Abstract
Mice lacking factor XII (fXII) or factor XI (fXI) are resistant to experimentally–induced thrombosis, suggesting fXIIa activation of fXI contributes to thrombus formation in vivo. It is not clear whether this reaction has relevance for thrombosis in pri mates. In 2 carotid artery injury models (FeCl3 and Rose Bengal/laser), fXII-deficient mice are more resistant to thrombosis than fXI- or factor IX (fIX)–deficient mice, raising the possibility that fXII and fXI function in distinct pathways. Antibody 14E11 binds fXI from a variety of mammals and interferes with fXI activation by fXIIa in vitro. In mice, 14E11 prevented arterial occlusion induced by FeCl3 to a similar degree to total fXI deficiency. 14E11 also had a modest beneficial effect in a tissue factor–induced pulmonary embolism model, indicating fXI and fXII contribute to thrombus formation even when factor VIIa/tissue factor initiates thrombosis. In baboons, 14E11 reduced platelet-rich thrombus growth in collagen-coated grafts inserted into an arteriovenous shunt. These data support the hypothesis that fXIIa-mediated fXI activation contributes to thrombus formation in rodents and primates. Since fXII deficiency does not impair hemostasis, targeted inhibition of fXI activation by fXIIa may be a useful antithrombotic strategy associated with a low risk of bleeding complications.
Introduction
Initiation of fibrin formation by contact activation requires proteolytic conversion of plasma factor XII (fXII) to the protease factor XIIa (fXIIa) on a surface.1-3 FXIIa activates the next zymogen in the coagulation cascade, factor XI (fXI), to factor XIa (fXIa), which in turn converts factor IX (fIX) to factor IXaβ (fIXaβ). This series of reactions, referred to as the intrinsic pathway of coagulation, drives thrombin generation and fibrin formation in the activated partial thromboplastin time (aPTT) assay used by clinical laboratories. A role for fIX in hemostasis is not in question, as its deficiency causes the severe bleeding disorder hemophilia B. However, the importance of the intrinsic pathway, as a whole, to clot formation and stability at a site of injury is probably limited, as fXII deficiency is not associated with abnormal bleeding,1,2 and fXI-deficient patients have a variable hemorrhagic disorder with milder symptoms than hemophiliacs.2,4 Current models of thrombin generation address these phenotypic differences by incorporating additional mechanisms for protease activation. Thus, fIX is activated by the factor VIIa/tissue factor complex in addition to fXIa,3,5 while fXI can be activated by thrombin.3,6
Mice lacking fXII, like their human counterparts, do not have a demonstrable bleeding abnormality,7 supporting the premise that fXIIa activation of fXI is not required for hemostasis.8 Given this, it was surprising to observe that mice lacking fXII9 or fXI10 were resistant to arterial thrombotic occlusion. While this suggested contact activation might play an important role in pathologic coagulation, if not hemostasis, it was not clear that fXIIa was mediating its prothrombotic effect through fXI. We developed an antibody against mouse fXI (14E11) that prolongs time to clot formation in plasma by interfering with fXI activation by fXIIa. Based on the phenotypes of fXII- and fXI-deficient mice, we postulated that 14E11 would inhibit thrombus formation in vivo, despite selectively interfering with a reaction not required for hemostasis. Here we show that 14E11 affects fXIIa-dependent coagulation in plasmas from multiple species and report on its effects in mouse and primate thrombosis models.
Methods
Reagents
Pooled normal and fXII-deficient plasmas were from George King Bio-Medical. fIX, fXI, and fXIa were from Haematologic Technologies. fXIIa, high-molecular-weight kininogen (HK), and corn trypsin inhibitor (CTI) were from Enzyme Research Laboratories. Z-Gly-Gly-Arg-AMC was from Bachem. Dioleoylphosphatidylcholine:dioleoylphosphatidylserine (7:3 w/w) was from Avanti Polar Lipids. S-2366 was from Diapharma. Bovine serum albumin (BSA), rabbit brain cephalin (RBC), kaolin, and O-phenylenediamine (OPD) were from Sigma-Aldrich.
Monoclonal antibodies
FXI-deficient Balb-C mice were immunized with 25 μg of recombinant mouse fXI11 diluted 1:1 with Freund adjuvant (200 μL total) by intraperitoneal injection. Two 25-μg booster doses in incomplete Freund adjuvant were given 3 and 7 weeks later, and hybridomas were generated by standard protocols. Media were tested for capacity to recognize mouse fXI by enzyme-linked immunosorbent assay and to prolong the aPTT of mouse and human plasmas. Clones of interest were subcloned twice by limiting dilution. Clone 14E11 was expanded in a CL1000 bioreactor (Integra Biosciences), and immunoglobulin G (IgG) was purified by cation exchange and thiophilic agarose chromatography. 14E11 was biotinylated using an EZ-link sulfo-NHS-biotinylation kit (Thermo Scientific). Generation and characterization of monoclonal IgG O1A6, which binds to the A3 domain of human fXI and interferes with activation of factor IX by fXIa, has been described.6,12
14E11 binding to fXI and fXIa
FXI or fXIa (2 μg/mL, 100 μL/well) in 50mM Na2CO3 pH 9.6 was incubated overnight at 4°C in Immulon 2HB microtiter plates (Thermo Scientific). Wells were blocked with 150 μL of phosphate-buffered saline (PBS) with 2% BSA for 1 hour at room temperature (RT). One hundred microliters of biotinylated 14E11 (100 to 10−7μM) in 90mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) pH 7.2, 100mM NaCl, 0.1% BSA, 0.1% Tween-20 (HBS) were added, with incubation for 90 minutes at RT. After washing with PBS-0.1% Tween-20 (PBST), 100 μL of strepavidin-horseradish peroxidase (HRP; Thermo Scientific, 1:8000 dilution in HBS) was added, with incubation at RT for 90 minutes. After washing, 100 μL of substrate solution (12 mL of 30mM citric acid, 100mM Na2HPO4 pH 5.0, one tablet OPD, 12 μL of 30% H2O2) was added. Reactions were stopped after 10 minutes with 50 μL of 2.5M H2SO4. Absorbance at 495 nm was measured on a SpectroMax 340 microplate reader (Molecular Devices).
14E11 binding site on fXI
Biotinylated 14E11 was the primary antibody in Western blots of recombinant fXI with individual apple domains replaced with corresponding domains from human prekallikrein (PK),13,14 or individual fXI apple domains attached to tissue plasminogen activator (tPA).15 Detection was with strepavidin-HRP and chemiluminescence.
Effect of 14E11 on plasma coagulation
aPTT assays.
Plasma (50 μL) anticoagulated with 0.38% sodium citrate was mixed with 50 μL of PBS (with or without 14E11 [0 to 320nM]) and 50 μL of aPTT reagent (aPTT-ES; Helena Laboratories) at 37°C for 3 minutes. Fifty microliters of CaCl2 (20mM) was added and time to clot formation determined on an Amelung KC4 Analyzer (Sigma-Aldrich).
Modified fXIa PTT.
FXIa (30nM) in 25mM Tris-HCl pH 7.4, 100mM NaCl (TBS) with 0.1% BSA (TBSA) was incubated at RT for 30 minutes, with or without 14E11 (300nM). FXIa (65 μL) was mixed with 65 μL of fXI-deficient plasma and 65 μL of RBC at 37°C. After 30 seconds, 25mM CaCl2 (65 μL) was added and time to clot formation measured.
Modified fXIIa PTT.
65 μL of fXIIa (1-100nM) in TBSA was mixed with 65 μL of normal plasma (with or without 14E11 [300nM]), and 65 μL of RBC at 37°C. After 30 seconds, 25mM CaCl2 (65 μL) was added and time to clot formation determined.
FIX activation by fXIa
FIX (100nM) was incubated with fXIa (1nM) in TBS with 5mM CaCl2 at RT with or without 200nM 14E11 or O1A6. At intervals, 20-μL samples were placed in sodium dodecyl sulfate (SDS) sample buffer. Samples were size-fractionated on 10% nonreducing SDS-polyacrylamide gels and transferred to polyvinylidene fluoride membranes. Chemiluminescent Western blotting was performed using goat anti-human fIX IgG (Affinity Biologicals).
HK-fXI binding assay
Immulon 2HB microtiter plates were coated with HK (30nM) in 50mM carbonate buffer pH 9.6 (100 μL per well) overnight at 4°C. Wells were blocked with 150 μL of PBS-2% BSA for 1 hour at RT. Biotinylated fXI (10nM) and unlabeled 14E11 (25pM to 1μM) were added to the wells (total volume 100 μL) and incubated at RT for 90 minutes. After washing with PBST, 100 μL of strepavidin-HRP (1:8000 in HBS) was added and incubated at RT for 90 minutes. After washing, 100 μL of Substrate Solution was added. Reactions were stopped after 10 minutes with 50 μL of 2.5M H2SO4, and absorbance at 495 nm measured.
Chromogenic assay for fXI activation
FXI (170nM) in PBS with 1 mg/mL BSA was incubated at 37°C for 15 minutes with or without 600nM 14E11. At time zero, fXIIa, HK, and kaolin, all in PBS with 1 mg/mL BSA, were added so that final concentrations were fXI (85nM), fXIIa (17nM), HK (70nM), and kaolin (0.5 mg/mL). At various time points, 50-μL of aliquots were supplemented with CTI (600nM final concentration) and then mixed with an equal volume of 1.0mM S-2366. Changes in absorbance at 405 nm were measured.
Thrombin generation assay
Thrombin generation was measured by following cleavage of Z-Gly-Gly-Arg-AMC (415μM) in fXII-deficient plasma on a Thrombinoscope (Thrombinoscope BV),6 in the presence of 14E11 (300nM) or vehicle. Plasma (80 μL) was mixed with 20 μL of Tyrode buffer pH 7.4 containing PC:PS vesicles (30μM) and either tissue factor (2.3pM) or fXIIa (10 or 100nM). CTI was added to a final concentration of 4μM for reactions with tissue factor (TF). Final concentrations: 5μM PC:PS vesicles, 0.23pM TF or 1-10nM fXIIa. Clotting was started with 20 μL of 20mM HEPES pH 7.4, 100mM CaCl2, 6% BSA, and fluorescence was monitored (emission λ 460 nm). Assays were run twice in triplicate, and endogenous thrombin potential (ETP) was determined with Thrombinoscope Analysis software, version 3.0.
Platelet adhesion assay
Glass coverslips were incubated with fXI, fXIa, or fibrinogen (50 μg/mL) for 1 hour at RT.16 Surfaces were blocked with denatured fatty acid–free BSA (5 mg/mL) for 1 hour, then incubated with vehicle, 14E11 or nonspecific IgG (20 μg/mL) for 20 minutes. Purified human platelets (2 × 107/mL) were incubated on protein-coated coverslips for 45 minutes at 37°C and imaged using Köhler illuminated Nomarski differential interference contrast optics with a Zeiss 63× oil immersion 1.40 NA plan-apochromat lens on a Zeiss Axiovert 200M microscope (Carl Zeiss). Numbers of adherent platelets was computed using ImageJ software (National Institutes of Health).16
Immunoprecipitation of plasma fXI
Plasma anticoagulated with sodium citrate or EDTA was obtained from the following species: African elephant, (Loxodonta africana), Amur tiger (Panthera tigris altaica), baboon (Papio anubis), cattle (Bos taurus), chicken (Gallus gallus domesticus), dog (Canis lupus familiaris), domestic cat (Felis silvestris catus), giant anteater (Myrmecophaga tridactyla), horse (Equus ferus caballus), llama (Lama glama), pig (Sus scrofa domestica), rabbit (Oryctolagus cuniculus), and raccoon (Procyon lotor). Fifty microliters of Affigel-10 beads (BioRad) bound with 14E11 (3 mg/mL) were rocked at RT for 2 hour with 500 μL of plasma and 500 μL of TBS. Beads were washed with 1 mL of TBS and then eluted with 50 μL of SDS-nonreducing sample buffer. Eluates were size fractionated on 7.5% SDS-polyacrylamide gels, followed by Western blotting using biotinylated 14E11 antibody.
Mouse carotid artery thrombosis models
Procedures for mice were approved by the Institutional Animal Care and Use Committee of Vanderbilt University. C57Bl/6 mice deficient in fIX,17 fXI,18 or fXII7 were described. Mice with combined fXI and fXII deficiencies were generated from these lines. Anesthesia was with 50 mg/kg intraperitoneal pentobarbital. The right common carotid artery was fitted with a Doppler flow probe (Model 0.5 VB; Transonic System). 14E11 (1.0 mg/kg) was infused into the internal jugular vein 15 minutes before injury. In the ferric chloride model,10 thrombus formation was induced by applying two 1 × 1.5-mm filter papers (GB003; Schleicher & Schuell) saturated with FeCl3 (2.5% to 15% solution) to opposite sides of the artery for 3 minutes, followed by rinsing with normal saline. Flow was monitored for 30 minutes. For the laser injury model,19 Rose Bengal (75 mg/kg) was infused through the internal jugular vein, and the carotid artery was illuminated with a 1.5-mW 540-nm laser (Melles Griot) positioned 6 cm from the vessel. Flow was monitored for 120 minutes.
Mouse pulmonary embolism model
Mice anesthetized with 50 mg/kg intraperitoneal pentobarbital were fitted with an MP100 transducer (AD Instruments) to monitor respiration, and 14E11 (1.0 mg/kg) or vehicle was infused into the internal jugular vein. After 15 minutes, 100 μL of a 1:4 dilution of STA-Neoplastin CI Plus (∼ 1nM TF; Diagnostica Stago) was infused into the inferior vena cava over 30 seconds.20 Time to cessation of breathing was determined.
Baboon thrombosis model
Nonterminal thrombosis studies were performed on a male baboon (Papio anubis) with an exteriorized femoral arteriovenous shunt.11,21 Protocols were approved by the Institutional Animal Care and Use Committee of the Oregon Health and Sciences University. Briefly, thrombus formation was initiated by introducing a 20-mm long by 4-mm diameter vascular graft (polytetrafluorethylene [Gore-Tex]; Gore & Co.) coated with collagen into the shunt. Flow rate through the shunt was restricted to 100 mL/min (mean shear rate 265/s). Thrombus formation was assessed by quantitative imaging of 111In-labeled platelet accumulation within, and downstream of the graft (10 cm), using a GE-400T gamma scintillation camera interfaced with a NuQuest InteCam computer system. Fibrin deposition in the graft was determined by direct measurement of 125I-labeled fibrinogen as described.21
Results
Antibody 14E11
Antibodies were raised against mouse fXI (Figure 1A) in fXI-deficient mice and screened for ability to prolong the aPTT of mouse plasma. The IgG 14E11 was selected for further study because it also prolonged the aPTT of human plasma. On Western blots, 14E11 recognizes a single band of appropriate size (∼ 160 kDa) in normal mouse (Figure 1B) and human (Figure 1C) plasma, but not in fXI-deficient plasma. 14E11 has a high affinity for mouse and human fXI, and human fXIa in a solid phase binding assay (apparent Kd ∼ 2-3pM; Figure 1D). fXI is a dimer of 80-kDa subunits, each containing 4 apple domains (A1-A4) and a protease domain. PK, a monomeric homolog of fXI, has an identical structure. Previously, we used human fXI/PK chimeras to show that the A2 domain is required for 14E11 binding to fXI (Figure 1E).6 Western blots using individual fXI apple domains indicate the 14E11 binding site is likely located entirely within A2 (Figure 1F).
Binding properties of 14E11. (A) Coomassie blue–stained 10% polyacrylamide gel of human (H) and mouse (M) recombinant fXI. (B-C) Western blots of nonreducing 10% polyacrylamide gels of mouse (B) and human (C) normal (N) and fXI-deficient (XI−/−) plasmas, using biotinylated-14E11 for detection. rXI in panel B indicates recombinant mouse fXI control. (D) Binding of biotinylated-14E11 to immobilized mouse fXI (○), human fXI (●), or human fXIa (□), as described in “Methods.” (E) Western blot of nonreducing 10% polyacrylamide gel of human fXI (hXI), human prekallikrein (PK), and human fXI in which the A1, A2, A3, or A4 domain has been replaced with the corresponding domain from PK. Position for fXI dimer is indicated to the right by “D,” and for monomeric PK by “M.” Note fXI with the PK A4 domain is a monomer because A4 mediates fXI dimer formation. (F) Western blot (left panel) of a nonreducing 10% polyacrylamide gel of human fXI (hXI) and individual human fXI apple domains linked to tPA. The right panel is a stained gel showing the recombinant apple domain-tPA chimeras. Note the A4 chimera forms a dimer. For panels A-C and E, positions of molecular mass standards in kDa are at the left of the figures, and for panel F at the right.
Binding properties of 14E11. (A) Coomassie blue–stained 10% polyacrylamide gel of human (H) and mouse (M) recombinant fXI. (B-C) Western blots of nonreducing 10% polyacrylamide gels of mouse (B) and human (C) normal (N) and fXI-deficient (XI−/−) plasmas, using biotinylated-14E11 for detection. rXI in panel B indicates recombinant mouse fXI control. (D) Binding of biotinylated-14E11 to immobilized mouse fXI (○), human fXI (●), or human fXIa (□), as described in “Methods.” (E) Western blot of nonreducing 10% polyacrylamide gel of human fXI (hXI), human prekallikrein (PK), and human fXI in which the A1, A2, A3, or A4 domain has been replaced with the corresponding domain from PK. Position for fXI dimer is indicated to the right by “D,” and for monomeric PK by “M.” Note fXI with the PK A4 domain is a monomer because A4 mediates fXI dimer formation. (F) Western blot (left panel) of a nonreducing 10% polyacrylamide gel of human fXI (hXI) and individual human fXI apple domains linked to tPA. The right panel is a stained gel showing the recombinant apple domain-tPA chimeras. Note the A4 chimera forms a dimer. For panels A-C and E, positions of molecular mass standards in kDa are at the left of the figures, and for panel F at the right.
Anticoagulant properties of 14E11
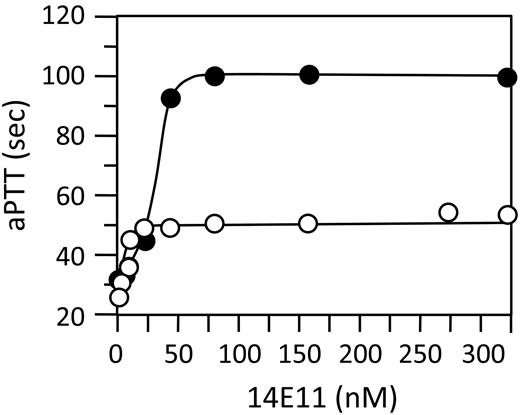
14E11 prolonged the aPTT of mouse and human plasma (Figure 2) with maximum inhibition at 25-50nM. This is similar to the plasma fXI concentration in humans (20-45nM),2 and probably in mice.12,18 The prolongation of the clotting times were similar to those of fXI-deficient plasmas. The aPTT of fXI-deficient mouse plasma, or plasma from a wild-type mouse treated with 14E11 is 48-70 seconds. This modest prolongation, relative to human fXI-deficient plasma, is a consistent finding. 14E11 did not affect the prothrombin time of human plasma. In an assay using fXIa to trigger clotting, 14E11 did not affect clotting times, indicating it does not interfere with fXIa activation of fIX. This was confirmed by Western blot analysis in a purified protein system (Figure 3A). In contrast, fIX activation is reduced by antibody O1A6 (Figure 3A), which binds to the A3 domain6 near an area required for fIX activation.13 Cumulatively, these data indicate 14E11 interferes with fXI activation, but not fXIa activity. This was supported by studies in which clotting was triggered by addition of fXIIa. Here, 14E11 prolonged clotting times to an extent consistent with 90% inhibition of fXI activation by fXIIa.
Effect of 14E11 on the aPTT assay. Mouse (○) or human (●) plasma supplemented with 14E11 (10−5 to 100μM) were tested in an aPTT assay, as described in “Methods.”
Effect of 14E11 on the aPTT assay. Mouse (○) or human (●) plasma supplemented with 14E11 (10−5 to 100μM) were tested in an aPTT assay, as described in “Methods.”
Activities of antibody 14E11. (A) Nonreducing Western blots showing fIX (100nM) activation by fXIa (1nM) in the presence of control vehicle (top), 200nM 14E11 (middle), or 200nM O1A6 (bottom). Blots were developed with a polyclonal anti-human fIX IgG. Arrows to the right of each blot indicate zymogen fIX (IX) and fIXaβ (IXa). Time of incubation in minutes is shown at the bottom, and positions of molecular mass standards are at the left of the figure. (B) Competition binding assay in which biotinylated fXI is allowed to bind to immobilized HK in the presence of 14E11 (10−5 to 100μM). Bound fXI was detected with strepavidin-HRP, as described in “Methods.” Each symbol is the average of results for duplicate experiments. (C) Factor XI (85nM) was activated by fXIIa (17nM) in the presence of HK (70nM and kaolin (0.5 mg/mL) in the presence (●) or absence (○) of 300nM 14E11. At various time points, samples were removed from the reaction into CTI, and fXIa generated was determined with a chromogenic substrate, as described in “Methods.” Each symbol is the average of results for duplicate experiments. (D) Thrombin generation in fXII deficient plasma initiated by addition of Ca2+ and fXIIa (10nM curves 1 and 3, or 1nM curves 2 and 4) in the absence (curves 1 and 2) or presence (curves 3 and 4) of 300nM 14E11. (E) Thrombin generation in fXII deficient plasma initiated by addition of Ca2+ in the absence (curve 1) or presence (curvess 2, 3, and 4) of tissue factor (0.23pM). Reaction for curves 2, 3, and 4 included control vehicle, 300nM 14E11, or 300nM O1A6, respectively.
Activities of antibody 14E11. (A) Nonreducing Western blots showing fIX (100nM) activation by fXIa (1nM) in the presence of control vehicle (top), 200nM 14E11 (middle), or 200nM O1A6 (bottom). Blots were developed with a polyclonal anti-human fIX IgG. Arrows to the right of each blot indicate zymogen fIX (IX) and fIXaβ (IXa). Time of incubation in minutes is shown at the bottom, and positions of molecular mass standards are at the left of the figure. (B) Competition binding assay in which biotinylated fXI is allowed to bind to immobilized HK in the presence of 14E11 (10−5 to 100μM). Bound fXI was detected with strepavidin-HRP, as described in “Methods.” Each symbol is the average of results for duplicate experiments. (C) Factor XI (85nM) was activated by fXIIa (17nM) in the presence of HK (70nM and kaolin (0.5 mg/mL) in the presence (●) or absence (○) of 300nM 14E11. At various time points, samples were removed from the reaction into CTI, and fXIa generated was determined with a chromogenic substrate, as described in “Methods.” Each symbol is the average of results for duplicate experiments. (D) Thrombin generation in fXII deficient plasma initiated by addition of Ca2+ and fXIIa (10nM curves 1 and 3, or 1nM curves 2 and 4) in the absence (curves 1 and 2) or presence (curves 3 and 4) of 300nM 14E11. (E) Thrombin generation in fXII deficient plasma initiated by addition of Ca2+ in the absence (curve 1) or presence (curvess 2, 3, and 4) of tissue factor (0.23pM). Reaction for curves 2, 3, and 4 included control vehicle, 300nM 14E11, or 300nM O1A6, respectively.
We previously reported that 14E11 effects fXI activation by fXIIa in a purified protein system (rate reduced ∼ 50%),6 but does not completely explain its potent effect in the aPTT assay. fXI forms a complex in plasma with HK.14,22,23 In the aPTT, HK enhances fXI activation by fXIIa by facilitating fXI binding to the contact surface.1,2 In a solid phase competition assay, 14E11 inhibited binding of fXI to immobilized HK (apparent Ki of ∼ 10-20nM; Figure 3B). A modification of the method of Baglia et al24 was used to measure activation of fXI by fXIIa in the presence of HK on a kaolin (aluminum silicate) surface. 14E11 significantly reduced the rate of fXI activation in this HK-dependent system (Figure 3C).
14E11 significantly reduced thrombin generation in a plasma thrombin generation assay when coagulation is initiated by adding fXIIa in the absence of surface (Figure 3D). Previous work with this system showed that fXI can be activated in the absence of fXIIa, most likely by thrombin.6 This pathway, which is thought to serve a role in hemostasis,6,8 is demonstrated in Figure 3E. Here, a low concentration of TF (0.23pM) produced a burst of thrombin generation in the presence of fXI (ETP 1092nM) that was not affected by 14E11 (ETP 1118nM). This is consistent with the selective effect of 14E11 on fXIIa-mediated fXI activation. In contrast, O1A6 reduced thrombin generation approximately 50% (ETP 547nM), as expected with inhibition of fIX activation by fXIa. Taken as a whole, the data indicate 14E11 exerts an inhibitory effect on fXI activation, but not fXIa activity, in plasma in the absence or presence of a surface. In the absence of a surface, this may be due to direct inhibition of fXIIa activation of fXI. In the presence of a surface (contact activation), inhibition of the fXI-HK interaction likely also contributes.
14E11 recognizes fXI from multiple species
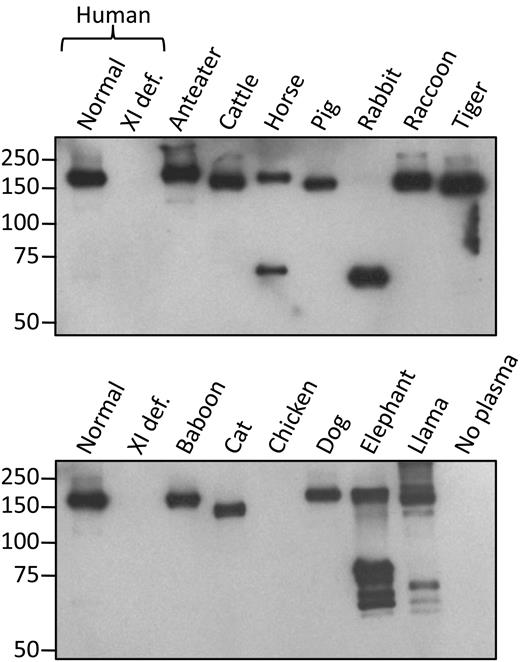
Give the unexpectedly high affinity of 14E11 for human fXI, we tested the antibody in plasmas from other species used in vascular research. 14E11 had a potent effect on the aPTT assay in pig, rabbit, and baboon plasma (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), and in rhesus macaque and rat plasma (not shown). 14E11 immunoprecipitated fXI from plasmas of a variety of placental mammals (Figure 4). Rabbit fXI migrates at a relatively low molecular mass because it lacks Cys321, which forms the interchain bond connecting the fXI dimer subunits.25 While rabbit fXI is a noncovalently associated dimer, this is not apparent under the denaturing conditions of SDS-gel electrophoresis. Horse fXI also has a substantial fraction of protein without the interchain bond. As the horse cDNA codes for Cys321, formation of the interchain bond is likely incomplete. The pattern for the African elephant is unexplained, but was noted in 3 unrelated members of the species. FXI is a relatively recent addition to the coagulation mechanism, making its appearance during mammalian evolution.26 The negative result with chicken plasma is expected, as birds do not have a fXI gene.26
14E11 immunoprecipitates of fXI from mammalian plasmas. Western blots of nonreducing 7.5% polyacrylamide gels of fXI from mammal plasmas immunoprecipitated with 14E11 linked to agarose beads. The primary detection antibody was biotinylated-14E11. Positions of molecular mass standards are shown on the left.
14E11 immunoprecipitates of fXI from mammalian plasmas. Western blots of nonreducing 7.5% polyacrylamide gels of fXI from mammal plasmas immunoprecipitated with 14E11 linked to agarose beads. The primary detection antibody was biotinylated-14E11. Positions of molecular mass standards are shown on the left.
Mouse arterial thrombosis models
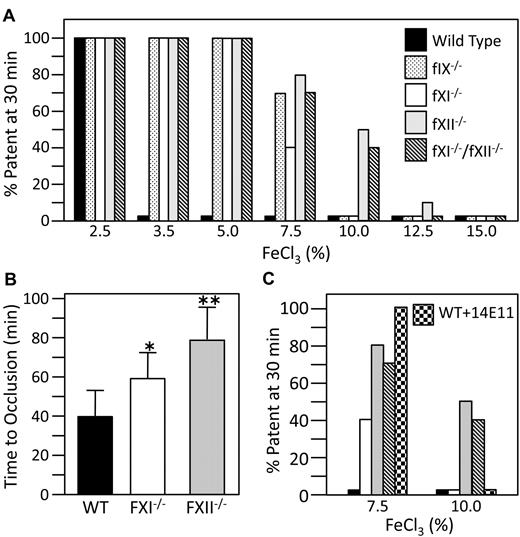
Application of FeCl3 to the exterior of blood vessels causes severe endothelial damage and occlusion by platelet-rich thrombi.27 fXII- and fXI-deficient mice are resistant to mesenteric artery occlusion induced by a 20% FeCl3 solution.9 We studied carotid artery occlusion in fIX-, fXI-, or fXII-deficient mice using graduated FeCl3 concentrations (Figure 5A).10 In our hands, the artery typically occludes in 5-15 minutes or not at all. Because our mouse lines were maintained for many years, they were rebackcrossed through 6 generations to C57/Bl6 mice from a single vendor to compensate for genetic drift. Vessel occlusion occurs consistently in wild-type mice treated with 3.5% FeCl3, but not 2.5% FeCl3. Mice lacking fIX, fXI, or fXII were resistant to occlusion at 5% FeCl3, with some mice occluding at 7.5% FeCl3. fIX and fXI null mice all occluded at 10% FeCl3, but half of fXII-null mice tested at 10% FeCl3, and one at 12.5% FeCl3 did not. fXII- and fXI-deficient mice were tested in a model where carotid artery thrombosis is induced with a laser after infusion of Rose Bengal (Figure 5B).19 In this model, there is probably less endothelial damage and collagen exposure than with FeCl3.27 Mean time to vessel occlusion in wild type mice (40 ± 13 minutes, ± 1 standard deviation, n = 16) was significantly shorter than for fXI-deficient mice (59 ± 13 minutes, n = 12,P = .0012) or fXII-deficient mice (79 ± 17 minutes, n = 15 P = .0001). Time to occlusion was also significantly longer for fXII-deficient mice than for fXI-deficient mice (P = .0031)
Mouse arterial thrombosis models. (A) C57Bl/6 mice of the genotypes indicated at the right of the panel were tested in a carotid artery thrombosis model at FeCl3 concentrations from 2.5% to 15% (10 animals per concentration), as described in “Methods.” Bar heights indicate percent of mice in each group with patent arteries 30 minutes after applying FeCl3. (B) Time to carotid arterial occlusion in wild-type (n = 16), fXI-deficient (n = 12), or fXII-deficient (n = 15) mice in a Rose Bengal-laser injury model, as described in “Methods.” Error bars indicate 1 standard deviation. Time to occlusion is significantly longer for fXI−/− than wild-type mice (*P = .0012), for fXII−/− than wild-type mice (**P = .0001) and for fXII−/− than fXI−/− mice (**P = .0031). (C) Results from the FeCl3 carotid artery thrombosis model (panel A), comparing the effect of treatment with an intravenous infusion of 14E11 (1.0 mg/kg) into wild-type mice ( ) to results for 7.5% and 10% FeCl3 from panel A.
) to results for 7.5% and 10% FeCl3 from panel A.
Mouse arterial thrombosis models. (A) C57Bl/6 mice of the genotypes indicated at the right of the panel were tested in a carotid artery thrombosis model at FeCl3 concentrations from 2.5% to 15% (10 animals per concentration), as described in “Methods.” Bar heights indicate percent of mice in each group with patent arteries 30 minutes after applying FeCl3. (B) Time to carotid arterial occlusion in wild-type (n = 16), fXI-deficient (n = 12), or fXII-deficient (n = 15) mice in a Rose Bengal-laser injury model, as described in “Methods.” Error bars indicate 1 standard deviation. Time to occlusion is significantly longer for fXI−/− than wild-type mice (*P = .0012), for fXII−/− than wild-type mice (**P = .0001) and for fXII−/− than fXI−/− mice (**P = .0031). (C) Results from the FeCl3 carotid artery thrombosis model (panel A), comparing the effect of treatment with an intravenous infusion of 14E11 (1.0 mg/kg) into wild-type mice ( ) to results for 7.5% and 10% FeCl3 from panel A.
) to results for 7.5% and 10% FeCl3 from panel A.
The results are consistent with several possible mechanisms. fXIIa may contribute to thrombus formation through fXI activation. However, given the different degrees of response of fXI- and fXII-deficient mice to vessel injury, the proteins could contribute through distinct pathways. Alternatively, fXIIa may work through both fXI-dependent and -independent processes. We tested mice with combined fXI- and fXII-deficiency in the FeCl3 model to look for an additive effect indicating the proteins operate in distinct pathways. Results for these animals were similar to those for fXII-deficient mice (Figure 4A), consistent with fXIIa activating fXI. As 14E11 selectively interferes with fXIIa-mediated fXI activation in vitro, we tested it in the FeCl3 model. Intravenous infusion of 1.0 mg/kg 14E11 into wild-type mice prolonged the aPTT to a similar degree as adding 14E11 directly to plasma. The same effect was obtained 5 hours after intraperitoneal administration. Western blots of plasma obtained at intervals after 14E11 infusion indicate the antibody does not clear fXI protein from plasma (supplemental Figure 2). In the FeCl3 model, 1.0 mg/kg 14E11 protected mice from carotid occlusion induced by 7.5% FeCl3, but not 10% FeCl3 (Figure 5C), with the antithrombotic effect lasting approximately 48 hours. Smaller doses of 14E11 were less effective at preventing thrombosis (supplemental Figure 3). Cumulatively, the results are consistent with the premise that fXI is activated by fXIIa in the thrombosis models. However, it seems likely that fXIIa is influencing thrombus formation partly by a fXI-independent mechanisms.
Pulmonary embolism model
We predicted that thrombosis triggered by a high concentration of TF would be relatively unaffected by fXI or fXII deficiency and, therefore, unresponsive to 14E11. C57Bl/6 mice were studied in a model of lethal pulmonary embolism. Wild-type mice typically developed respiratory arrest in 100-300 seconds (mean 195 ± 97 seconds) after infusion of rabbit TF into the inferior vena cava (Figure 6).20,28 As expected, most fXI-deficient mice succumbed to TF infusion; however, time to respiratory arrest (392 ± 273 seconds) was significantly longer than for wild-type mice (P = .0065), and a similar trend was seen in fXII-null mice (462 ± 318 seconds, P = .0024). Infusion of 14E11 before TF infusion also significantly prolonged survival (430 ± 285 seconds, P = .0027) compared with untreated wild-type animals. Comparable results were obtained with human TF (data not shown).
Mouse pulmonary embolism model. C57Bl/6 mice were given a single 100-μL bolus of rabbit brain tissue factor (∼ 1nM solution), and time to cessation of respiration was measured. The mice tested are wild-type (WT: ●), fXI-deficient (fXI−/−: ■), fXII-deficient (fXII−/−: ▴) and WT treated with 1.0 mg/kg 14E11 (▾). Each symbol represents one animal, and bars within the column of symbols indicate the mean occlusion time. The box plots indicate the 25th, 50th, and 75th percentile for each group, with the whiskers showing the 10th and 90th percentile.
Mouse pulmonary embolism model. C57Bl/6 mice were given a single 100-μL bolus of rabbit brain tissue factor (∼ 1nM solution), and time to cessation of respiration was measured. The mice tested are wild-type (WT: ●), fXI-deficient (fXI−/−: ■), fXII-deficient (fXII−/−: ▴) and WT treated with 1.0 mg/kg 14E11 (▾). Each symbol represents one animal, and bars within the column of symbols indicate the mean occlusion time. The box plots indicate the 25th, 50th, and 75th percentile for each group, with the whiskers showing the 10th and 90th percentile.
14E11 in a primate thrombosis model
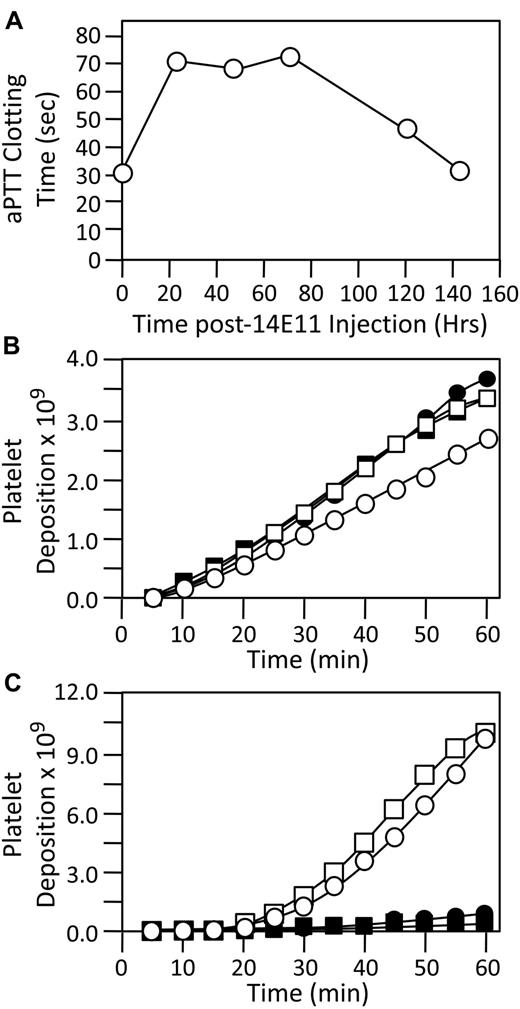
Previously, we described the effects of anti-fXI antibodies on thrombus formation in collagen-coated Gore-Tex grafts inserted into arteriovenous shunts in baboons.12,21 Antibody O1A6, which blocks fIX activation by fXIa, had a limited effect on platelet binding to the graft, but caused a major defect in growth of platelet-rich thrombi.12 14E11 was tested in this model in a male baboon. Twenty-four hours after a subcutaneous injection of 14E11 (1 mg/kg), the aPTT increased from 31.4 seconds to 71.9 seconds and remained above baseline for 4 days (Figure 7A). When collagen-coated grafts were introduced into the shunt before 14E11 treatment, 111In-labeled platelets bound to the grafts (Figure 7B), with robust downstream formation of platelet-rich thrombi (Figure 7C). 14E11 did not affect platelet deposition on the graft surface (Figure 7B), similar to prior results with heparin or aspirin.21 However, there was almost complete absence of downstream thrombus growth (Figure 7C). Fibrin deposition within the grafts was reduced approximately 40% after 14E11 treatment compared with pretreatment controls (average 1.15 mg and 1.74 mg, respectively).
Baboon AV shunt thrombosis model. (A) Shown are aPTT clotting times (see “Methods”) of plasma from a male baboon treated with a single subcutaneous injection of 14E11 (1 mg/kg) at time zero. (B and C) Platelet deposition over time for 4 separate collagen coated grafts, 2 inserted into the shunt before treatment with 14E11 (○, □) and 2 inserted 24 hours after a 1-mg/kg subcutaneous dose of 14E11 (●, ■). Deposition of 111I-labeled platelets was measured with a gamma scintillation camera, as described in “Methods.” Panel B shows accumulation of platelets within the graft, while panel C shows the bulk of platelet accumulation in a thrombus forming in the 10 cm immediately downstream of the graft.
Baboon AV shunt thrombosis model. (A) Shown are aPTT clotting times (see “Methods”) of plasma from a male baboon treated with a single subcutaneous injection of 14E11 (1 mg/kg) at time zero. (B and C) Platelet deposition over time for 4 separate collagen coated grafts, 2 inserted into the shunt before treatment with 14E11 (○, □) and 2 inserted 24 hours after a 1-mg/kg subcutaneous dose of 14E11 (●, ■). Deposition of 111I-labeled platelets was measured with a gamma scintillation camera, as described in “Methods.” Panel B shows accumulation of platelets within the graft, while panel C shows the bulk of platelet accumulation in a thrombus forming in the 10 cm immediately downstream of the graft.
Discussion
Patients with severe fXI deficiency are predisposed to excessive bleeding after trauma to sites such as the oropharynx or urinary tract.2,4 FXII deficiency does not compromise hemostasis.1,2 Taking these established observations into account, current models of thrombin generation often omit fXII, with fXI activation mediated by another protease such as thrombin.3,6,29 These hypotheses are being reconsidered, at least as they pertain to pathologic coagulation, in light of the finding that fXII-deficient mice are resistant to thrombosis.9 fXI-deficient mice are also resistant to thrombosis,9,10 consistent with the idea that thrombin generation driven by fXIIa activation of fXI supports occlusive thrombus growth. However, it has not been established that fXI is the major (or sole) target of fXIIa, nor is it clear that the mouse data are relevant to thrombus formation in humans. The antibody 14E11 provides us with a tool to begin to address these questions, which have major implications for development of therapeutic inhibitors that target the intrinsic pathway.
fIX-, fXI-, and fXII-deficient mice have not previously been compared in a single study. In our hands, fXII-deficient mice are relatively more resistant to arterial occlusion than fIX- or fXI-deficient animals, suggesting at the very least that fXII also makes a fXI-independent contribution to thrombosis. It is even conceivable that fXII and fXI operate in distinct pathways. As 14E11 selectively targets the fXIIa-mediated fXI activation in vitro, we tested it in the FeCl3 model. In wild-type mice, 14E11 had a comparable effect to fXI deficiency, without clearing fXI from the plasma, supporting the hypothesis that thrombus formation in this model requires fXI activation by fXIIa.
14E11 has a partial inhibitory effect on fXI activation by fXIIa in a purified protein system.6 Inhibition of this reaction is more robust in plasma, particularly when coagulation is triggered through contact activation by addition of a surface. The proposed binding site on fXI for fXIIa is on the A4 domain,30 at a considerable distance from the 14E11 binding site on A2. FXI forms a complex with HK in plasma, and 14E11 inhibits this interaction. The 4 fXI apple domains form a disk-like structure,31 and HK is predicted to bind along a channel on the surface of A2 that extends between A1 and A4.14,31,32 HK-deficient mice have delayed time to thrombus formation in the Rose Bengal-laser injury model,33 consistent with the premise that interfering with the fXI-HK interaction could affect thrombus formation in vivo. Considering the available data, 14E11 appears to have a complex effect on contact activation-initiated coagulation, directly interfering with fXIIa activation of fXI, and the fXI-HK interaction required for binding fXI to the contact surface. It must be considered that 14E11 could interfere with a fXI function in vivo not required in the aPTT or thrombin generation assay, such as fXI binding to platelets.34 FXI binds to 2 platelet receptors: glycoprotein Ib (GPIb)35 and ApoER2.16 Previously, we showed that blocking ApoER2 prevents platelet binding to fXI;16 however, 14E11 does not interfere with this interaction in vitro (supplemental Figure 4).
While the data for 14E11 in mice support the premise that fXIIa activates fXI, the resistance of fXII-deficient mice to thrombosis is not completely explained by one pathway. The consistent observation that the aPTT of fXII-deficient plasma is longer than that of fXI-deficient plasma suggests there are prothrombotic targets for fXIIa other than fXI, although they have not been defined in vivo. For example, fXIIa can activate factor VII, priming the extrinsic pathway of coagulation.36,37 Platelets have been shown to support fXII activation38,39 ; however, recent work raises the intriguing possibility that fXIIa activates platelets. Four protease-activated receptors (PARs) have been described that are cleaved by thrombin or other proteases. Mouse platelets primarily express PAR3 and PAR4. Thrombin cleaved PAR3 functions as a cofactor, supporting PAR4 cleavage and subsequent platelet activation.40 Mao et al41 recently reported that fXIIa cleaves PAR3, activating PAR4-null murine platelets. As PAR3-null mice are resistant to FeCl3- or TF-induced thrombosis,20 fXIIa cleavage of PAR3 may explain the differences we observed between fXII- and fXI-deficient mice in arterial thrombosis models. If this is the case, it is possible that fXII makes a greater contribution to thrombosis in mouse models than in humans, as human platelets primarily express PARs 1 and 4, with PAR3 serving a lesser role.40
Indeed, in contrast to fXI, a direct relationship between fXII and thrombosis in humans is not established. High plasma fXI levels are associated with increased risks for arterial42 and venous thrombosis43 , and incidences of stroke44 and deep venous thrombosis45 are reduced in severe fXI deficiency. Inhibition of fIX activation by fXIa produces an antithrombotic effect in several species,46 including primates,12,21 supporting an important role for fXIa in thrombus formation in humans. Paradoxically, a link has been suggested between fXII deficiency and thrombosis dating back to the death of the first described fXII-deficient patient from a pulmonary embolism.47 Recently, Doggen et al42 described an inverse relationship between fXII levels and risk of myocardial infarction, bringing the issue back to the fore. A large study by Endler et al48 also showed mortality from cardiovascular disease generally increases as fXII levels decrease. Bradford et al49 noted that fXII inhibits thrombin-induced platelet aggregation by interfering with thrombin binding to GP1b. These data do not make a strong case for fXII contributing to thrombosis, and raise the possibility that therapeutic intervention directed at fXII/XIIa could actually increase thrombotic risk. The affinity of 14E11 for baboon fXI provided us with an opportunity to test the hypothesis that fXIIa-mediated activation of fXI contributes to thrombus formation in primates.
The pan-specific nature of 14E11 is surely a consequence of raising antibodies in fXI-deficient mice, producing clones that recognize conserved epitopes that are not antigenic in wild-type animals. 14E11 should prove useful in thrombosis models in a variety of mammalian species. Previously, we showed that blocking fIX activation by fXIa, a reaction required for hemostasis in humans, reduced platelet accumulation in a baboon thrombosis model.12 14E11 had a similar impressive effect in this model, indicating a role for fXIIa in thrombosis in primates. While these data support targeting fXI activation as an antithrombotic strategy, they do not directly address the concerns raised regarding the safety of directly inhibiting fXII/fXIIa as a means to this end. The study by Endler et al48 contained a subset of patients with severe fXII deficiency (< 10% of normal plasma level). Surprisingly, the risk of cardiovascular death in this group was similar to that for the population mean, and not a continuation of the trend of increased mortality with lower fXII levels observed within the normal fXII range. This suggests severe fXII deficiency (and perhaps the state produced by a potent fXII/XIIa inhibitor) is distinctly different from a low fXII level within the normal range in humans. Development of specific fXIIa inhibitors will be important for definitively establishing the contributions of fXII and fXI to thrombosis in nonhuman primates (and ultimately in humans) and the relative safety of fXIIa inhibition.
Finally, we tested 14E11 in a TF-induced pulmonary embolism model, with the expectation that it would have a little effect. We reasoned that a bolus of TF large enough to produce respiratory arrest in under 5 minutes would induce substantial fIX activation through factor VIIa/TF, overwhelming contributions from fXIa. Interestingly, fXI and fXII deficiency, as well as 14E11, had a modest beneficial effect. Previously, we showed that XI inhibition reduces propagation of thrombi initiated by TF-coated surfaces in baboons.21 More recently, Dow et al28 described a locus on chromosome 11 in Cast/EiJ mice that confers resistance to the lethal effects of TF infusion. Congenic C57Bl/6 mice carrying Cast/EiJ chromosome 11 were significantly more resistant to TF infusion than C57Bl/6 controls. The single plasma abnormality identified in the congenic strain was reduced fXI activity.28 These results raise the possibility that feedback activation of fXI and fXII after initiation of coagulation by factor VIIa/TF, or contact activation on the clot surface itself, contribute to thrombus growth even in situations where TF is a primary driver of thrombosis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr. Joost Meijers (Academic University, Amsterdam, The Netherlands) for fXI apple domain-tPA fusion constructs, Dr. Darrell Stafford (University of North Carolina, Chapel Hill, NC) for fIX-deficient mice, and Dr. Sally Nofs (Nashville Zoo at Grassmere, Nashville, TN) for mammal plasma samples. We are also grateful to Drs. Douglas Tollefsen and Li He (Washington University, St. Louis, MO) for consultation on the Rose Bengal-laser injury thrombosis model.
This work was supported by awards HL81326 and HL58837 from the National Heart, Lung, and Blood Institute (D.G.), and 09GRNT2150003 and 0910025G from the American Heart Association (O.J.T.M.).
National Institutes of Health
Authorship
Contribution: Q.C. conducted FeCl3, TF-pulmonary embolism, Rose Bengal-laser injury studies, and analyzed data; E.I.T. developed 14E11 and tested it in baboons and coagulation assays; M.S.P. performed FeCl3 studies, fXI immunoprecipitation, and Western blot analyses; I.S. performed FeCl3 and TF pulmonary embolism studies; A.M. performed thrombin generation studies; M-f.S. prepared mouse fXI and performed HK binding and fIX activation studies; T.C.W.-A. performed platelet adhesion assays; S.A.S. provided veterinary consultation, designed experiments, and prepared mammal plasma; S.R.H. assisted and consulted on 14E11 studies in baboons; O.J.T.M. characterized 14E11 properties in platelet-based systems; T.R. developed fXII-deficient mice, analyzed data, and assisted in manuscript writing; A.G. developed the 14E11 antibody, performed baboon studies, analyzed data, and assisted in manuscript writing; and D.G. analyzed data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Gailani, M.D., Division of Hematology/Oncology, Vanderbilt University, 777 Preston Research Bldg, 2220 Pierce Ave, Nashville, TN 37232-6305; e-mail: dave.gailani@vanderbilt.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal