Abstract
Immune reconstitution inflammatory syndrome (IRIS) is a considerable problem in the treatment of HIV-infected patients. To identify immunologic correlates of IRIS, we characterized T-cell phenotypic markers and serum cytokine levels in HIV patients with a range of different AIDS-defining illnesses, before and at regular time points after initiation of antiretroviral therapy. Patients developing IRIS episodes displayed higher frequencies of effector memory, PD-1+, HLA-DR+, and Ki67+ CD4+ T cells than patients without IRIS. Moreover, PD-1+ CD4+ T cells in IRIS patients expressed increased levels of LAG-3, CTLA-4, and ICOS and had a Th1/Th17 skewed cytokine profile upon polyclonal stimulation. Elevated PD-1 and Ki67 expression was also seen in regulatory T cells of IRIS patients. Furthermore, IRIS patients displayed higher serum interferon-γ, compared with non-IRIS patients, near the time of their IRIS events and higher serum interleukin-7 levels, suggesting that the T-cell populations are also exposed to augmented homeostatic signals. In conclusion, our findings indicate that IRIS appears to be a predominantly CD4-mediated phenomenon with reconstituting effector and regulatory T cells showing evidence of increased activation from antigenic exposure. These studies are registered online at http://clinicaltrials.gov as NCT00557570 and NCT00286767.
Introduction
Immune reconstitution inflammatory syndrome (IRIS) is a term used to describe the paradoxical worsening or unmasking of infections or tumors after antiretroviral therapy (ART) initiation.1 Two clinical predictors for the development of IRIS have been identified in clinical studies: severe CD4+ T-cell lymphopenia before ART initiation and the presence of opportunistic diseases either symptomatic (paradoxical IRIS) or occult (unmasking IRIS).2,3 Some studies have also shown a significant association between shorter duration of treatment of underlying infection and ART initiation with paradoxical IRIS.3 Although a high proportion of IRIS events are related to underlying mycobacterial diseases (eg, Mycobacterium tuberculosis and M avium complex),4 a variety of other opportunistic infections and AIDS-associated conditions have also been identified as predisposing factors.
The pathogenesis of IRIS is still unclear. The best evidence comes from studies suggesting an exuberant Th1 response and increased proportions of killer inhibitory receptor (KIR)−γδ+ T cells post-ART in patients with tuberculosis (TB)-IRIS, compared with patients with TB, but no IRIS event.5,6 Subsequent studies, though, did not show a clear association of TB-IRIS, with more pronounced restoration of Th1 pathogen-specific response for TB.7 The possibility of a lack of appropriate regulatory T-cell (Treg) response because of inadequate numbers of Treg has also been investigated. Phenotypic studies examining Treg frequency in peripheral blood have showed similar proportions of these cells in patients who developed TB-IRIS and those who did not.7 Another study found evidence of potential Treg dysfunction in IRIS patients with M avium infection, but the number of patients studied was too small to draw definitive conclusions.8
The development of IRIS at high frequencies in severely lymphopenic patients after ART initiation suggests that lymphopenia-induced T-cell homeostatic mechanisms may contribute to the pathogenesis of this syndrome. It is also plausible that all lymphopenic patients are at high risk for IRIS because of severe immunosuppression, and the presence of excess foreign antigen tips the balance of regulatory mechanisms. Although specific pathways may differ depending on the underlying antigen (ie, mycobacterial, fungal, viral, parasitic, or self) or live pathogens vs. antigen (ie, unmasking vs paradoxical), we hypothesized that the severe lymphopenia and resultant profound immunosuppression in the presence of high antigenic exposure and homeostatic forces may share a common thread. We thus sought to determine T-cell phenotypic changes and serum cytokine levels that occur before, and at different times after, ART initiation in individuals with a variety of different AIDS-defining illnesses, with the goal to identify distinguishing patterns in patients who develop IRIS. To examine general mechanisms not confined to TB-IRIS, namely, the effects of lymphopenia and the presence of foreign antigen, individuals with a variety of different AIDS-defining illnesses were included in this study.9
Our findings support the hypothesis that a highly activated, predominantly CD4, T-cell phenotype is characteristic of IRIS patients before and during the IRIS episodes. This is likely caused by antigenic stimulation revealed by high interferon γ (IFN-γ) production and high expression of both inhibitory and costimulatory molecules, with the possible contribution of homeostatic signals revealed by high interleukin-7 (IL-7) levels. These findings are in agreement with clinical observations suggesting that, untreated, occult, or symptomatic, infections increase the risk of IRIS, and that reversal of severe CD4 lymphopenia, with its resultant immunosuppression, is a significant risk factor.
Methods
Patients
This retrospective study was performed at the Clinical Center, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) under an institutional review board–approved protocol. All study subjects had signed informed consent in accordance with the Declaration of Helsinki.
HIV-infected patients were included in the study if they were ARV-naïve, had CD4 T-cell counts lower than 100 cells/μL, and achieved suppression of HIV-RNA to < 50 copies/mL by 24 weeks of ART initiation. Samples [peripheral blood mononuclear cells (PBMCs) and serum] were collected at baseline (pre-ART) and approximately 1, 3, 6, 12, and 24 months after ART initiation. The patients who developed IRIS also had specimens collected during the episode. The diagnosis of IRIS was based on a previously described definition by the AIDS Clinical Trial Group (ACTG 2004) and was recently described in detail by Porter et al.9 Criteria included: initiation of ART; evidence of HIV virologic suppression at the time of event; the emergence of an infectious or inflammatory process that could not be explained by the course of the existing infection; a new infection/diagnosis or the side effects of therapy; and could be attributed to a specific pathogen. The classification was discussed and agreed upon by clinical members of the study team. Specimens from HIV-negative subjects were collected under NIH-approved protocols and were analyzed for the same variables as the HIV-infected patients.
T-cell immunophenotyping and intracellular cytokine measurement
Cryopreserved PBMCs were thawed in RPMI 1640 (Sigma-Aldrich) supplemented with 10% fetal calf serum (FCS) and benzonase nuclease (25 U/mL; Novagen). Cells were stained directly after thawing, as described below, or first cultured for 5 hours in the presence of 10 ng/mL phorbol acetate myristate (PMA), 1 μg/mL ionomycin (both from Sigma-Aldrich), and Brefeldin-A (BD Biosciences) in RPMI 1640 supplemented with 10% FCS. PBMCs were washed in phosphate-buffered saline (PBS) (Invitrogen) and then incubated with ViViD (Invitrogen) for dead cell exclusion. Six antibody panels were used for analyzing T-cell activation, Treg, and intracellular cytokine production (supplemental Table 2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). They included the following: anti-CD14 (clone M5E2) -pacific blue, anti-CD3 (clone SK7) -APC-Cy7, anti-CCR5 (clone 2D7/CCR5) -biotin (BD Pharmingen), anti-CD27 (clone 1A4-CD27) -APC-Alexa 700 (Immunotech-Coulter), anti-CD127 (clone R34.34)–phycoerythrin (PE) (Coulter), anti-CD25 (clone BC96) -PE-Cy7, anti-inducible costimulatory molecule (ICOS) (clone ISA-3) -PE-Cy7 (eBioscience), anti-TIM-3 (clone 344823) -PE, anti-LAG3 (clone 333221) -PE and anti-CCR4 (clone 205410)–allophycocyanin (APC; R&D Systems), purified anti-CD19 (clone HIB19), anti-CD45RO (clone UCHL1), anti-CD8 (clone RPA-T8), anti-CD57 (clone NK-1), anti-CD4 (clone RPA-T4), anti-CD27 (clone M-T271), anti-CD45RA (clone 5H9), and anti-HLA-DR (clone G46-6) (BD Pharmingen) were conjugated in-house with pacific blue, quantum dot (QD) 545, QD585, QD705, QD800, QD655, Alexa-594 (Invitrogen), and PE-Cy5 (PE–Prozyme and Cy5; Amershan Life Science), respectively. Anti-CCR7 (clone 150503) (R&D Systems) was conjugated with Alexa-594 and Alexa-680 (Invitrogen), and anti-CD38 (clone OKT10) (Maine Biotech Services) was conjugated with PE (Prozyme). Anti-PD-1 biotin (clone EH12.2H7) was a kind gift from Gordon Freeman (Dana-Farber Cancer Institute, Boston, MA). Subsequently, PBMCs were washed, fixed, and permeabilized (FoxP3 Staining Buffer Set; eBiosciences), according to the manufacturer's instructions. The cells were then stained with anti-Ki67 (clone 35)–fluorescein isothiocyanate (FITC), CTLA-4 (clone BNI3) -APC (BD Pharmingen), anti-granzyme-B (GrB; clone GB12)–APC (Caltag), anti-FoxP3 (clone PCH101) -PE-Cy5 (eBioscience), streptavidin-QD605 (Invitrogen), anti-IFN-γ-Alexa488 (clone AS.B3) (ReaMetrix), anti-IL-17-PerCP-Cy5.5 (clone BL168) (Biolegend), anti-IL-4-PE (clone MP4-25D2), anti–tumor necrosis factor alpha (TNF-α)–Alexa594 (clone MAb11), and anti-IL-2-APC (clone MQ1-17H12) (BD Pharmingem). CD4 (clone S3.5) (Caltag) and CD3 (anti-CD4-PE-Cy5.5, and anti-CD3-Cy7APC) were also stained intracellularly after the cells were cultured for 5 hours due to down-regulation of their expression. Cells were washed with permeabilization buffer and acquired on an LSR II. Data were analyzed using FlowJo Version 8.8.2 (TreeStar). A forward scatter area (FSC-A) versus forward scatter height (FSC-H) gate was used initially to remove doublets, and then cells were gated based on FSC-A and SSC-A to capture lymphocytes. Nonviable and CD14- and CD19-expressing cells were excluded using a dump channel vs. CD3. After this, events were sequentially gated on CD3+, CD4+, or CD8+, and more detailed analysis of naive and memory subpopulations were done based on CD27 and CD45RO expression: naive cells (CD45RO−CD27+), central memory cells (CD45RO+CD27+), effector memory cells (CD45RO+CD27−), and effector cells (CD45RO−CD27−). The activation markers, PD-1, CD38, human leukocyte antigen (HLA)-DR, CCR5, CD57, Gr-B, and Ki67, were analyzed within CD4+ and CD8+ T cells and also within each memory subpopulation.
Regulatory T cells were analyzed after dead-cell exclusion and based on the high expression of CD25, low expression of CD127, and expression of Foxp3 (supplemental Figure 1). Ki67 and PD-1 expression was analyzed within Treg in all or a subset of patients, respectively.
Intracellular cytokines were analyzed, after dead-cell exclusion, in CD4+ and CD8+ T-cell subpopulations. The cytokine production was compared between PD-1+ and PD-1− T cells.
GraphPad Prism Version 5.0b (GraphPad Software), Pestle Version 1.6.1, and Spice Version 4.2.2 (both by M.R., Vaccine Research Center, NIAID, NIH) were used for data analysis and graphic presentation.
Serum cytokine measurements
Th1 and Th2 cytokines (IFN-γ, IL-10, and TNF-α) were measured in cryopreserved serum using the meso scale discovery electroluminescence multiarray method, as recommended by the manufacturer (MSD). IL-7 levels were measured with the human IL-7 Quantikine enzyme-linked immunosorbent assay (ELISA) kit (limit of detection 0.27 pg/mL; R&D Systems), according to the manufacturer's recommendation.
Statistical analysis
The significance of paired differences (changes from baseline within each group) was determined by the sign test. Unpaired analyses (comparisons between the 2 groups at each time point) were performed using the Wilcoxon rank-sum test. Median values with bars representing interquartile ranges (IQRs) are reported in the figures. Overall P values to assess differences between the 2 groups over time were based on mixed-model, repeated-measures analyses (SAS Version 9.1). The mixed model considered measurements before and up to 24 months after ART initiation. Because of the exploratory nature of the study, there was no correction for multiple comparisons, and only unadjusted P ≤ .10 is reported.
Results
Study population
A group of 45 HIV-infected patients were identified between 2002 and 2007. Among these, 16 patients met the definition of confirmed (11/45; 24%) or suspected (5/45; 11%) IRIS and presented with at least one IRIS episode occurring between 9 and 197 days after ART initiation (median: 35; IQR: 28-97). All IRIS patients had samples of their blood collected before ART initiation (n = 16), and 15, 12, 12, and 10 patients had blood collected at 1, 3, 6, and 12 months after ART initiation, respectively. Twenty-nine HIV-infected patients who never developed IRIS had blood collected before and 1 month after ART initiation, and 28, 25, and 17 patients had blood collected 3, 6, and 12 months after ART initiation, respectively. Opportunistic infections, presented by patients who developed IRIS and associated with the episodes, are shown in supplemental Table 1. There was one IRIS case that manifested as autoimmune disease (alopecia universalis; the same patient later developed Graves disease).10 The 2 groups were balanced with respect to all baseline characteristics, including proportion with opportunistic diseases at time of ART initiation. The detailed clinical description of the cohort, and of all the IRIS cases, was the subject of a separate report.9
T-cell reconstitution in response to ART
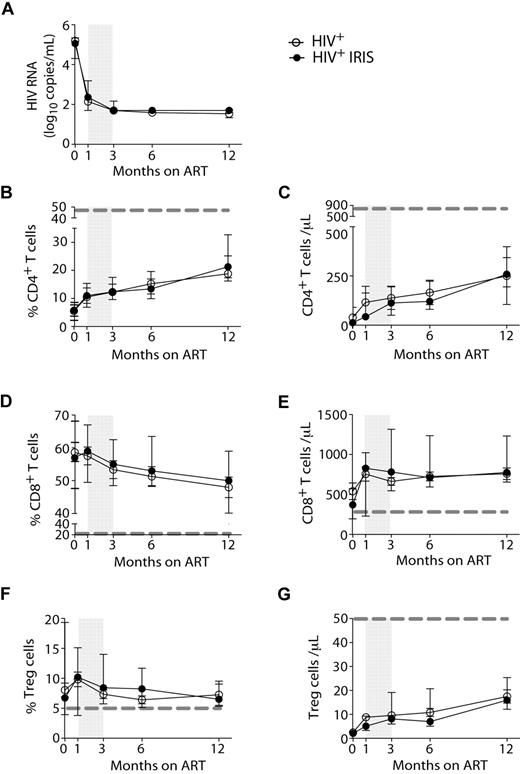
HIV-1 RNA levels (Figure 1A) and the proportions and numbers of CD4+ T cells (Figure 1B-C) were not different between IRIS and non-IRIS groups at baseline (pre-ART) or at any time point after ART initiation. Whereas the frequencies of CD4+ T cells increased after initiation of ART, the frequencies of CD8+ T cells slightly decreased (Figure 1D) in both IRIS and non-IRIS patients. Immediately after ART initiation, an increase in the absolute numbers of CD8+ T cells was observed in the patients who developed IRIS; however, no significant differences between the 2 groups were found (Figure 1E).
HIV-1+ patients developing IRIS and non-IRIS HIV+ controls display the same HIV-RNA levels and T-cell reconstitution in response to ART. HIV-RNA was measured before and 1, 3, 6, and 12 months after ART initiation (A). Proportion and absolute numbers of CD4+ (B-C), CD8+ (D-E), and regulatory T (F-G) cells were measured before and several months after ART initiation, as indicated. Open and filled symbols represent HIV+ control patients and HIV+ patients who developed at least one IRIS episode, respectively. The shaded area represents the IQR of the time of initiation of IRIS episodes. Dotted lines represent median values of the given measurements in healthy donors.
HIV-1+ patients developing IRIS and non-IRIS HIV+ controls display the same HIV-RNA levels and T-cell reconstitution in response to ART. HIV-RNA was measured before and 1, 3, 6, and 12 months after ART initiation (A). Proportion and absolute numbers of CD4+ (B-C), CD8+ (D-E), and regulatory T (F-G) cells were measured before and several months after ART initiation, as indicated. Open and filled symbols represent HIV+ control patients and HIV+ patients who developed at least one IRIS episode, respectively. The shaded area represents the IQR of the time of initiation of IRIS episodes. Dotted lines represent median values of the given measurements in healthy donors.
The frequencies of Treg, gated as described in supplemental Figure 1, did not differ between IRIS and non-IRIS patients at any time point analyzed (Figure 1F), but were significantly higher than healthy controls (dashed gray line). No major differences were observed when the absolute numbers of Treg were compared between the 2 groups at all time points (Figure 1G).
Proportions of naive and memory T-cell subsets during immune reconstitution after ART initiation
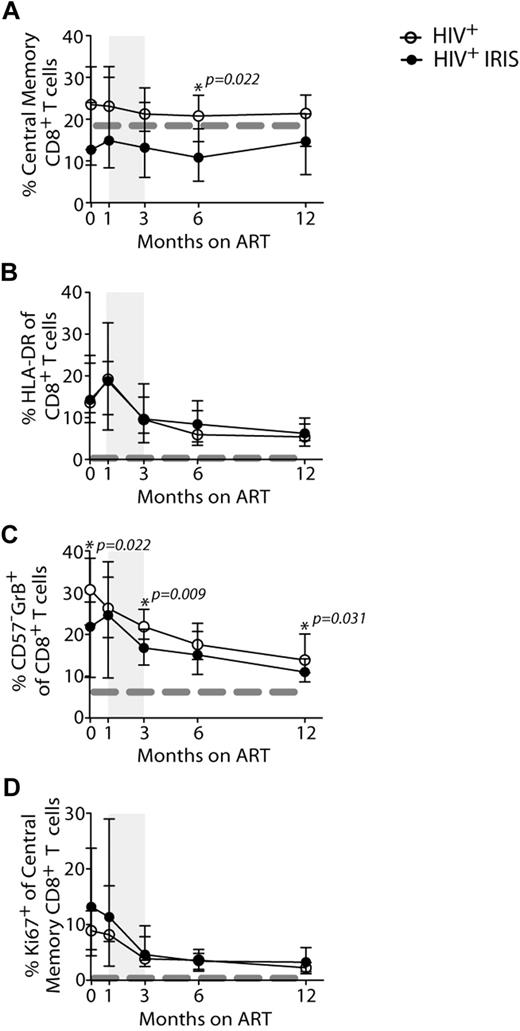
The proportion of naive and memory T-cell subsets were analyzed based on CD45RO and CD27 expression, as outlined in Methods (Figures 2A,B,E, 3A, and supplemental Figure 2). The frequencies of CD4+ T cells with naive and effector memory phenotypes did not differ significantly between the IRIS and non-IRIS groups at baseline, 1, and 3 months after ART initiation (Figure 2A,B,E). However, after 6 months on ART and after most IRIS events, the patients who had developed IRIS ended up with a higher proportion of effector memory CD4+ T cells, compared with patients who never developed the syndrome (38% vs 22%, P = .046) (Figure 2B). Conversely, at the same time point, the non-IRIS group presented a higher proportion of naive CD4+ T cells, compared with the IRIS group (25% vs 5.3%, P = .009) (Figure 2A). The absolute numbers of this subpopulation were also higher in non-IRIS, compared with IRIS, patients (supplemental Figure 2C). The absolute numbers of effector memory CD4+ T cells did not differ significantly between IRIS and non-IRIS patients (supplemental Figure 2D). The mixed-model/repeated-measures analysis revealed an overall difference in the kinetics of naive CD4+ T cells between the 2 groups (P = .01). Thus, non-IRIS patients reconstituted their naive CD4+ T-cell compartment earlier than IRIS patients, whereas IRIS patients retained higher frequencies of effector memory T cells. An overall difference in the distribution of CD4+ T-cell memory subsets between IRIS and non-IRIS patients was observed at month 6 (supplemental Figure 2A). After 12 months on ART, though, the naive and memory subset compartments were similar between the 2 groups. IRIS subgroups based on presentation (ie, paradoxical or unmasking) and on underlying pathogen (ie, fungal/mycobacterial versus viral) had similar proportions of naive and effector memory CD4+ T cells at all study time points (data not shown).
IRIS patients display a distinct activation status during reconstitution after ART therapy. The percentage of naive (CD45RO−CD27+; A) effector memory (CD45RO+CD27−; B) and HLA-DR+ (C) cells within CD4+ T lymphocytes, and Ki67+ cells within central memory CD4+ T lymphocytes (D) in HIV+ controls (open symbols) and HIV+ IRIS patients (filled symbols) were measured before and during ART. The shaded area represents the IQR of the time of initiation of IRIS episode. Dotted lines represent median values of the given measurements in healthy donors. Representative dot plots showing proportions of naive (Nv), central memory (CM), effector memory (EM), and effector (Ef) CD4+ T cells (E) and frequencies of Ki67+ cells within central memory CD4+ T lymphocytes (F) from a single HIV+ non-IRIS (left panel) and a single HIV+ IRIS patient (right panel) are shown.
IRIS patients display a distinct activation status during reconstitution after ART therapy. The percentage of naive (CD45RO−CD27+; A) effector memory (CD45RO+CD27−; B) and HLA-DR+ (C) cells within CD4+ T lymphocytes, and Ki67+ cells within central memory CD4+ T lymphocytes (D) in HIV+ controls (open symbols) and HIV+ IRIS patients (filled symbols) were measured before and during ART. The shaded area represents the IQR of the time of initiation of IRIS episode. Dotted lines represent median values of the given measurements in healthy donors. Representative dot plots showing proportions of naive (Nv), central memory (CM), effector memory (EM), and effector (Ef) CD4+ T cells (E) and frequencies of Ki67+ cells within central memory CD4+ T lymphocytes (F) from a single HIV+ non-IRIS (left panel) and a single HIV+ IRIS patient (right panel) are shown.
Activation status of CD8+ T cells during reconstitution in HIV+ non-IRIS and HIV+ IRIS patients before and during ART. The percentage of central memory (CD45RO+CD27+; A), HLA-DR+ (B), and CD57−GrB+ (C) cells within CD8+ T lymphocytes and Ki67+ (E) cells within central memory CD8+ T lymphocytes in HIV+ non-IRIS (open symbols) and HIV+ IRIS patients (filled symbols) were measured before and after ART initiation. The shaded area represents the IQR of IRIS episodes. Dotted lines represent median of the given measurement in healthy donors.
Activation status of CD8+ T cells during reconstitution in HIV+ non-IRIS and HIV+ IRIS patients before and during ART. The percentage of central memory (CD45RO+CD27+; A), HLA-DR+ (B), and CD57−GrB+ (C) cells within CD8+ T lymphocytes and Ki67+ (E) cells within central memory CD8+ T lymphocytes in HIV+ non-IRIS (open symbols) and HIV+ IRIS patients (filled symbols) were measured before and after ART initiation. The shaded area represents the IQR of IRIS episodes. Dotted lines represent median of the given measurement in healthy donors.
At 6 months after ART initiation, the frequencies of central memory CD8+ T cells were higher in non-IRIS patients, compared with IRIS (21% vs 11%, P = .022; Figure 3A). No differences were observed in the distribution of other CD8+ T-cell subsets between IRIS and non-IRIS patients (supplemental Figure 2).
Changes in the expression of T-cell activation markers during immune reconstitution after ART initiation
Expression of various activation markers, including HLA-DR, CD38, and Ki67 (marker of cycling), were analyzed on CD4+ (Figure 2) and CD8+ (Figure 3) T cells. IRIS and non-IRIS patients displayed similar levels of expression of HLA-DR (Figure 2C) at baseline and similar kinetics of CD38-expressing CD4+ T cells throughout the study (data not shown). HLA-DR expression slightly increased in both groups shortly after treatment initiation and started to decrease after 1 month on ART (Figure 2C). At month 6, the frequencies of HLA-DR–expressing CD4+ T cells were significantly higher in IRIS, compared with non-IRIS, patients (21% vs 12%; P = .017). Moreover, when the mixed-model/repeated-measures analysis was used, an overall difference in the kinetics of HLA-DR–expressing cells was found between the 2 groups (P = .02). HLA-DR expression on CD8+ T cells slightly increased shortly after treatment initiation and started to decrease after 1 month on ART, but was similar between IRIS and non-IRIS patients (Figure 3B). IRIS and non-IRIS patients had comparable frequencies of Ki67-expressing CD4+ T cells before ART initiation (Figure 2D). However, 1 month after treatment initiation, the expression of Ki67 in central memory CD4+ T cells was significantly higher in IRIS patients than non-IRIS controls (14% vs 7.4%; P = .019; Figure 2D-E). The expression of Ki67 in CD8+ T cells did not differ between IRIS and non-IRIS groups at any time point (Figure 3D).
HIV patients who developed IRIS had a lower frequency of CD57−GrB+-expressing CD8+ T cells (23% vs 30%; P = .022) at baseline (Figure 3C). This phenotype has been previously described as representing effector cells capable of IFN-γ production.11 The frequency of CD57−GrB+ CD8+ T cells was also lower at 3 (16% vs 22%; P = .009) and 12 months (10% vs 14%; P = .031) after ART initiation in patients who developed IRIS, compared with non-IRIS patients (Figure 3C). The difference in the frequencies of CD57−GrB+-expressing CD8+ T cells was also observed by repeated measures analysis (P = .04).
IRIS patients displayed higher frequencies of T cells expressing PD-1 before ART initiation
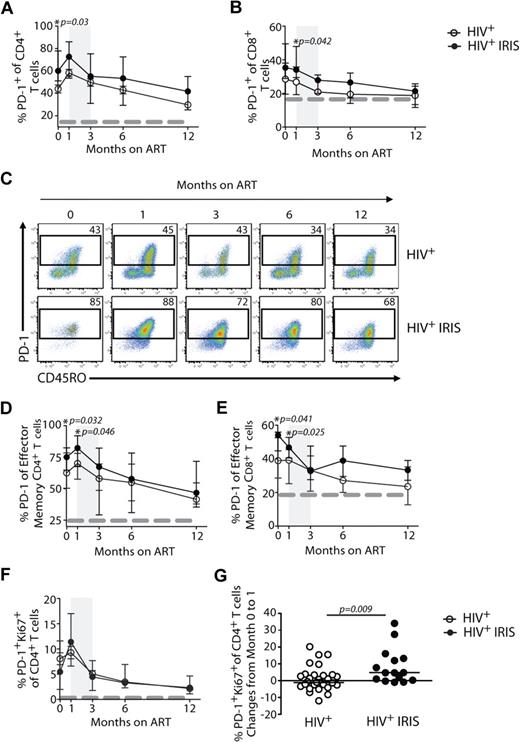
We next investigated the expression of PD-1 on T cells, an inhibitory receptor known to be up-regulated on mouse and human cells after antigenic stimulation that can lead to defects in T-cell function.12 IRIS patients displayed significantly higher frequencies of CD4+ T cells expressing PD-1 before ART initiation, compared with non-IRIS patients (60% vs 46%; P = .03; Figure 4A,C). PD-1 expression on CD4+ T cells slightly increased after treatment initiation in both groups and started to decrease after month 1, though an overall difference in the kinetics of PD-1–expressing CD4+ T cells was found between the 2 groups when the mixed-model/repeated-measures analysis was used (P = .05). When PD-1 expression was analyzed in the effector memory subset, its expression was also significantly higher in IRIS, compared with non-IRIS, patients before treatment (75% vs 62%; P = .032) and 1 month after ART initiation (82% vs 70%; P = .046; Figure 4D). Although there were no significant differences in the proportion of PD-1+Ki67+CD4+ T cells between IRIS and non-IRIS patients at different time points after ART initiation (Figure 4F), the change of these cells from baseline to month 1 was significantly higher in IRIS patients, compared with the non-IRIS group (+4.7% vs −0.6%; P = .009; Figure 4G). There was no difference in the proportion of PD-1+ CD4+ T cells in subgroups of IRIS based on underlying pathogen (ie, mycobacterial or fungal vs. viral) or based on pre-ART presence of opportunistic infection (OI) diagnosis (paradoxical vs unmasking; data not shown).
Higher frequencies of PD-1 expressing cells are found in IRIS patients before ART initiation. The percentage of PD-1 expression within CD4+ T and CD8+ T lymphocytes in HIV+ controls (open symbols) and HIV+ IRIS patients (filled symbols) were measured before and after ART initiation (A-B). Representative dot plots showing PD-1 expression on CD4+ T cells throughout study follow-up. Upper dot plots represent a single HIV+ control patient and lower dot plots represent a single HIV+ patient who developed one IRIS episode (C). The percentage of PD-1 expression within the effector memory compartment of CD4+ T and CD8+ T cells in HIV+ controls (open symbols) and HIV+ IRIS patients (filled symbols) were measured before and after ART initiation (D-E). Percentage of PD-1+Ki67+ expression within CD4+ T lymphocytes was measured before and 1, 3, 6, and 12 months after ART initiation. The dotted line represents median of the given measurement from healthy donors (F). The shaded area represents the IQR of the time of initiation of IRIS episodes, and dotted lines represent median of the given measurements from healthy donors (A-B, D-F). Symbols represent changes in the frequency of PD-1+Ki67+ cells among CD4+ T cells in individual patients from baseline to month 1 after ART initiation (G).
Higher frequencies of PD-1 expressing cells are found in IRIS patients before ART initiation. The percentage of PD-1 expression within CD4+ T and CD8+ T lymphocytes in HIV+ controls (open symbols) and HIV+ IRIS patients (filled symbols) were measured before and after ART initiation (A-B). Representative dot plots showing PD-1 expression on CD4+ T cells throughout study follow-up. Upper dot plots represent a single HIV+ control patient and lower dot plots represent a single HIV+ patient who developed one IRIS episode (C). The percentage of PD-1 expression within the effector memory compartment of CD4+ T and CD8+ T cells in HIV+ controls (open symbols) and HIV+ IRIS patients (filled symbols) were measured before and after ART initiation (D-E). Percentage of PD-1+Ki67+ expression within CD4+ T lymphocytes was measured before and 1, 3, 6, and 12 months after ART initiation. The dotted line represents median of the given measurement from healthy donors (F). The shaded area represents the IQR of the time of initiation of IRIS episodes, and dotted lines represent median of the given measurements from healthy donors (A-B, D-F). Symbols represent changes in the frequency of PD-1+Ki67+ cells among CD4+ T cells in individual patients from baseline to month 1 after ART initiation (G).
Differences were also observed in the expression of PD-1 on CD8+ T cells 1 month after ART initiation (34% vs 27%; P = .042) when IRIS and non-IRIS patients were compared (Figure 4B). Higher frequencies of PD-1–expressing effector memory CD8+ T cells were observed in IRIS, compared with non-IRIS, patients before ART initiation (54% vs 39%; P = .041) and 1 month on treatment (47% vs 39%; P = .025; Figure 4E). An overall difference in the frequencies of PD-1–expressing effector memory CD8+ T cells was seen between the groups throughout the study (mixed-model; P = .04).
IRIS patients had higher frequencies of regulatory T cells coexpressing Ki67 and PD-1, and PD-1+CD4+ T cells expressing CTLA-4, LAG-3, or ICOS before ART initiation
The molecular pathways involved in T-cell regulation remain poorly understood, and recent reports show that this regulation is a result of the coexpression of multiple inhibitory receptors.13 Inasmuch as PD-1 was up-regulated in IRIS patients before IRIS initiation, we also evaluated the expression of CTLA-4, LAG-3, and TIM-3 on T cells.
At baseline, IRIS patients displayed significantly higher frequencies of PD-1+CD4+ T cells expressing CTLA-4 (37% vs 4.3%; P = .028) and LAG-3 (19% vs 8.9%; P = .028), compared with non-IRIS patients (Figure 5A-B). The expression of TIM-314 was not different in IRIS, compared with non-IRIS, patients (data not shown). We also observed higher frequencies of PD-1+CD4+ T cells expressing the costimulatory molecule, ICOS (54% vs 32%; P = .015) in IRIS, compared with non-IRIS, patients before ART initiation (Figure 5C).
Higher frequencies of PD-1+CD4+ T cells expressing CTLA-4, LAG-3, or ICOS are found in IRIS patients before ART initiation. The percentage of CTLA-4+ (A), LAG-3+ (B), and ICOS+ (C) cells within PD-1+CD4+ T lymphocytes in HIV+ controls (open symbols), HIV+ IRIS patients (black symbols) were measured before ART initiation. The same molecules were measured in healthy donors (gray symbols). Percentage of PD-1+Ki67+ expression within Treg was also measured (D).
Higher frequencies of PD-1+CD4+ T cells expressing CTLA-4, LAG-3, or ICOS are found in IRIS patients before ART initiation. The percentage of CTLA-4+ (A), LAG-3+ (B), and ICOS+ (C) cells within PD-1+CD4+ T lymphocytes in HIV+ controls (open symbols), HIV+ IRIS patients (black symbols) were measured before ART initiation. The same molecules were measured in healthy donors (gray symbols). Percentage of PD-1+Ki67+ expression within Treg was also measured (D).
The expression of PD-1 on Treg cells was not different between the 2 groups (data not shown). However, IRIS patients displayed significantly higher frequencies of Treg cells coexpressing PD-1 and Ki67, compared with non-IRIS controls (39% vs 12%; P = .002; Figure 5D).
Changes in the levels of serum cytokines during immune reconstitution after ART initiation
Although IFN-γ levels remained stable after ART initiation in non-IRIS patients, an increase was observed at month 1 in IRIS patients, close to when most of the IRIS episodes happened. At this time point, the levels of IFN-γ were significantly higher in IRIS, compared with non-IRIS, patients (7.1 vs 3.1 pg/mL; P = .033; Figure 6A). Serum levels of IL-10 and TNF-α in IRIS and non-IRIS patients did not differ significantly at baseline or at any other time point analyzed (Figure 6B-C), although TNF-α levels were, overall, higher in the IRIS group throughout the study (mixed-model; P = .08).
IRIS patients display higher levels of serum IFN-γ and IL-7 than non-IRIS patients. The cytokines, IFN-γ (A), IL-10 (B), TNF-α (C), and IL-7 (D), were measured in the serum of HIV+ controls (open symbols) and HIV+ IRIS patients (filled symbols) before and 1, 3, 6, and 12 months after ART initiation. The shaded area represents the IQR of the time of initiation of IRIS episodes. Dotted lines represent medians of the given measurements from healthy donors.
IRIS patients display higher levels of serum IFN-γ and IL-7 than non-IRIS patients. The cytokines, IFN-γ (A), IL-10 (B), TNF-α (C), and IL-7 (D), were measured in the serum of HIV+ controls (open symbols) and HIV+ IRIS patients (filled symbols) before and 1, 3, 6, and 12 months after ART initiation. The shaded area represents the IQR of the time of initiation of IRIS episodes. Dotted lines represent medians of the given measurements from healthy donors.
IRIS and non-IRIS patients had comparable levels of IL-7 before ART initiation. However, 1 month after treatment, the levels of this cytokine started to decrease in non-IRIS patients, but remained elevated and were significantly higher in IRIS patients at months 1, 3, and 6 after ART initiation, compared with non-IRIS patients (37 vs 27 pg/mL; P = .031; 36 vs 25 pg/mL; P < .001; and 38 vs 23 pg/mL; P = .005). By month 12, the levels of IL-7 in IRIS patients decreased, and differences were no longer significant between the 2 groups (Figure 6D). An overall difference in the levels of serum IL-7 was found between the 2 groups when the mixed-model/repeated-measures analysis was applied (P = .01).
Higher frequencies of inflammatory cytokine–producing cells were found within PD-1+ CD4+ T cells, compared with their PD-1− counterparts
Inasmuch as patients who developed IRIS displayed higher frequencies of PD-1+CD4+ T cells before ART initiation, and IFN-γ levels were increased in the same patients just after ART initiation (month 1), we next asked if the PD-1–expressing CD4+ subset had the same potential to produce cytokines upon in vitro mitogenic stimulation (Figure 7). When the frequencies of PD-1+CD4+ and PD-1−CD4+ cells producing cytokines were compared between IRIS and non-IRIS patients, no significant differences were observed, and for this reason, the 2 groups were analyzed together. Higher frequencies of IFN-γ–producing cells were found among the PD-1+ CD4+ T cells than in the PD-1− subpopulation in healthy donors (27% vs 10%; P = .031), but the difference did not reach statistical significance in cells from HIV patients before ART initiation (35% vs 23%; P = .057) or 3 months after (Figure 7A). Overall higher frequencies of IFN-γ–producing cells were found among the PD-1+ than in the PD-1− CD8+ T-cell subpopulation before ART initiation and 3 months after (data not shown). PD-1+ and PD-1− cells produced comparable TNF-α (Figure 7B) and IL-2 (Figure 7C) before and 3 months after ART initiation. The same frequencies of IL-4–producing PD-1+ and PD-1− cells were observed before ART, though PD-1+ CD4+ T cells were able to produce more IL-4 than PD-1− 3 months after ART initiation (3.49% vs 1.32%; P = .013) (Figure 7D). The proportion of IL-17-producing cells was also higher in PD-1+ than the PD-1− CD4+ T cells before ART (0.36% vs 0.02; P = .001) as well as 3 months after treatment initiation (1% vs 0.26; P = .002) (Figure 7E).
PD-1 expressing cells produce more IFN-γ in response to polyclonal stimulation in comparison to PD-1− CD4+ T cells. Cryopreserved PBMCs were thawed and cultured for 5 hours in the presence of PMA, ionomycin, and Brefeldin-A. The percentage of IFN-γ (A), TNF-α (B), IL-2 (C), IL-4 (D), and IL-17 (E) expression within PD-1+ (filled symbols) and PD-1− (open symbols) CD4+ T lymphocytes in HIV+ (controls and IRIS) patients were measured before and 3 months after ART initiation, as well as in healthy donors (HD). Symbols represent frequencies of cytokine-producing cells after PMA/ionomycin stimulation minus background cytokine production in unstimulated controls.
PD-1 expressing cells produce more IFN-γ in response to polyclonal stimulation in comparison to PD-1− CD4+ T cells. Cryopreserved PBMCs were thawed and cultured for 5 hours in the presence of PMA, ionomycin, and Brefeldin-A. The percentage of IFN-γ (A), TNF-α (B), IL-2 (C), IL-4 (D), and IL-17 (E) expression within PD-1+ (filled symbols) and PD-1− (open symbols) CD4+ T lymphocytes in HIV+ (controls and IRIS) patients were measured before and 3 months after ART initiation, as well as in healthy donors (HD). Symbols represent frequencies of cytokine-producing cells after PMA/ionomycin stimulation minus background cytokine production in unstimulated controls.
Moreover, upon HIV Gag peptide pool stimulation, the proportions of IFN-γ–, TNF-α–, and IL-2–producing cells were higher among the PD-1+, CD4+, and CD8+ T cells than in the PD-1− subpopulations both at baseline and 3 months after ART initiation (supplemental Figure 3A-F). The HIV-specific responses did not differ significantly between the IRIS and non-IRIS groups (data not shown).
Discussion
The pathogenesis of immune reconstitution syndrome in HIV infection remains unclear, but the 2 major clinical predictors, infection and severe CD4 lymphopenia,1 point to the role of emerging dysregulated T-cell responses to antigen as severe immunosuppression reverses. Our data, based on subjects with IRIS events due to fungal, mycobacterial, and viral infections, support the hypothesis of effector responses leading to significant T-cell activation, possibly in the absence of adequate Treg control with a potential contribution of homeostatic signals.
Evidence from TB-IRIS studies have previously shown a prominent role of Th1 cytokines produced by either αβ or γδ T cells in the development of IRIS.5,6 Our data are in agreement with that of Bourgarit et al5 in demonstrating high serum levels of IFN-γ shortly after ART initiation in the IRIS group, and by showing that CD4+PD-1+ T cells were higher in IRIS patients and, overall, enriched in Th1 (IFN-γ–producing) cells.
The highly activated phenotype of the CD4+ T cells of both groups (IRIS and non-IRIS) was anticipated, because our patients were untreated and profoundly lymphopenic (with low CD4 and CD8 T cells) and the majority of them had an AIDS-defining disease. The immune activation seen in untreated patients is thought to stem from both Ag-specific responses and compensatory homeostatic responses supported by IL-7 and IL-1515–20. The presence of active viral replication hampers both of these proliferative stimuli, but when the HIV insult is blocked by ART, T cells can become responsive and restore both their Ag-specific responses and, gradually, their homeostatic responses.21 Although our 2 groups were equally lymphopenic and had similar opportunistic diseases, patients who developed IRIS had clear evidence of more pronounced activation and effector responses by higher levels of PD-1 expression, predominantly in CD4+, but also in CD8+ T cells, as well as higher levels of CD4+ T cells expressing HLA-DR (suggesting Ag-specific responses). This could imply a higher antigenic load in these patients or a greater imbalance of Ag and effector responses. The former is supported by clinical observations (longer therapy of OIs before ART may lower the incidence of IRIS1 ), although the role of HIV itself has not been fully investigated. The latter is supported by the presence of high proportions of PD-1+ T cells, many coexpressing other inhibitory receptors, suggesting hyperactivation with functional impairment that, likely, quickly reverses with ART.22 Although it seems counterintuitive to see more robust, dysregulated responses in patients with higher proportions of PD-1+ T cells, it probably represents evidence of higher antigen load and higher frequencies of Ag-specific T cells ready to get mobilized once immunosuppression reverses. Although specific pathways may diverge based on the underlying pathogen or the presence of live versus dead organisms, we found no differences in subgroup analysis of unmasking or paradoxical IRIS mycobacterial/fungal versus viral IRIS, suggesting that the T-cell activation markers and the naive/effector memory restoration represent unifying patterns that relate more to high antigen exposure in the setting of lymphopenia and immunosuppression that reverses with ART. The HIV-specific T-cell responses did not seem to differ between the 2 groups, but it would be essential to study the Ag-specific T responses against the opportunistic pathogens of these patients, where differences may emerge between unmasking and paradoxical IRIS, as suggested in a recent study of TB-IRIS.23
Lymphopenia-induced proliferation (LIP) and activation of T cells can be observed in animal models, as well as in humans with lymphopenia, that is associated with HIV infection24,25 or chemotherapy,26 or is idiopathic.27 Although cytokines such as IL-7 and IL-15 are important for LIP, T-cell receptor triggering through self-antigens is also considered essential.28 If foreign antigens are found in abundance because of infection, LIP can be skewed and T cells recognizing the existing Ag will predominate.29 It is possible that a similar phenomenon occurs, to some extent, in IRIS. The high proportion of effector CD4+ T cells expressing inhibitory and costimulatory molecules that lead the immune restoration while the naïve CD4 cell restoration lagged behind would support primarily Ag-experienced T cells driving the IRIS events. The high levels of IL-7 in the IRIS group could be secondary to decreased receptor-mediated clearance (fewer naive cells). An alternative explanation could be the increased production or release of this cytokine. Regardless of the mechanism, the wider availability of IL-7 in the IRIS group could have supported a more pronounced response to proliferative signals.30,31 Interestingly, some similarities are emerging between graft-versus-host disease seen in stem cell transplant recipients and IRIS, namely, high serum levels of IL-7 and the predominance of effector CD4+ T cells.32,33
The role of Treg could be crucial in IRIS, because they can suppress both Ag-specific effector responses and control homeostatic T-cell responses.34,35 In our study, we did not see differences, between subjects with or without IRIS, in the numbers or proportions of Treg. In agreement with other groups, we did see a higher proportion of Treg in HIV patients, compared with healthy controls.36 The limited availability of samples precluded functional studies for our patients. It is thus still conceivable that Treg dysregulation plays a role in IRIS; some evidence for this was the observation that Treg with coexpression of both Ki67 and PD-1 were higher in the IRIS group. It has been shown that PD-1 expression can adversely affect the suppressive function of Treg in humans.37,38 Similarly, activation of Treg in severe infection in an animal model of lethal infection with Toxoplasma gondii led to loss of suppressive function and acquisition of effector phenotype with IFN-γ production.39
One of our main observations was the increased PD-1 expression on CD4+ T cells and, to a smaller extent, effector memory CD8+ T cells. Although PD-1 has received a lot of attention for being a marker of T-cell exhaustion of CD8+ T cells,40 it has also been shown that PD-1+ T cells can maintain effector functions, and that expression or coexpression of other inhibitory molecules influences the overall functional capacity of T cells.12,41–43 Furthermore, T-cell function depends on nonredundant coexpression of multiple inhibitory receptors that has been associated with greater T-cell exhaustion and more severe infection.14 We found significantly higher proportions of PD-1+CD4+ T cells expressing ICOS, LAG3, and CTLA-4 in IRIS patients, suggesting that the CD4+ T cells of the IRIS patients are more activated and, possibly, with some functional impairment. It is unclear if this can be the result or the cause of higher antigenic load, but our results suggest that PD-1 expression may predict the pathogenic T cells before therapy that overcome their exhaustion during reconstitution. Despite the coexpression of many inhibitory molecules, PD-1+ CD4 T cells were able to produce cytokines after both mitogenic and HIV Gag peptide stimulation. This was in agreement with the higher levels of IFN-γ found in serum at month 1, when most of the IRIS events occurred. Again, our study was limited to peripheral blood, so it is unclear if the same differences would be observed in the relevant tissues.44
In conclusion, our study shows that IRIS patients have a higher proportion of activated T cells even before ART initiation, including a higher proportion of activated and, possibly, dysfunctional Treg, all suggestive of a higher antigenic burden. Although specific mechanisms may differ based on the underlying pathogen, it appears that IRIS events are accompanied by high serum levels of IFN-γ and IL-7, suggesting a dysregulated effector response as profound lymphopenia and immunosuppression reverse.
This work was presented, in part, in abstract format at Keystone Symposium, HIV Immunobiology, March 2009, Keystone, CO, and at the Conference on Retroviruses and Opportunistic Infections, February 2010, San Francisco, CA.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge the staff of the Clinic OP8 at NIH Clinical Center for their assistance with patient recruitment and care and all study participants for their support of clinical research. We also thank Drs Joanne Yu and Pratip Chattopadhyay for reagent manufacture and qualification and members of the ImmunoTechnology Section, Vaccine Research Center, for their valuable assistance.
This work was funded through the intramural research program of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (Bethesda, MD) and, in part, with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
National Institutes of Health
Authorship
Contribution: L.R.A., I.S., Y.M., M.R., D.B., and A.S. designed the experiments and assisted in data analysis and interpretation; L.R.A. and J.H. performed research and analyzed data; Y.M. and J.G. performed experiments and data analysis; B.O.P. helped with clinical data extraction, and performed experiments and data acquisition; R.D.S. performed the statistical analysis; G.R. and J.M. assisted with patient care and case identification; L.R.A. and I.S. wrote the manuscript; and all the coauthors assisted in manuscript preparation.
Conflict-of-interest disclosure: D.L.B. receives royalties and is listed as coinventor in a patent on the role of inhibition of the PD-1 pathway in cancers and infections. The remaining authors declare no competing financial interests.
The current affiliation for L.R.V.A. is Laboratory of Immunopathology, René Rachou Research Center, FIOCRUZ, Belo Horizonte, Brazil.
Correspondence: Irini Sereti, Laboratory of Immunoregulation, Bldg 10–Magnuson Clinical Center, Rm 11B07A, 10 Center Dr, Bethesda, MD 20892; e-mail: isereti@niaid.nih.gov.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal