Abstract
Granulysin is a cytolytic and proinflammatory peptide contained in the cytotoxic granules of NK cells and CTL. Unexpectedly, granulysin also recruits and activates dendritic cells via TLR4/Myd88, playing the role of an immune adjuvant. Hence, in contrast to classical alarmins released by early leukocytes, granulysin is the first alarmin to be released from lymphocytes.
The tantalizing network properties and subtle regulation of the immune system are achieved by the existence of multiple distinct cell types that engage in mutual interactions, as well as by the action of a large variety of mediators that allow for the exchange of information between immune effectors at autocrine, paracrine, and endocrine levels. Typically, such mediators are ligands that act on specific high-affinity receptors. In an elegant study published in this issue of Blood, Tewary et al1 report the unexpected finding that granulysin can act as an alarmin, an agent that rapidly galvanizes antigen-presenting cells (APCs) and activates innate and adaptive immune responses.
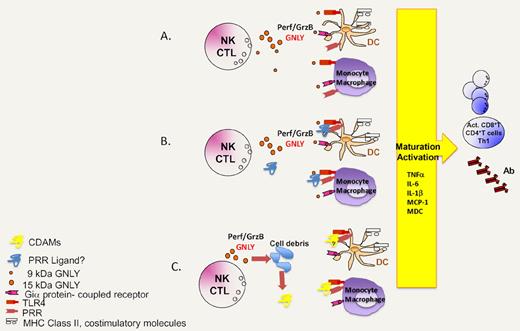
Hypothetical scenarios explaining the immunoadjuvant action of granulysin. (A) Granulysin directly acts on TLR4 and/or a Gi protein–coupled receptor to mediate chemotaxis and DC activation/maturation. (B) Granulysin interacts with other molecules to form molecular complexes that then stimulate TLR4 or other pattern recognition receptors (PRR). (C) Granulysin kills a fraction of vulnerable cells, thereby triggering the exposure or release of cell death–associated molecules (CDAMs), which act on TLR4 and perhaps other PRR.
Hypothetical scenarios explaining the immunoadjuvant action of granulysin. (A) Granulysin directly acts on TLR4 and/or a Gi protein–coupled receptor to mediate chemotaxis and DC activation/maturation. (B) Granulysin interacts with other molecules to form molecular complexes that then stimulate TLR4 or other pattern recognition receptors (PRR). (C) Granulysin kills a fraction of vulnerable cells, thereby triggering the exposure or release of cell death–associated molecules (CDAMs), which act on TLR4 and perhaps other PRR.
Granulysin belongs to the family of saposin-like, lipid-binding proteins and colocalizes in the granular compartments of human cytotoxic T lymphocytes (CTL) and natural killer (NK) cells along with granzymes and perforin. The “mature” granulysin protein (9 kDa), which is degranulated along with granzymes and perforin, results from the proteolytic maturation of a “secretory” 15-kDa precursor. Granulysin is highly cationic and folded as a 5-helix bundle stabilized by 2 highly conserved intramolecular disulfide bonds.2 Based on its cationic ampholytic structure, granulysin has the capacity to lyse bacterial membranes (which generally contain negatively charged lipids) and hence mediates a broad bactericidal activity. In addition, granulysin can kill human cells that are attacked by CTL or NK, by virtue of their membrane-permeabilizing effects on mitochondria,3 whose inner membrane resembles that of bacteria insofar as it contains more negatively charged lipids than any other cellular membrane. In addition, granulysin may induce lysosomal membrane permeabilization, which constitutes another potentially lethal event.4 The blister fluids from patients with Stevens- Johnson syndrome and toxic epidermal necrolysis contain abnormally high levels of secretory 15-kDa granulysin, which mediate disseminated keratinocyte necrosis,5 underscoring that granulysin can exert broad, pathophysiologically relevant cytotoxic effects.
Granulysin has been known to act as a chemoattractant for T lymphocytes, monocytes, and other inflammatory cells and to stimulate the expression of several cytokines, including RANTES, MCP-1, MCP-3, MIP-1α, IL-1, IL-6, IL-10, and IFNα.6 Tewary et al1 now show that granulysin (be it the 9-kDa or 15-kDa forms) is chemotactic for human monocyte-derived dendritic cells (Mo-DC) and mouse bone marrow–derived DC (BMDC). This effect was inhibited by pertussis toxin, indicating that granulysin activates a hitherto elusive Gi protein–coupled receptor. When injected into the peritoneal cavity of mice, granulysin also induced a substantial increase in intraperitoneal inflammatory leukocytes,1 underscoring the likewise physiologic relevance of this chemotactic effect.
When added to human Mo-DC or CD1c+ peripheral blood myeloid DC, the 15-kDa form of granulysin induced the surface expression of CD80, CD83, CD86, and MHC class II and the secretion of IL-6, IL-8, IL-12, IL-10, MCP1, MDC, and TNFα. Concomitantly, granulysin enhanced the capacity of Mo-DC to stimulate allogeneic T-cell proliferation in vitro. BMDC from wild-type, but not TLR4 or Myd88 mutant, mice responded to granulysin by expressing maturation markers (such as CD80) and producing cytokines (such as IL-6 and TNFα). Supernatants of human degranulated NK cells also induced the phenotypic maturation (increase of CD80 and CD86) of and cytokine production (IL-6 and TNFα) by human Mo-DC, and this effect was abolished by blocking TLR4 with an antibody or by immunodepletion of granulysin.1
In a further twist of their study, Tewary et al1 showed that both the 9-kDa and 15-kDa forms of granulysin act as adjuvants in vivo. When coadministered with the model antigen ovalbumin (OVA), granulysin enhanced the proliferation and Th1 (and to a lesser extent Th2) cytokine production in response to OVA and increased the production of OVA-specific antibodies. This adjuvant effect was observed in TLR4-sufficient but not in TLR4-deficient mice,1 underscoring the importance of TLR4 for sensing the immunostimulatory effects of granulysin.
The results by Tewary et al1 suggest for the first time that a constituent of NK and CTL granules may act as an alarmin, that is as a cytokine-like molecule that alerts innate immune effectors. Until now, it has been conceived that alarmins (such as cathelicidins, α-defensins, eosinophil-associated ribonucleases, iron-binding proteins or HMGB1) are released by neutrophils, phagocytes or epithelial cells, not by lymphocytes. Hence, granulysin might constitute a novel link between effector cells (such as NK cells and CTL) and the innate immune system that participates in the local amplification of the inflammatory or immune response.
One of the major incognita that emerge from the study by Tewary et al1 concerns the precise mechanisms through which granulysin recruits and activates innate immune effectors including DC. Although Tewary et al1 imply a yet-to-be-defined Gi protein–coupled receptor and TLR4 in response to granulysin, it may be premature to assume that granulysin acts directly on such receptors (see ‘A’ in figure). As a possibility, granulysin might engage in electrostatic interactions with negatively charged molecules (such as nucleic acids, anionic lipids, or other proteins) to form complexes that then activate TLR4 or other pattern recognition receptors (see ‘B’ in figure). One precedent for this scenario is provided by another alarmin, HMGB1, which forms highly inflammatory complexes with single-stranded DNA, LPS, IL-1β, and nucleosomes, that then interact with TLR9, TLR4, IL-1R, and TLR2 receptors, respectively.7 As a third possibility, that is supported by the relatively high (and potentially cytotoxic) levels of granulysin required to induce DC activation and maturation,1 the effects of granulysin might be highly indirect (see ‘C’ in figure). If granulysin killed a fraction of cells upon its in vitro or in vivo administration by apoptosis3 or by necrosis,4,5 these cell deaths—with the consequent exposure and release of cell death–associated molecules including other alarmins8 —might account for its immuno-stimulatory effects. Future work will discriminate between these possibilities.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal