PD-L2 was expressed on AAM in the lung of N brasiliensis–infected mice, and blockade of PD-L2 caused an enhanced Th2 response.
Methods
Mice
BALB/c mice, Stat6−/− mice, and Ly5.1 mice (B6.SJL-Ptprca Pepcb/BoyJ) were originally obtained from The Jackson Laboratory. CD4Cre mice were obtained from Taconic Farms. IL-4/enhanced green fluorescent protein (eGFP) reporter mice (4get mice) were kindly provided by R. Locksley (University of California San Francisco).19 Conditional IL-4/IL-13–deficient mice (IL-4/IL-13F/F mice) have recently been described.20 IL-4/IL-13−/− mice were kindly provided by A. McKenzie (MRC Laboratory of Molecular Biology, Cambridge, United Kingdom). All mice were backcrossed at least 9 generations to BALB/c background, housed according to institutional guidelines, and used between 6 and 12 weeks of age. The animal experiments were approved by the Regierung von Oberbayern.
Generation and stimulation of macrophages
Macrophages were differentiated from bone marrow (BM) cells in RPMI 1640 (PanBiotech) supplemented with 10% fetal calf serum (FCS; Invitrogen), 2mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin (Biochrom AG), and 5 × 10−5M β-mercaptoethanol (Merck) for 7 days in the presence of 10% supernatant from the macrophage colony-stimulating factor producing fibroblast cell line L929. After removal of nonadherent cells, macrophages (> 90% purity) were detached from Petri dishes using 5mM ethylenediaminetetraacetic acid treatment, washed, counted, and replated at a density of 106 cells/mL for 24 hours with medium (control), IL-4 (10 ng/mL; R&D Systems), or lipopolysaccharide (LPS, 1 μg/mL; Sigma-Aldrich).
In vitro cell culture
Splenocytes from BALB/c mice were labeled with 10μM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen) and cultured either alone or at indicated ratios with BM macrophages from BALB/c or Stat6−/− mice or with supernatant of cultured macrophages in 96-well flat bottom plates, which had been precoated with 0.5 μg/mL anti-βT-cell receptor (βTCR, H57-597; Biolegend) and 0.2 μg/mL anti-CD28 (37.51; eBioscience) antibodies. Cells were cultured at a density of 5 × 105 cells/well in supplemented RPMI 1640 and 20 ng/mL recombinant IL-2 (ImmunoTools). After 4 days, nonadherent cells were collected, stained for CD4 and CD8, and analyzed by flow cytometry.
In vitro blockade of PD-L2 on alternatively activated macrophages
Splenocytes from BALB/c mice were labeled with 10μM CFSE and were either cultured alone or with IL-4 (10 ng/mL)-treated BM macrophages from BALB/c mice as described above. Anti-PD-L2 (122; 5 μg/mL) blocking antibody or isotype control (immunoglobulin G2a [IgG2a]; 5 μg/mL) were added to the culture. After 4 days, nonadherent cells were collected, stained for CD4, and analyzed by flow cytometry.
Coculture with retrovirally transfected macrophages
The retroviral vector encoding murine PD-L2 was kindly provided by H. Kuipers (Crucell Holland BV, Leiden, The Netherlands).21 Virus-containing supernatants were produced and collected from transfected Phoenix E cells using standard procedures. BM of Stat6−/− mice were cultured for 2 days in supplemented RPMI 1640 with 10% L929 supernatant before cells were transduced by spin infection (1320g for 2 hours at 32°C) with retroviral supernatant containing 2 μg/mL polybrene (Sigma-Aldrich). The transduced cells were cultured for 5 days in the presence of 10% L929 supernatant before GFP+ macrophages were sorted and cocultured with CFSE-labeled BALB/c splenocytes at a ratio of 1:3 as described above. Nonadherent cells were collected after 4 days, stained for CD4 and CD8, and analyzed by flow cytometry.
Flow cytometry and cell sorting
Single cell suspensions were prepared by liberase CI and DNase digestion (Roche) or by mechanical disruption in 70-μm cell strainers (BD Falcon). Cells were washed once in fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline [PBS]/2% FCS/1 mg/mL sodium azide), incubated with anti-CD16/CD32 blocking antibody (2.4G2) for 5 minutes at room temperature, and stained with diluted antibody mixtures. The following monoclonal antibodies were purchased from eBioscience, unless otherwise indicated: phycoerythrin (PE)–labeled anti-CD3 (145-2C11), fluorescein isothiocyanate– or PE-Cy5.5– or PerCP-Cy5.5– or PE-labeled anti-CD4 (RM4-5), allophycocyanin (APC)–labeled anti-CD8 (53-6.7), PE- or APC-labeled anti-F4/80 (BM8), PE-labeled or biotinylated anti-PD-L2, (122), PE- or PE-Cy5.5–labeled anti-CD11c (N418), PE-labeled anti-CD11b (M1/70), Alexa Fluor 647–labeled anti-CD19 (eBio1D3), biotinylated anti–B7-1 (16-10A1), fluorescein isothiocyanate–labeled anti-CD45R (RA3-6B2), biotinylated anti–B7-2 (GL1), APC-labeled or biotinylated anti–major histocompatibility complex class II (MHC-II, I-A/I-E; M5/114.15.2), biotinylated anti–PD-L1 (1-111A), biotinylated anti–PD-1 (PMP1-30), APC-labeled anti-NK1.1 (PK 136), Alexa Fluor 647–labeled anti–PDCA-1 (eBio 927), Alexa Fluor 647–labeled CD45.1 (A20; Biolegend), PE-labeled anti–Siglec-F (E50-2440; BD Pharmingen), APC-labeled donkey anti–rat IgG (H+L; polyclonal), PE-Cy7–labeled streptavidin (BD Pharmingen), APC-labeled streptavidin (SouthernBiotech), PE-labeled or biotinylated rat IgG2a isotype control. Samples were acquired on a FACSCanto II instrument (BD Immunocytometry Systems) and analyzed by FlowJo software Version 6.4 (TreeStar). Cells were sorted using a high-speed cell sorter (FACSAria; BD Immunocytometry Systems). Macrophages in lung and bronchoalveolar lavage (BAL) were identified as autofluorescencehiSSChiFSChiCD11chiF4/80+, whereas macrophages in spleen were identified as CD11bhiF4/80hi. We excluded dead cells by using DAPI (4′,6-diamidino-2-phenylindole).
N brasiliensis infection
Third-stage larvae (L3) of N brasiliensis were recovered from the cultured feces of infected rats, washed extensively in sterile 0.9% saline (37°C), and injected (500 organisms) into mice subcutaneously at the base of the tail. Mice were provided antibiotica-containing water (2 g/L neomycin sulfate, 100 mg/L polymyxin B sulfate; Sigma-Aldrich) for the first 5 days after infection.
In vivo blockade of PD-L2
Mice (4get/Thy1.1) received intraperitoneal injections of 500 μg anti–mouse PD-L2 monoclonal antibody (mAb; TY25, rat IgG2a; Bioxcell) or control rat IgG2a (2A3; Bioxcell) on day 5 after N brasiliensis infection, followed by 250 μg on day 8.
Mixed BM chimeras
BM cells were prepared from the tibia and femur of BALB/c/Ly5.1 and Stat6−/−/Ly5.2 mice, mixed at equal ratios, and washed in PBS. Recipient BALB/c/Ly5.1 mice were lethally irradiated with 2 doses of 550 rad given 5 hours apart followed by reconstitution with 5 × 106 mixed BM cells intravenously. Mice were treated for 8 weeks with antibiotics in the drinking water (2 g/L neomycin sulfate, 100 mg/L polymyxin B sulfate).
BrdU staining
5-Bromo-2′-deoxyuridine (BrdU; 1 mg) was injected intraperitoneally in 100 μL PBS at 0, 12, 24, and 36 hours. Single-cell suspensions from total lung tissue, spleen, peritoneum, and BAL were stained for F4/80, CD11c or CD11b, and Ly5.1 followed by intracellular staining for BrdU as described.22
Immunohistochemistry
Cryostat sections (6 μm thick) of acetone-fixed lungs of BALB/c and Stat6−/− mice that had been infected with N brasiliensis 13 days before were treated with streptavidin/biotin blocking kit (Vector Laboratories). Sections were stained with biotinylated anti-PD-L2 (TY25; Biolegend) or purified rabbit anti–mouse Ym1 (StemCell Technologies) followed by biotinylated goat anti–rabbit IgG secondary antibody (Invitrogen). For detection, we used avidin-horseradish peroxidase (Vector Laboratories) or avidin-horseradish phosphatase (Vector Laboratories) and the corresponding substrates according to the manufacturer's instructions. Sections were counterstained with hematoxylin (Vector Laboratories). Pictures were acquired on a Leitz Laborlux O microscope (Leica) equipped with an Olympus E330 camera. Original magnification of the objective was ×40.
Real-time quantitative PCR
RNA was extracted from sorted macrophages using the RNeasy Mini kit (Qiagen), and 2 μg total RNA were used for first-strand cDNA synthesis. Reverse transcription polymerase chain reaction (RT-PCR) was performed using the LightCycler (Roche Diagnostics) with the primers mouse β-actin: forward 5′-ATGGATGACGATATCGCT-3′, reverse 5′-ATGAGGTAGTCTGTCAGGT-3′; mouse PD-L2: forward 5′-GAGCCAGTTTGCAGAAGGTAG-3′, reverse 5′-ATCCGACTCAGAGGGTCAATG-3′; mouse Rentla: forward 5′-CCATAGAGAGATTATCGTGGA-3′, reverse 5′-TGGTCGAGTCAACGAGTAAG-3′; mouse Arginase1: forward 5′-GTATGACGTGAGAGACCACG-3′, reverse 5′-CTCGCAAGCCAATGTACACG-3′; mouse Chi3l3: forward 5′-TGGAATTGGTGCCCCTACAA-3′, reverse 5′-AACTTGCACTGTGTATATTG-3′.
Microarrays
Gene expression profiling was performed on Affymetrix Mouse Gene ST 1.0 high-density oligonucleotide arrays. Total cellular RNA was isolated from sorted cells using RNA isolation kit (Fluka), labeled, fragmented, and hybridized to the arrays according to the manufacturer's recommendations. Raw data analysis was performed with the Affymetrix Expression Console software Version 1.1, using RMA-sketch for normalization as implemented. Data were exported as table-delimited text files to Microsoft Excel for differential gene expression analysis. Microarray data have been submitted to the Gene Expression Omnibus database (National Center for Biotechnology Information) and have the accession number GSE20030.
Statistical analysis
P values were calculated with Student t test using SigmaPlot software Version 6 (Systat Inc).
Results
The inhibitory effect of AAM on T-cell proliferation is Stat6-dependent and requires cell-cell contact
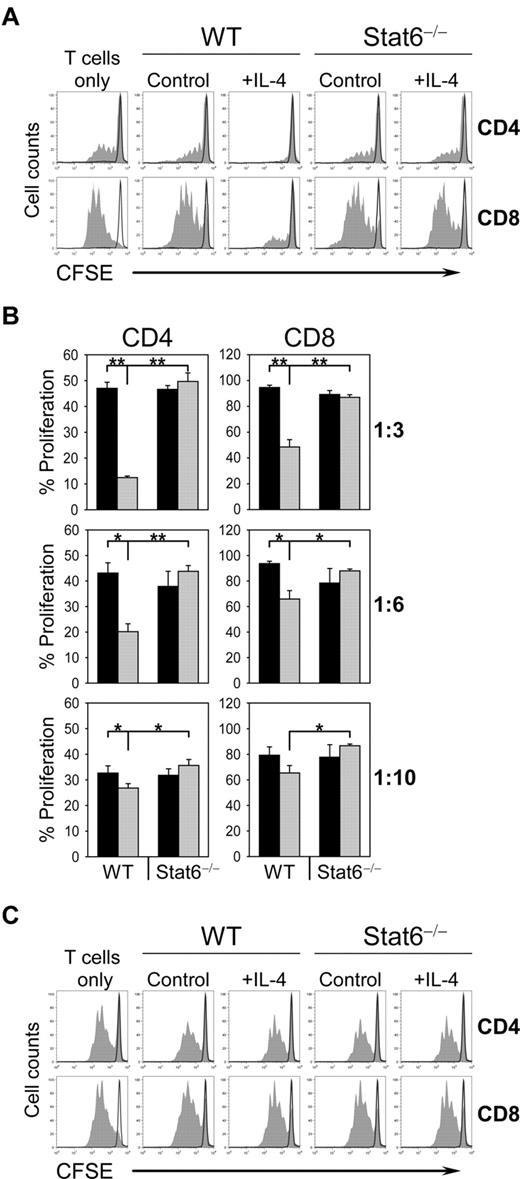
Macrophages differentiate to AAM and acquire an inhibitory activity against T cells after exposure to IL-4 or IL-13. Since downstream signaling can occur by activation of Stat6 or other pathways including the MAPK or PI3K pathways, we determined the requirement of Stat6 for AAM-mediated inhibition of T-cell proliferation. BM-derived macrophages (BMDM) from wild-type or Stat6−/− mice were precultured in the presence or absence (control) of IL-4 and then cultured with CFSE-labeled splenocytes from naive wild-type mice on α-TCR/α-CD28–coated plates. T cells exhibited high levels of proliferation in the presence of control macrophages from Stat6−/− or wild-type mice. In contrast, IL-4 exposed macrophages from wild-type but not from Stat6−/− mice markedly suppressed proliferation of CD4 and CD8 T cells, especially at a macrophage:splenocyte ratio of 1:3 (Figure 1A-B and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
IL-4–exposed macrophages suppress T-cell proliferation in a cell-cell contact–dependent manner. (A) CFSE-labeled wild-type (WT) splenocytes were left untreated (black line) or were stimulated with plate-bound anti-TCR/anti-CD28 (filled) and were either cultured alone (T cells only) or were cocultured with control or IL-4–treated (10 ng/mL) BMDM from WT or Stat6−/− mice at ratio of 1:3 (BMDM:splenocytes). Cells were collected after 4 days, stained for CD4 and CD8 and analyzed by flow cytometry. Histograms show the CFSE profile of gated CD4 and CD8 T cells. (B) CFSE-labeled WT splenocytes were cultured with untreated BMDM (black bars) or IL-4–treated BMDM (gray bars) from WT or Stat6−/− mice at ratios of 1:3, 1:6, and 1:10 (BMDM:splenocytes). Bars show the mean ± SD from 3 separate cultures per condition (**P < .001; *P < .05). (C) T-cell suppression is dependent on cell-cell contact. Supernatants of untreated or IL-4–treated WT and Stat6−/− BMDM were collected after 24 hours of stimulation and added to CFSE-labeled WT splenocytes that were left untreated (black line) or were stimulated (filled) as described in panel A. “T cells only” indicates cultures without addition of supernatant. The data are representative of 3 independent experiments.
IL-4–exposed macrophages suppress T-cell proliferation in a cell-cell contact–dependent manner. (A) CFSE-labeled wild-type (WT) splenocytes were left untreated (black line) or were stimulated with plate-bound anti-TCR/anti-CD28 (filled) and were either cultured alone (T cells only) or were cocultured with control or IL-4–treated (10 ng/mL) BMDM from WT or Stat6−/− mice at ratio of 1:3 (BMDM:splenocytes). Cells were collected after 4 days, stained for CD4 and CD8 and analyzed by flow cytometry. Histograms show the CFSE profile of gated CD4 and CD8 T cells. (B) CFSE-labeled WT splenocytes were cultured with untreated BMDM (black bars) or IL-4–treated BMDM (gray bars) from WT or Stat6−/− mice at ratios of 1:3, 1:6, and 1:10 (BMDM:splenocytes). Bars show the mean ± SD from 3 separate cultures per condition (**P < .001; *P < .05). (C) T-cell suppression is dependent on cell-cell contact. Supernatants of untreated or IL-4–treated WT and Stat6−/− BMDM were collected after 24 hours of stimulation and added to CFSE-labeled WT splenocytes that were left untreated (black line) or were stimulated (filled) as described in panel A. “T cells only” indicates cultures without addition of supernatant. The data are representative of 3 independent experiments.
To further address whether AAM acts directly on T cells to modulate T-cell proliferation or whether it is mediated by soluble factors, we stimulated T cells in the presence of supernatants from Stat6−/− or wild-type BMDM, which had been cultured with or without IL-4. No inhibitory activity could be detected in supernatants from control or IL-4–exposed Stat6−/− or wild-type macrophages (Figure 1C). This result indicates that AAM-mediated suppression of T-cell proliferation requires expression of Stat6 in macrophages and depends on cell-cell contact.
PD-L2 is expressed on AAM in a Stat6-dependent manner
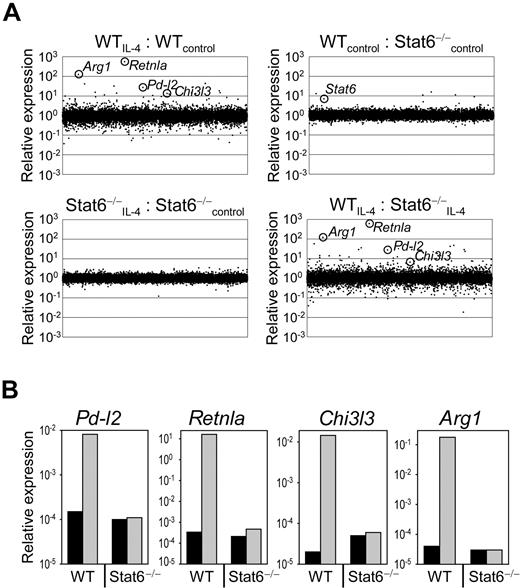
The activity of T cells can be regulated by several inhibitory receptors including CTLA-4, PD-1, Tim-3, and LAG-3. To identify expression of potential inhibitory ligands for these receptors on AAM, which could be involved in mediating T-cell inhibition, we analyzed the gene expression profile of Stat6−/− or wild-type BMDM before and after exposure to IL-4 using high-density oligonucleotide arrays (Figure 2A). Direct comparison of macrophages from wild-type and Stat6−/− mice before IL-4 treatment revealed that 17 genes showed > 3-fold higher expression and 13 genes showed > 3-fold lower expression in wild-type compared with Stat6−/− macrophages. Exposure of wild-type macrophages to IL-4 induced 113 genes > 3-fold and repressed 394 genes > 3-fold in comparison to untreated wild-type macrophages. Seventy-nine percent of the up-regulated genes and 53% of the down-regulated genes were controlled by Stat6. Among the highly up-regulated genes were typical signature genes for AAM like Retnla (558-fold up, encodes for Relm-α/Fizz1), Arg1 (124-fold up, encodes for arginase 1), and Chi3l3 (13-fold up, encodes for Ym1; Figure 2A; WTIL-4:WTcontrol). Expression of these genes was dependent on Stat6 and confirms previous reports (Figure 2A; WTIL-4:Stat6−/−IL-4).23,24 We further observed that PD-L2, an inhibitory ligand for PD-1, was strongly (27-fold) induced by IL-4 in a Stat6-dependent manner in AAM (Figure 2A). The Stat6-dependent expression of Retnla, Arg1, Chi3l3, and Pd-l2 was confirmed by quantitative RT-PCR analysis (Figure 2B).
Stat6-regulated gene expression profile of macrophages. (A) RNA was isolated from F4/80+-sorted (purity 99%), untreated (control), or IL-4–treated (10 ng/mL) wild-type (WT) and Stat6−/− BMDM. Gene expression profile was performed on high-density oligonucleotide arrays (Affymetrix). (B) Quantitative RT-PCR analysis of selected genes that appeared differently expressed in untreated (black bars) or IL-4–treated (gray bars) WT and Stat6−/− BMDM based on the microarray experiment. Samples were normalized to β-actin expression. The microarray data have been deposited to the Gene Expression Omnibus database under the accession number GSE20030.
Stat6-regulated gene expression profile of macrophages. (A) RNA was isolated from F4/80+-sorted (purity 99%), untreated (control), or IL-4–treated (10 ng/mL) wild-type (WT) and Stat6−/− BMDM. Gene expression profile was performed on high-density oligonucleotide arrays (Affymetrix). (B) Quantitative RT-PCR analysis of selected genes that appeared differently expressed in untreated (black bars) or IL-4–treated (gray bars) WT and Stat6−/− BMDM based on the microarray experiment. Samples were normalized to β-actin expression. The microarray data have been deposited to the Gene Expression Omnibus database under the accession number GSE20030.
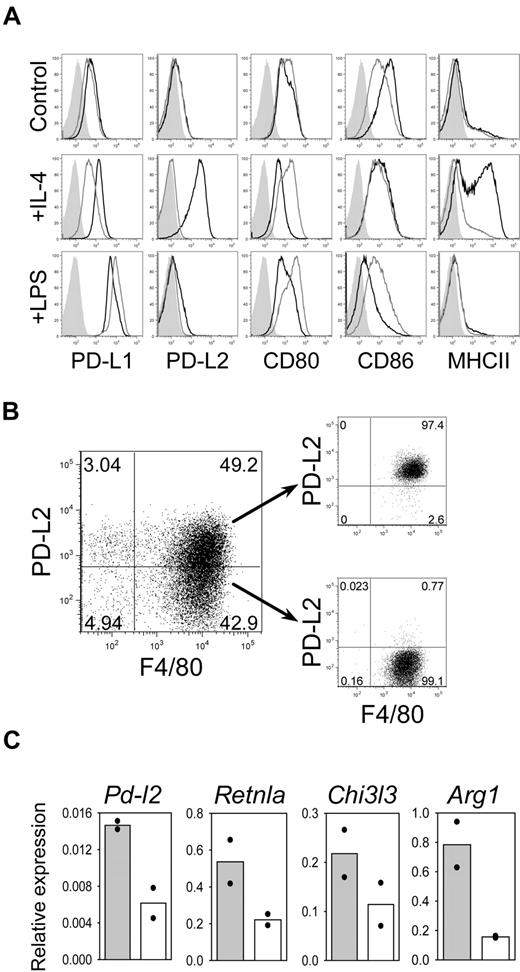
Next, we compared the IL-4–induced expression of PD-L2 to other potentially inhibitory ligands of the B7 family by flow cytometry. BMDM from wild-type and Stat6−/− mice were either left untreated or cultured for 24 hours with LPS (to induce CAM) or IL-4 (to induce AAM). Untreated macrophages showed no difference in expression of PD-L1, PD-L2, CD80, and CD86 between wild-type and Stat6−/− macrophages (Figure 3A). The expression level of MHC-II, which is known to be regulated by Stat6 in macrophages,25 was also comparable. IL-4 induced strong up-regulation of PD-L2 and MHC-II on wild-type but not Stat6−/− macrophages. In contrast, LPS induced strong up-regulation of PD-L1 in macrophages from both mouse strains. Furthermore, CD86 was down-regulated on LPS-exposed wild-type but not Stat6−/− macrophages (Figure 3A). To determine whether PD-L2 surface expression correlates with established markers for AAM, we stimulated BMDM for 6 hours with IL-4, sorted PD-L2+ and PD-L2− macrophages, and performed quantitative RT-PCR analysis. PD-L2+ sorted cells showed 2- to 4-fold higher expression levels of the AAM markers Retnla, Chi3l3, and Arg1 compared with PD-L2− cells (Figure 3B-C). We conclude from these results that PD-L2 can indeed serve as a reliable surface marker for AAM.
PD-L2 is the only B7 family member that is induced by IL-4 on BMDM in a Stat6-dependent manner. (A) BMDM from wild-type mice (black line) and Stat6−/− mice (gray line) were cultured in medium alone (control) or were activated for 24 hours with IL-4 (10 ng/mL) or LPS (1 μg/mL). The cells were double-stained for F4/80 and PD-L1, PD-L2, CD80, CD86, or MHC-II. Histograms are gated on F4/80+ macrophages. Filled histograms show isotype control staining. Data shown are representative of 3 independent experiments. (B) BMDM from WT mice were cultured for 6 hours with IL-4 and sorted into PD-L2+ and PD-L2− populations. (C) PD-L2 expression correlates with higher expression levels of the AAM markers Retnla, Chi3l3, and Arg1. RNA was isolated from PD-L2+ (solid bars) or PD-L2− (open bars) BMDM and analyzed by quantitative RT-PCR. The graphs show means of 2 independent experiments, normalized to β-actin expression.
PD-L2 is the only B7 family member that is induced by IL-4 on BMDM in a Stat6-dependent manner. (A) BMDM from wild-type mice (black line) and Stat6−/− mice (gray line) were cultured in medium alone (control) or were activated for 24 hours with IL-4 (10 ng/mL) or LPS (1 μg/mL). The cells were double-stained for F4/80 and PD-L1, PD-L2, CD80, CD86, or MHC-II. Histograms are gated on F4/80+ macrophages. Filled histograms show isotype control staining. Data shown are representative of 3 independent experiments. (B) BMDM from WT mice were cultured for 6 hours with IL-4 and sorted into PD-L2+ and PD-L2− populations. (C) PD-L2 expression correlates with higher expression levels of the AAM markers Retnla, Chi3l3, and Arg1. RNA was isolated from PD-L2+ (solid bars) or PD-L2− (open bars) BMDM and analyzed by quantitative RT-PCR. The graphs show means of 2 independent experiments, normalized to β-actin expression.
Kinetics, Stat6 dependence, and plasticity of PD-L2 expression
To further characterize the expression kinetics of PD-L2 on AAM, we measured PD-L2 expression by flow cytometry at different time points after IL-4 stimulation of BMDM. PD-L2 was induced already 2 hours after stimulation with a rapid increase after 6 hours and a maximum of 97% positive cells at 24 hours (supplemental Figure 2A). Interestingly, although Stat6 appears to be required for the IL-4–mediated PD-L2 up-regulation in AAM, there are no Stat6-binding sites present in the PD-L2 promoter.26 Therefore, it remains unclear whether Stat6-mediated signaling is directly required in PD-L2 expressing macrophages or whether Stat6-induced factors mediate PD-L2 expression on bystander macrophages. To distinguish between these 2 possibilities, we cocultured congenic wild-type (Ly5.1+) and Stat6−/− (Ly5.1−) BMDM in the presence of IL-4. PD-L2 was only induced in wild-type cells, clearly demonstrating the cell-intrinsic requirement of Stat6 for PD-L2 expression (supplemental Figure 2B).
Next, we addressed the plasticity of PD-L2 and PD-L1 expression on AAM and CAM. We first sorted PD-L2+ AAM and PD-L1high CAM and cultured these cells for 24 hours under the opposite polarizing conditions (ie, AAM were treated with LPS, and CAM were treated with IL-4). IL-4 induced expression of PD-L2 on CAM, while LPS induced expression of PD-L1 on AAM (supplemental Figure 2C). These results demonstrate that the differentiation state of macrophages is flexible, and the cells can rapidly respond to environmental changes.
PD-L2 is required and sufficient to mediate the inhibitory activity of AAM
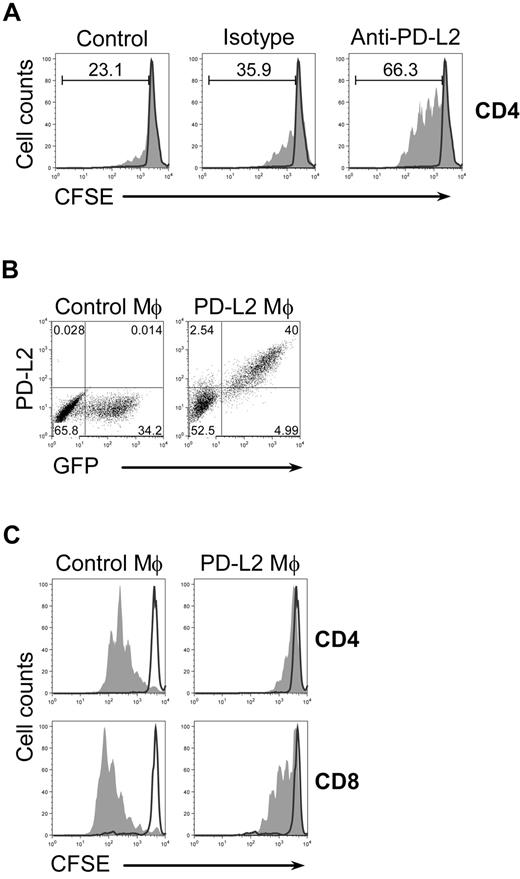
The association between PD-L2 expression and the inhibitory activity of AAM suggested that inhibition could be mediated by interaction between the inhibitory receptor PD-1 on T cells and PD-L2 on AAM. To directly address this possibility, we performed coculture experiments with AAM- and CFSE-labeled T cells stimulated with α-TCR/α-CD28 mAb in the presence of blocking antibodies against PD-L2. T-cell proliferation was increased in anti-PD-L2–treated cocultures in comparison to untreated cultures (Figure 4A). This effect was specific for PD-L2, since isotype control antibodies had no effect. Anti-PD-L2 administration in the absence of AAM did not enhance the proliferation, indicating that PD-L2 on AAM is responsible for the inhibitory effect on T-cell proliferation (supplemental Figure 3).
PD-L2 on AAM is required and sufficient to mediate inhibition. (A) Untreated (black line) or anti-TCR/anti-CD28 stimulated (filled) CFSE-labeled wild-type splenocytes were cultured with IL-4–treated (10 ng/mL) BMDM from wild-type mice in the absence (control) or presence of anti-PD-L2 (5 μg/mL) or isotype control (5 μg/mL) and analyzed after 4 days. Histograms are gated on CD4+ T cells. The experiment has been repeated with similar results. (B) BMDM from Stat6−/− mice were transduced with a bicistronic retroviral PD-L2/GFP expression vector (PD-L2 Mϕ) or empty GFP control vector (control Mϕ). Cells were stained for F4/80 and PD-L2. Dot plots are gated on F4/80+ cells. F4/80+GFP+ macrophages were sorted with > 94% purity. (C) CFSE-labeled wild-type splenocytes were left untreated (black line) or were stimulated with plate-bound anti-TCR/anti-CD28 (filled), cultured in the presence of GFP+-sorted macrophages and analyzed after 4 days. Histograms are gated on CD4+ or CD8+ cells as indicated. The data are representative of 3 independent experiments.
PD-L2 on AAM is required and sufficient to mediate inhibition. (A) Untreated (black line) or anti-TCR/anti-CD28 stimulated (filled) CFSE-labeled wild-type splenocytes were cultured with IL-4–treated (10 ng/mL) BMDM from wild-type mice in the absence (control) or presence of anti-PD-L2 (5 μg/mL) or isotype control (5 μg/mL) and analyzed after 4 days. Histograms are gated on CD4+ T cells. The experiment has been repeated with similar results. (B) BMDM from Stat6−/− mice were transduced with a bicistronic retroviral PD-L2/GFP expression vector (PD-L2 Mϕ) or empty GFP control vector (control Mϕ). Cells were stained for F4/80 and PD-L2. Dot plots are gated on F4/80+ cells. F4/80+GFP+ macrophages were sorted with > 94% purity. (C) CFSE-labeled wild-type splenocytes were left untreated (black line) or were stimulated with plate-bound anti-TCR/anti-CD28 (filled), cultured in the presence of GFP+-sorted macrophages and analyzed after 4 days. Histograms are gated on CD4+ or CD8+ cells as indicated. The data are representative of 3 independent experiments.
Because Stat6 is required for PD-L2 expression and for the inhibitory activity of AAM in general, we sought to determine whether forced expression of PD-L2 in Stat6−/− macrophages would be sufficient to mediate suppression of T-cell proliferation. We transduced BMDM from Stat6−/− mice with a bicistronic retroviral PD-L2/GFP expression vector or empty GFP control vector (Figure 4B). CFSE-labeled splenocytes from naive wild-type mice were stimulated with α-TCR/α-CD28 mAb in the presence of GFP+-sorted macrophages for 4 days. PD-L2–transduced macrophages blocked the proliferation of CD4 and CD8 T cells, whereas control macrophages had no suppressive activity (Figure 4C). This result indicates that the interaction between PD-1 and PD-L2 is a relevant inhibitory mechanism by which AAM suppress T cells, and other Stat6-dependent proteins including Arg1 or Relm-α/Fizz1 are not required for this effect.
PD-L2 is induced on macrophages in vivo during helminth infection
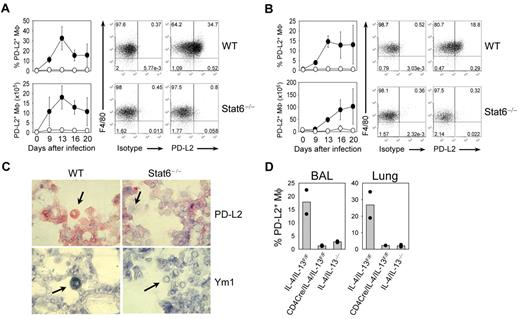
To extend our in vitro findings to a physiologic in vivo model, we decided to determine PD-L2 expression on alveolar macrophages after infection of mice with the helminth N brasiliensis, which induces a strong type 2 immune response in the lung. To determine whether PD-L2+ macrophages accumulate in the lung, we infected wild-type and Stat6−/− mice with N brasiliensis and analyzed PD-L2 expression on macrophages in lung and BAL at different days after infection. PD-L2 was induced in macrophages from wild-type mice, with a maximum expression between day 13 and 16 after infection in lung and BAL (Figure 5A-B). In contrast, Stat6−/− macrophages showed no specific staining in both lung and BAL (Figure 5A-B). The expression level of PD-L1 was comparable between wild-type and Stat6−/− mice (supplemental Figure 4). Immunohistochemical analysis of lung tissue from N brasiliensis-infected mice revealed that Ym1 and PD-L2 were expressed in alveolar macrophages of wild-type but not Stat6−/− mice (Figure 5C).
Analysis of PD-L2 expression on macrophages in vivo during N brasiliensis infection. Lung (A) and BAL (B) from wild-type (WT) and Stat6−/− mice were analyzed before or at indicated time points after N brasiliensis infection for PD-L2 expression by flow cytometry. Dot plots show staining of F4/80 and PD-L2 or isotype control on CD11c+ autofluorescencehi-gated cells at day 13 after N brasiliensis infection. Graphs show the frequency and absolute number of PD-L2+ macrophages in WT (filled circles) and Stat6−/− (open circles) mice as mean ± SE with 3 individual mice from 2 independent experiments. (C) Detection of Ym1 (blue) and PD-L2 (red) expression in alveolar macrophages (arrows) in the lung of WT but not Stat6−/− mice that had been infected with N brasiliensis 13 days before. Original magnification of the objective was ×40. (D) T-cell–derived IL-4/IL-13 is required for PD-L2 expression on AAM in vivo. Analysis of PD-L2 expression on macrophages in BAL and lung (F4/80+ CD11c+ autofluorescencehi) in complete IL-4/IL-13–deficient mice (IL-4/IL-13−/−), mice deficient of IL-4/IL-13 only in T cells (CD4Cre/IL-4/IL-13F/F), and control mice (IL-4/IL-13F/F) 13 days after N brasiliensis infection. Bars show the frequency of PD-L2+ macrophages as means of 2 mice (dots).
Analysis of PD-L2 expression on macrophages in vivo during N brasiliensis infection. Lung (A) and BAL (B) from wild-type (WT) and Stat6−/− mice were analyzed before or at indicated time points after N brasiliensis infection for PD-L2 expression by flow cytometry. Dot plots show staining of F4/80 and PD-L2 or isotype control on CD11c+ autofluorescencehi-gated cells at day 13 after N brasiliensis infection. Graphs show the frequency and absolute number of PD-L2+ macrophages in WT (filled circles) and Stat6−/− (open circles) mice as mean ± SE with 3 individual mice from 2 independent experiments. (C) Detection of Ym1 (blue) and PD-L2 (red) expression in alveolar macrophages (arrows) in the lung of WT but not Stat6−/− mice that had been infected with N brasiliensis 13 days before. Original magnification of the objective was ×40. (D) T-cell–derived IL-4/IL-13 is required for PD-L2 expression on AAM in vivo. Analysis of PD-L2 expression on macrophages in BAL and lung (F4/80+ CD11c+ autofluorescencehi) in complete IL-4/IL-13–deficient mice (IL-4/IL-13−/−), mice deficient of IL-4/IL-13 only in T cells (CD4Cre/IL-4/IL-13F/F), and control mice (IL-4/IL-13F/F) 13 days after N brasiliensis infection. Bars show the frequency of PD-L2+ macrophages as means of 2 mice (dots).
IL-4/IL-13 has been shown to be required for the differentiation of AAM,14 although the cellular source of these cytokines in vivo has not been determined. Therefore, we analyzed whether expression of IL-4/IL-13 is required from T cells or non-T cells, including eosinophils, basophils, and mast cells. We used complete IL-4/IL-13–deficient mice (IL-4/IL-13−/−) or mice that lack IL-4/IL-13 only in T cells (CD4Cre × IL-4/IL-13F/F) and control mice with normal expression of both cytokines (IL-4/IL-13F/F mice). When BAL and lung were analyzed on day 13 after N brasiliensis infection, IL-4/IL-13F/F control mice showed abundant expression of PD-L2 on macrophages. However, both IL-4/IL-13−/− and CD4Cre/IL-4/IL-13F/F mice lacked PD-L2 expression (Figure 5D). This indicates that PD-L2 expression on macrophages requires IL-4/IL-13 from Th2 cells.
Accumulation of PD-L2+ macrophages in the lung of N brasiliensis-infected mice is not due to Stat6-dependent mobilization of monocytes
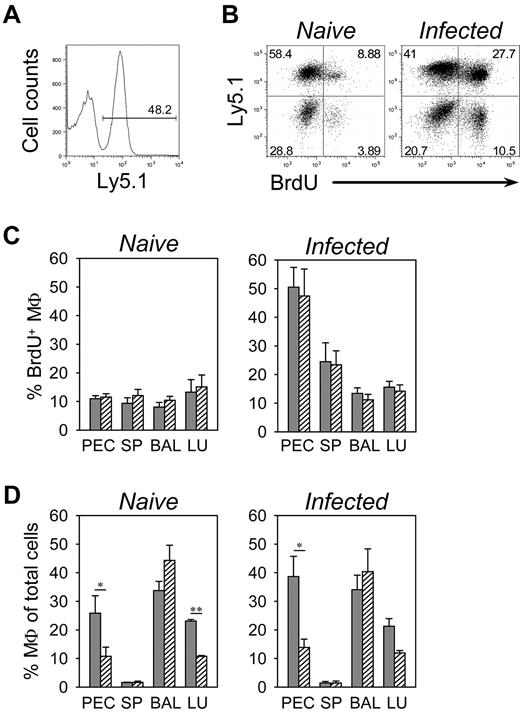
To exclude the possibility that lack of PD-L2+ macrophages in Stat6−/− mice was due to indirect effects including lack of IgE, fewer Th2 cells, or reduced mobilization and recruitment of monocytes to the lung compartment, we generated congenic mixed BM chimeras with BM from wild-type (Ly5.1+) and Stat6−/− (Ly5.1−) mice that we transferred into lethally irradiated wild-type recipient mice to analyze wild-type and Stat6−/− macrophages side-by-side in the same environment. Eight weeks after reconstitution, we observed efficient engraftment of recipient mice with BM from both donors (Figure 6A). We used these mixed chimeras to determine the turnover of wild-type and Stat6−/− macrophages in different tissues within the same animal by flow cytometric analysis of BrdU incorporation. Mice were either left untreated or had been infected with N brasiliensis 9 days before BrdU administration and analyzed 36 hours later. We observed no difference in BrdU incorporation between wild-type and Stat6−/− macrophages in all tissues analyzed. In naive mice, BrdU was incorporated in approximately 10% of macrophages in peritoneum, spleen, BAL, and lung. The incorporation rate increased 2-fold in splenic macrophages and 5-fold in peritoneal macrophages but remained unchanged in the lung of day 9 N brasiliensis-infected mice (Figure 6B-C). Despite equal BrdU incorporation, we observed preferential accumulation of wild-type macrophages in the lung and peritoneum but not in BAL and spleen (Figure 6D). This suggests that Stat6-dependent cell–intrinsic factors are not required for infection-induced mobilization of macrophages, although Stat6 seems to promote recruitment and prolong the lifespan of macrophages in selected tissues.
Analysis of Stat6 requirement for accumulation of macrophages during N brasiliensis infection. (A) Congenic mixed BM chimeras were generated with BM cells from wild-type (Ly5.1+) and Stat6−/− (Ly5.1−) donor mice to analyze whether Stat6 expression in macrophages was required for survival and recruitment to the effector sites. The histogram demonstrates equal engraftment of both donor marrows at 8 weeks after reconstitution in the peripheral blood. (B) Peritoneal cells of naive mice (left dot plot) or of day 9 N brasiliensis-infected mice (right dot plot) that had been given BrdU for the past 36 hours were stained for F4/80, Ly5.1, and BrdU. Dot plots show F4/80-gated macrophages. (C-D) Macrophages in peritoneum (PEC), spleen (SP), BAL, and lung (LU) of wild-type (filled bars) and Stat6−/− (hatched bars) mice that had been infected with N brasiliensis 9 days before (infected) or were left untreated (naive) were analyzed 36 hours after BrdU administration. Panel C shows the frequency of BrdU+ cells among total macrophages, and panel D shows the frequency of macrophages among total cells. Macrophages were identified by staining for F4/80 and CD11c (lung and BAL), F4/80 and CD11b (spleen), or F4/80 (PEC). The bars show mean ± SE of 7 individual mice from 4 independent experiments (**P ≤ .001; *P ≤ .05).
Analysis of Stat6 requirement for accumulation of macrophages during N brasiliensis infection. (A) Congenic mixed BM chimeras were generated with BM cells from wild-type (Ly5.1+) and Stat6−/− (Ly5.1−) donor mice to analyze whether Stat6 expression in macrophages was required for survival and recruitment to the effector sites. The histogram demonstrates equal engraftment of both donor marrows at 8 weeks after reconstitution in the peripheral blood. (B) Peritoneal cells of naive mice (left dot plot) or of day 9 N brasiliensis-infected mice (right dot plot) that had been given BrdU for the past 36 hours were stained for F4/80, Ly5.1, and BrdU. Dot plots show F4/80-gated macrophages. (C-D) Macrophages in peritoneum (PEC), spleen (SP), BAL, and lung (LU) of wild-type (filled bars) and Stat6−/− (hatched bars) mice that had been infected with N brasiliensis 9 days before (infected) or were left untreated (naive) were analyzed 36 hours after BrdU administration. Panel C shows the frequency of BrdU+ cells among total macrophages, and panel D shows the frequency of macrophages among total cells. Macrophages were identified by staining for F4/80 and CD11c (lung and BAL), F4/80 and CD11b (spleen), or F4/80 (PEC). The bars show mean ± SE of 7 individual mice from 4 independent experiments (**P ≤ .001; *P ≤ .05).
PD-L2 expression on macrophages correlates inversely with PD-1 expression on T cells
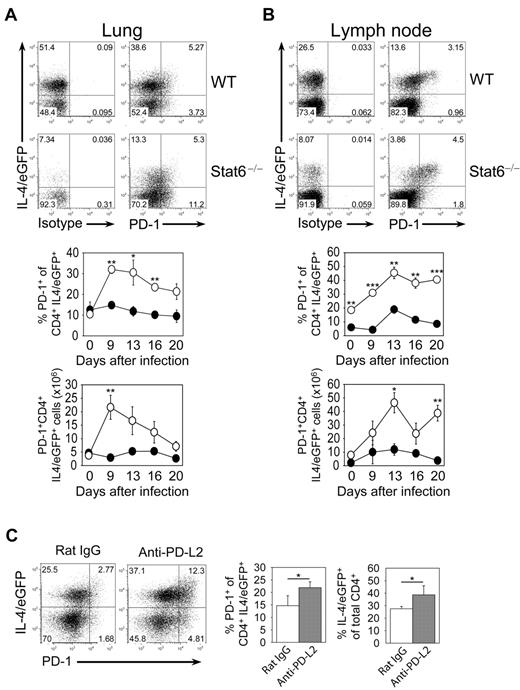
Because PD-L2 binds to PD-1, we thought that lack of PD-L2 expression on macrophages in Stat6−/− mice could modulate PD-1 expression on T cells. To address this possibility, we analyzed PD-1 expression on CD4 T cells in lung and mesenteric lymph nodes (MLN) from wild-type and Stat6−/− mice at different days after N brasiliensis infection. We used mice that had been crossed to IL-4/eGFP reporter mice (4get mice) to visualize Th2 cells. The frequency of Th2 cells (IL-4/eGFP+ cells) was higher in wild-type compared with Stat6−/− mice, reflecting the requirement of Stat6 for stable Th2 differentiation (Figure 7A-B). However, Stat6−/− mice showed an enhanced frequency of PD-1+ cells among IL-4/eGFP-expressing CD4 T cells and also contained significantly more PD-1+ IL-4/eGFP+ CD4 T cells compared with wild-type mice indicating that PD-L2 or other Stat6-dependent factors limit the accumulation of effector T cells in the lung (Figure 7A-B).
PD-1 expression on Th2 cells during N brasiliensis infection. Lung (A) and mesenteric lymph nodes (B) of 4get (WT) and 4get/Stat6−/− (Stat6−/−) mice were analyzed before or at indicated days after N brasiliensis infection. Dot plots are gated on CD4+ cells and show staining for PD-1 or isotype control versus expression of IL-4/eGFP at day 13 after N brasiliensis infection. The graphs show the frequency and absolute number of PD-1+ cells among Th2 cells (CD4+ IL-4/eGFP+ T cells) from WT (●) or Stat6−/− (○) mice as mean ± SE of 3 individual mice from 2 independent experiments (***P ≤ .001; **P ≤ .01; *P ≤ .05). (C) Effect of anti-PD-L2 mAb treatment during N brasiliensis infection on the Th2 response and PD-1 expression on T cells. Anti-PD-L2–treated or rat IgG-treated mice were analyzed at day 9 after infection. Dot plots are gated on CD4+ cells and show staining for PD-1 versus expression of IL-4/eGFP. Bar graphs show the frequency of PD-1+ cells among CD4+IL-4/eGFP+ cells (left) and the frequency of IL-4/eGFP+ cells among all CD4+ cells (right) as mean ± SE of 4 individual mice from 2 independent experiments (*P ≤ .05).
PD-1 expression on Th2 cells during N brasiliensis infection. Lung (A) and mesenteric lymph nodes (B) of 4get (WT) and 4get/Stat6−/− (Stat6−/−) mice were analyzed before or at indicated days after N brasiliensis infection. Dot plots are gated on CD4+ cells and show staining for PD-1 or isotype control versus expression of IL-4/eGFP at day 13 after N brasiliensis infection. The graphs show the frequency and absolute number of PD-1+ cells among Th2 cells (CD4+ IL-4/eGFP+ T cells) from WT (●) or Stat6−/− (○) mice as mean ± SE of 3 individual mice from 2 independent experiments (***P ≤ .001; **P ≤ .01; *P ≤ .05). (C) Effect of anti-PD-L2 mAb treatment during N brasiliensis infection on the Th2 response and PD-1 expression on T cells. Anti-PD-L2–treated or rat IgG-treated mice were analyzed at day 9 after infection. Dot plots are gated on CD4+ cells and show staining for PD-1 versus expression of IL-4/eGFP. Bar graphs show the frequency of PD-1+ cells among CD4+IL-4/eGFP+ cells (left) and the frequency of IL-4/eGFP+ cells among all CD4+ cells (right) as mean ± SE of 4 individual mice from 2 independent experiments (*P ≤ .05).
Anti-PD-L2 treatment results in more PD-1+ T cells and an enhanced Th2 response in the lung during N brasiliensis infection
To directly address whether PD-1+ cells increased due to the lack of PD-L2 expression in Stat6−/− mice, we efficiently blocked PD-L2 in wild-type mice during N brasiliensis infection by injection of an anti-PD-L2 antibody, which did not deplete AAM (supplemental Figure 5). Significantly more PD-1+ T cells were present in the lung of anti-PD-L2–treated compared with isotype control-treated mice (Figure 7C). Interestingly, the frequency of Th2 cells was also higher in anti-PD-L2–treated compared with control mice, suggesting that PD-L2 on AAM dampens the Th2 response in the lung by engaging the PD-1 receptor on T cells (Figure 7C).
Discussion
We defined expression of PD-L2 on AAM as one important mechanism by which AAM mediate suppression of T-cell proliferation. Our results demonstrate that PD-L2 is rapidly expressed in vitro and in vivo on AAM in a Stat6-dependent manner. Blockade of PD-L2 on AAM restored T-cell proliferation. Furthermore, retroviral transduction of PD-L2 in Stat6−/− macrophages was sufficient to inhibit T-cell proliferation. Therefore, PD-L2 expression by AAM appears to provide a nonredundant inhibitory signal against T cells.
Suppression of T-cell proliferation by ex vivo isolated AAM has first been described in B malayi-infected mice.27 Further experiments revealed that IL-4 was required to induce the inhibitory activity.14 Others have shown that the inhibitory activity was present in peritoneal macrophages from chronically Taenia crassiceps–infected wild-type but not from Stat6−/− mice.28 However, because Stat6 controls many genes in different cell types, it remained unclear whether Stat6 was required in macrophages themselves or in other cells to generate suppressive macrophages. Another unresolved issue was the requirement of Stat6 for mobilization and recruitment of macrophages. Earlier studies reported that Stat6−/− mice show increased numbers of progenitors of the myeloid lineage.29 Our experiments with mixed BM chimeras demonstrated that Stat6 was not required in macrophages for development or tissue recruitment, but wild-type macrophages seemed to survive better in lung and peritoneum. However, Stat6 appears critical in macrophages to induce PD-L2 and gain suppressive activity. Stat6-mediated signals may not be sufficient for PD-L2 induction, since it has been reported that only inflammatory (thioglycollate-elicited) but not resting peritoneal macrophages could be induced to up-regulate PD-L2 in a Stat6-dependent manner.26
The direct correlation of PD-L2 expression with other established markers for AAM facilitates now the identification and ex vivo isolation of viable AAM for further functional studies. Initially, the mannose receptor (CD206) has been proposed as surface marker for AAM, but in our hands the currently available antibodies against murine CD206 showed a relatively poor staining and were not suitable for flow cytometric studies.30 We previously reported the generation of an Arg1/YFP-reporter mouse that can be used to isolate Arg1-expressing macrophages.31 However, a more recent study showed that expression of Arg1 is not strictly Stat6-dependent and might also be induced by toll-like receptor (TLR) signals.32 Therefore, PD-L2 appears to be a more specific, useful, and distinctive marker for AAM.
Expression of PD-L2 on other cell types is mainly restricted to dendritic cells (DCs), BM-derived mast cells, and B1 B cells in the peritoneum, whereas PD-L1 is expressed constitutively on monocyte-derived cells, epithelial, and endothelial cells.33 Numerous in vivo models for autoimmunity, tumor immunity and viral infections demonstrated an important inhibitory role for PD-L1, whereas PD-L2 seemed to play a minor role probably due to the more restricted expression and requirement of Th2-biased immune responses for induction of PD-L2.33 However, a recent report demonstrated that PD-L2–deficient mice generated an enhanced type 2 immune response and accelerated worm expulsion after N brasiliensis infection.34 Interestingly, PD-L1 and PD-L2 can also act as costimulatory ligands as shown in cultures with T cells from PD-1−/− mice, and it has been proposed that a second receptor with activating properties exists for these ligands.35 In addition, retrograde signaling for both ligands has been proposed, which requires further investigation.36,37
The expression of PD-L2 on AAM during Th2-biased immune responses like helminth infections and allergic inflammation might serve to prevent uncontrolled activation of the immune system to avoid tissue damage. This hypothesis is supported by the observation that AAM are required to protect mice from lethal infection with S mansoni.11 We observed that the inhibitory receptor PD-1 was transiently induced on Th2 cells in vivo after infection of mice with N brasiliensis. PD-1 has been described as marker for exhausted CD8+ T cells38 and as a marker for CD4+ follicular helper T (TFH) cells.39 Consistent with our results, 2 recent studies showed that TFH cells develop from Th2 cells during helminth infection.40,41 Interestingly, we observed enhanced and prolonged expression of PD-1 on IL-4–expressing T cells of Stat6−/− mice. This could be due to delayed worm expulsion, which might cause prolonged activation of T cells. Alternatively, PD-L2 expression in wild-type mice could either inhibit expansion of PD-1+ CD4 T cells or cause down-regulation of PD-1. We observed that blocking of PD-L2 in vivo resulted in significantly higher levels of PD-1+ CD4 T cells and Th2 cells. The relatively late expression of PD-L2 during the immune response against N brasiliensis is consistent with the concept that the initial Th2 response should be fast and strong, whereas an overwhelming response needs to be controlled by inhibitory mechanisms at later time points.
AAM could be detected in Rag−/− mice after wounding or helminth infection, which indicates that innate IL-4/IL-13–producing cells are sufficient for their generation, although T cells were required to sustain AAM accumulation.42,43 We and others could previously show that activated basophils can induce AAM.22,44 As we demonstrate here by using conditional knockout mice, IL-4/IL-13 production from T cells was indeed required for AAM accumulation at the later stage of infection. Consistent with the late appearance of PD-L2+ macrophages, it has been shown that macrophages isolated from lungs at 13-15 days after N brasiliensis infection showed an increased capacity to inhibit proliferation of CD4+ T cells compared with macrophages isolated on day 4 postinfection.45
We could show that IL-4 induced the expression of PD-L2 on CAM, and LPS increased the expression of PD-L1 on AAM. Studies using mice infected with the nematode B malayi as a source for macrophages with an alternatively activated phenotype showed that these macrophages were still responsive to treatment with LPS/interferon-γ (IFN-γ), changed their gene expression profile, and acquired bactericidal activity.46 These findings provide evidence that polarized macrophages maintain phenotypic and functional plasticity that might be important to first respond quickly as CAM to viral or bacterial infections and later help to remodel injured tissues as AAM.
AAM can be a benefical and protective cell type during infection with helminth parasites.11,47 However, they are also associated with impaired clearance of the tapeworm T crassiceps, the protozoan parasite L major, and the fungus Cryptococcus neoformans consistent with their potent immunosuppressive activity.48-50 Further studies are required to clarify the role of AAM in different murine infection models but also in chronically infected patients. Unfortunately, relatively little is currently known about the role of AAM during chronic allergic disorders. Better understanding of their contribution to fibrosis, tissue remodeling, and immunosuppression might help to identify new therapeutic targets for patients suffering from chronic Th2-associated diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank A. Bol and W. Mertl for animal husbandry, S. Wirth and I. Schiedewitz for technical assistance, A. Seidl, M. Panzer, and G. Wirnsberger for helpful comments, and H. Kuipers for providing reagents.
This work was funded by the Emmy Noether Program (Vo944/2-2) and a collaborative research center (SFB 571) of the Deutsche Forschungsgemeinschaft.
Authorship
Contribution: S.H. and D.V. designed experiments and wrote the paper; S.H. performed experiments; F.M. generated and provided retroviral constructs; and R.H. performed microarray analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Voehringer, Institute for Immunology, Ludwig-Maximilians-University, Goethestrasse 31, 80336 Munich, Germany; e-mail: david.voehringer@med.uni-muenchen.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal