To the editor:
Two groups have recently proposed that type II anti-CD20 monoclonal antibodies (mAbs) B1 (tositumomab) and GA101, as well as anti–HLA-DR antibody (L243), induce strong homotypic adhesion and direct cell death. They claim, on the basis of annexin V, propidium iodide (PI), and flow cytometric analysis, that up to 80% of cell death occurs after 24 hours of treatment with these mAbs.1-3
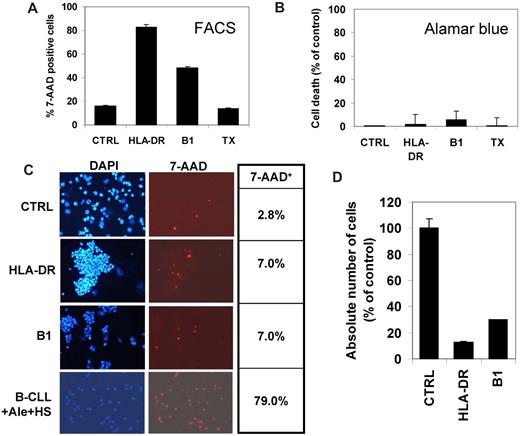
We have also observed, after treatment of B lymphoma cells in vitro with either anti–HLA-DR BK267 or anti-CD20 antibody B1 (BioGenex), an apparent 30% to 70% increase in cell death of different human B lymphoma cell lines by fluorescence-activated cell sorting (FACS) analysis of either 7-AAD–stained cells (Figure 1A and data not shown). However, in parallel observations in Bürker chambers, we found that only 5% of antibody-treated cells were stained with Trypan blue and most appeared alive (data not shown). We then observed in alamarBlue vital dye (AbD Serotec) assays that only 2% to 12% cell death could be measured after 24 hours of treatment of the same cell lines with anti–HLA-DR or B1 antibody (Figure 1B and data not shown). Seeing that the cells formed large and tight aggregates in the presence of the antibodies, which were resistant to vigorous pipetting, we thought that this aggregation could have significantly altered the flow cytometric data. We therefore performed cytospins of cells treated with mAbs and stained with 7-AAD and DAPI. Counting 7-AAD+ cells in cytospins showed very low mortality, with about 5% increased dead cells in antibody compared with controls (Figure 1C). This was markedly different from parallel flow cytometry (Figure 1A). Indeed, with Raji cells, 83% and 48.6% 7-AAD+ cells were revealed by FACS analysis after HLA-DR and B1 treatment, respectively, compared with only 7% in cytospins (Figure 1A,C). As positive control, B-CLL treated with alemtuzumab and human serum showed more than 70% cell death in cytospins (Figure 1C), consistent with the results obtained by FACS.4
The B lymphoma cell line Raji was treated with 10 μg/mL chimeric anti–HLA-DR antibody BK267, anti-CD20 antibody B1, or control antibody trastuzumab (TX). After 24 hours, cell death was evaluated (A) by 7-AAD staining and standard FACS analysis, (B) by incubation for 5 hours with alamarBlue vital dye, or (C) by performing 7-AAD staining followed by cytospin, fixing, and coloration of nuclei with DAPI. In panel C, photographs of cells stained with DAPI (left) or 7-AAD (right) are shown, as well as the percentage of 7-AAD+ cells with respect to total DAPI+ cells in the same experiment (table on the right). Control B-CLL cells treated with alemtuzumab and 20% human serum are shown in panel C. Finally the absolute number of cells scored by flow cytometry using reference beads was also determined by FACS (D). The data shown are the result of 1 representative experiment of at least 5 performed with 3 different cell lines. For panel C, slides were viewed with a Nikon Eclipse E800 microscope using a Nikon 20× Plan Fluor DICM lens at room temperature with Vectashield Medium (Vector Laboratories). Fluorochromes were CD19-FITC and 7-AAD (both BD Biosciences). Images were acquired using a Nikon Digital Sights DS-UI camera and were processed with Lucia Imaging Analysis Systems (Laboratory Imaging) and ImageJ software.
The B lymphoma cell line Raji was treated with 10 μg/mL chimeric anti–HLA-DR antibody BK267, anti-CD20 antibody B1, or control antibody trastuzumab (TX). After 24 hours, cell death was evaluated (A) by 7-AAD staining and standard FACS analysis, (B) by incubation for 5 hours with alamarBlue vital dye, or (C) by performing 7-AAD staining followed by cytospin, fixing, and coloration of nuclei with DAPI. In panel C, photographs of cells stained with DAPI (left) or 7-AAD (right) are shown, as well as the percentage of 7-AAD+ cells with respect to total DAPI+ cells in the same experiment (table on the right). Control B-CLL cells treated with alemtuzumab and 20% human serum are shown in panel C. Finally the absolute number of cells scored by flow cytometry using reference beads was also determined by FACS (D). The data shown are the result of 1 representative experiment of at least 5 performed with 3 different cell lines. For panel C, slides were viewed with a Nikon Eclipse E800 microscope using a Nikon 20× Plan Fluor DICM lens at room temperature with Vectashield Medium (Vector Laboratories). Fluorochromes were CD19-FITC and 7-AAD (both BD Biosciences). Images were acquired using a Nikon Digital Sights DS-UI camera and were processed with Lucia Imaging Analysis Systems (Laboratory Imaging) and ImageJ software.
We conclude that flow cytometry results with anti–HLA-DR or type II anti-CD20 antibodies are largely due to cell aggregation. Large aggregates containing 30 to 100 cells are probably mostly excluded from the analysis, falling outside the gate of scatter plots. Dead cells are in contrast enriched within the single-cell population because aggregation is an energy-dependent process. An apparent rapid loss of cells due to aggregation was confirmed in flow cytometry by counting the absolute number of events using calibrated beads. Indeed, 87% and 70% apparent decrease in total cell number was observed at 24 hours in anti–HLA-DR– and B1-treated samples, respectively (Figure 1D).
Altogether, these observations suggest that FACS analysis should be interpreted with caution in the functional study of antibodies that cause strong homotypic adhesion, unless means are found to disaggregate cells without causing cell death. They also suggest that anti–HLA-DR and anti-CD20 B1 antibody may induce much less direct cell death than previously suggested and the reason for their efficacy in vivo may reside in other properties, such as homotypic adhesion itself inducing opsonization or immune mediated mechanisms, as recently suggested for type II anti-CD20 mAbs.5
Authorship
Acknowledgments: The authors thank MAT Biopharma for BK267 purification.
This work was supported by Associazione Italiana per la Ricerca sul Cancro and European Economic Community (Specific Targeted Research Project, Bispecific Monoclonal Antibody Technology Concept project no. 518185).
Conflict-of-interest disclosure: L. Boumsell was a consultant for MAT Biopharma under French law article 25.2. The remaining authors declare no competing financial interests.
Correspondence: Josée T. Golay, c/o Presidio Matteo Rota, Laboratory Lanzani, Ospedali Riuniti, via Garibaldi 11-13, Bergamo, Italy 24128; e-mail: jgolay@ospedaliriuniti.bergamo.it.