Abstract
The transcription factor NF-E2 is overexpressed in the majority of patients with polycythemia vera (PV). Concomitantly, 95% of these patients carry the JAK2V617F mutation. Although NF-E2 levels correlate with JAK2V671F allele burden in some PV cohorts, the molecular mechanism causing aberrant NF-E2 expression has not been described. Here we show that NF-E2 expression is also increased in patients with essential thrombocythemia and primary myelofibrosis independent of the presence of the JAK2V617F mutation. Characterization of the NF-E2 promoter revealed multiple functional binding sites for AML1/RUNX-1. Chromatin immunoprecipitation demonstrated AML1 binding to the NF-E2 promoter in vivo. Moreover, AML1 binding to the NF-E2 promoter was significantly increased in granulocytes from PV patients compared with healthy controls. AML1 mRNA expression was elevated in patients with PV, essential thrombocythemia, and primary myelofibrosis both in the presence and absence of JAK2V617F. In addition, AML1 and NF-E2 expression were highly correlated. RNAi-mediated suppression of either AML1 or of its binding partner CBF-β significantly decreased NF-E2 expression. Moreover, expression of the leukemic fusion protein AML/ETO drastically decreased NF-E2 protein levels. Our data identify NF-E2 as a novel AML1 target gene and delineate a role for aberrant AML1 expression in mediating elevated NF-E2 expression in MPN patients.
Introduction
The molecular etiology of myeloproliferative neoplasms (MPNs) remains incompletely understood, despite recent advances incurred through the discovery of a point mutation in the JAK2 kinase (JAK2V617F) in a large proportion of patients.1-3 Several lines of evidence support the hypothesis that additional aberrations, either preceding or following acquisition of the JAK2V617F mutation, contribute to the pathophysiology of these disorders.4-7
Nuclear factor erythroid-2 (NF-E2), a hematopoietic transcription factor, is overexpressed in a large majority of patients with polycythemia vera (PV).8 NF-E2 is essential for platelet formation as knockout mice die perinatally of hemorrhage caused by thrombocytopenia.9 In addition, loss of NF-E2 also affects the erythroid lineage since surviving adult mice display mild anemia with compensatory reticulocytosis. We have recently shown that NF-E2 overexpression delays the early phase of erythroid maturation, resulting in an expansion of erythroid progenitors, thereby increasing the number of erythrocytes derived from one CD34+ cell.10 These data propose a role for NF-E2 in mediating the dysregulated erythrocytosis of PV.
In PV patients, NF-E2 expression levels were found to correlate with the JAK2V617F allele burden in some studies but not in others.11,12 Hence, it is not clear whether NF-E2 is a direct target of the aberrantly activated JAK2 kinase.
The NF-E2 gene is transcribed from 2 alternative promoters, termed 1A (“adult”) and 1F (“fetal”), which give rise to distinct mRNAs, encoding alternative first exons but containing identical open reading frames.13 Transcripts from the 1A promoter are detected both in myeloid and erythroid cells, whereas the 1F promoter appears to be predominantly erythroid.14,15 Consistently, the 1F promoter contains functional GATA-1 and SCL/Tal binding sites.14,16 By contrast, regulation of the NF-E2 1A promoter is not well studied. Because the molecular mechanism underlying NF-E2 overexpression in PV patients remains unclear, we sought to investigate the regulation of NF-E2 gene expression and determine its relationship to the presence or absence of the JAK2V617F mutation.
Methods
In silico analysis of the NF-E2 gene
Sequence of the human NF-E2 genomic locus (Ensembl:ENSG00000123405) was aligned and compared across species by use of the GenomeVista software (http://pipeline.lbl.gov/cgi-bin/GenomeVista).17 Potential transcription factor binding sites were predicted by use of the ChIP mapper search engine (http://mapper.chip.org).18
MPN patients and healthy controls
Peripheral blood samples were obtained from therapeutic phlebotomies of PV patients or from blood samples of ET patients, both fulfilling the World Health Organization criteria for diagnosis.19 Additional samples were obtained from the Tissue Bank of the Myeloproliferative Disease Research Consortium (MPD-RC), which uses the same diagnostic criteria. Patient characteristics are summarized in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Buffy coats of healthy volunteer blood donors were obtained from the University Hospital Freiburg Center for Blood Transfusion. The study protocol was approved by the local ethics committees (University Hospital Freiburg as well as the Member Institutions of the MPD-RC), and informed consent was obtained from all patients in accordance with the Declaration of Helsinki. Each patient was assigned a unique patient number, which was used thereafter for the protection of privacy. All patients were tested for the presence of the JAK2V617F mutation by quantitative reverse transcription polymerase chain reaction (qRT-PCR) as previously described.20
Granulocyte isolation
Peripheral blood granulocytes from PV patients and healthy controls were isolated by dextran sedimentation and Ficoll gradient centrifugation as previously described.21
Northern blot analysis
RNA was isolated by use of the Trizol reagent (Invitrogen) and assayed by Northern blot by the use of exon1A and 1F specific cDNA probes. The probes were generated by PCR amplification of genomic DNA by use of the following primers:
1A sense: 5′-TGGAGGAAGGACTGAGAACTCAGG-3′
1A anti-sense: 5′-GTTGTCTCTGGG GAGGCTGAG-3′
1F sense: 5′-GGGATCCTCAAGCATATACTGTC-3′,
1F anti-sense: 5′-CCAGGCAAAATGCCCACTCTT-3′.
A human GAPDH cDNA fragment was used to control for RNA loading.
Plasmid constructs
The −6.5-kb NF-E2 1A promoter construct was a kind gift of Dr Etsuro Ito.22 The −3606-, −1740-, −439-, and −167-bp promoter constructs were generated by restriction enzyme digestion or PCR amplification and cloned into pGL3 (Promega). Mutations of the AML1 binding site constructs were performed by the use of GeneTailor site-directed mutagenesis system (Invitrogen). AML1 binding sites I (TGCGGT), II (TGTGGC), and III (CGTGGT) were mutated to TGGCCT, TGACCC, and CGACCT, respectively.
Cell culture and transient transfections/nucleofections
Human erythroleukemia (HEL) and K562 cells were maintained in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS), l-glutamine, and penicillin/streptomycin (all from Invitrogen). UKE-1 cells were cultured in Iscove modified Dulbecco medium (Invitrogen) supplemented with 10% FBS, 10% horse serum (Biological Industries), 1μM hydrocortisone (Sigma-Aldrich), and penicillin/streptomycin. The human fibrosarcoma cell line γ-2A was a generous gift from Drs Stefan Constantinescou and Jean-Luc Villeval. The γ-2A cells were used because they do not express either JAK2 mRNA or protein and allow an analysis of transcriptional regulation without interference of JAK2 effects.23 The γ-2A cells were maintained in Dulbecco modified Eagle medium (Invitrogen) supplemented with 10% FBS, 400 μg/mL G418 (Sigma-Aldrich), and penicillin/streptomycin.
UKE-1 cells were a generous gift of Prof Dr W. Fiedler, Universitätsklinikum Hamburg-Eppendorf. K562 and UKE-1 were nucleofected by the use of a Nucleofector II device and the “Cell Line Nucleofector Solution V Kit” (Amaxa), according to manufacturer's recommendations. A total of 2 × 106 cells were conucleofected with 2.5 μg of various NF-E2-promoter-Luciferase reporter gene constructs or the pGL3 empty control vector as well as 0.5 μg of a TK-Renilla internal control plasmid (Promega) with the programs T-016 (K562) or X-013 (UKE-1), respectively.
γ-2A cells were transiently transfected with the “Superfect” reagent (QIAGEN). A total of 105 cells were cotransfected with 0.7 μg of various NF-E2-promoter–Luciferase reporter gene constructs or the pGL3 empty control vector, as well as 0.5 μg of either a AML1-pCMV6-XL4 expression vector (Origene) or an empty pCMV6-XL4 control vector in addition to 0.3 μg of TK-Renilla internal control vector. Cells were harvested 16 to 24 hours after transfection, and luciferase activity was determined using Dual Luciferase Reporter Assay System (Promega). Luciferase activity was normalized to the Renilla internal control to compensate for variations in transfection efficiency. Erythroid precursor cells from MPN patients and healthy controls were expanded from purified CD34+ cells as previously described.24
RNA interference
A total of 3 × 106 UKE-1 cells were nucleofected, as described in the previous section with 4μM siRNA against AML1 (Eurogentec), CBF-β (Eurogentec), JAK2 (#609; Ambion), or Negative Control #1 and #2 (AM4611, AM4613; Ambion) using the program X-003. Cells were harvested 72 to 96 hours after transfection. siRNA oligonucleotide sequences were as follows:
AML1 siRNA-sense: 5′-GAACCAGGUUGCAAGAUUUdTdT-3′
Antisense: 5′-AAAUCUUGCAACCUGGUUCdTdT-3′
CBF-β siRNA sense: 5′-GGACACGCGAAUUUGAAGAdTdT-3′
Antisense: 5′-UCUUCAAAUUCGCGUGUCCdTdT-3′
Immunoblotting
UKE-1 cells were lysed in RIPA buffer (50mM Tris, pH 8.0; 150mM NaCl; 1% NP-40; 0.5% sodiumdeoxychotate; and 0.1% sodium dodecyl sulfate [SDS]) supplemented with 2× Complete (Roche). The lysate was sonicated with an ultrasonic probe (Bandelin) and centrifuged, and the supernatant subsequently used for protein reduction and alkylation. Proteins were reduced and alkylated by boiling in 1× SDS sample loading buffer (250mM Tris, pH 6.8; 10% SDS; 0.5% bromophenylblue; and 50% glycerol) supplemented with 50mM dithio-dl-threitol (Sigma-Aldrich) for 7 minutes at 95°C. After cooling to room temperature, the samples were incubated for 2 minutes in the dark with the addition of 120mM iodoacetamide (Sigma-Aldrich). To quench excessive iodoacetamide, 20mM dithio-dl-threitol was added, followed by another 20-minute incubation in the dark.
UKE-1 cell lysates (80 μg) were subjected to SDS–polyacrylamide gel electrophoresis and Western blotting. Primary polyclonal antibodies against AML1 (39000; Active Motif), CBF-β (ab33516; Abcam), and NF-E28 were used. The blots were stripped and reprobed against β-Actin (A5441; Sigma-Aldrich) to control for equal loading. The immunocomplexes were detected with the use of chemiluminescence Western Blotting Reagents (GE Healthcare). Densitometric analyses were performed on a Photoscanner (Epson) with the use of the ImageJ software (National Institutes of Health). Results are depicted as percentages of control cells.
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) assays were performed following the protocol previously described by Shang et al.25 In brief, HEL cells or granulocytes from PV patients or healthy controls were treated with formaldehyde (1%) for 10 minutes at room temperature. Subsequently, cells were washed twice with cold phosphate-buffered saline and the cross-linked chromatin sonicated to obtain DNA fragments averaging in size from 500 to 1000 bp. Immnoprecipitations were performed with anti-AML1 antibody (Merck Bioscience) or an anti–normal immunoglobulin G (IgG; Diagenode) as a control. Two primer sets, spanning bp −3466 to −3192 and bp −3076 to −2752, respectively, of the NF-E2 1A promoter (sense 5′-CGTCTGTTGAGAGAGGAAGC-3′, anti-sense 5′-ACCCACTCCCTAAAAGATTCAC-3′ and sense 5′-GTGGCCGT GGTTTTCT-3′, anti-sense 5′-ATCTCACCCTCACTCCTCTC-3′), thereby covering the 3 predicted AML1 binding sites, were used in PCRs to compare the abundance of precipitated chromatin/DNA. As a control primers spanning bp −4589 to −4317 of the NF-E2 1A promoter, a segment not predicted to contain AML1 binding sites, were used (sense 5′-CGCGCCCGGCCTATTTTGT-3′, anti-sense 5′-TTTGGGAGGCTGAGGAAGGAGA-3). Primers spanning bp −332 to −114 of the human GAPDH promoter, which does not contain AML1 binding sites, were used as an additional control (sense 5′-GAAGGTGAAGGTCGGACTC-3′ and antisense 5′-GAAGATGGTGATGGGATTTC-3′).26
Quantitative RT-PCR assays
Quantitative RT-PCR experiments were performed using the following Assay on Demand (Applied Biosystems) products for gene expression analysis: Human NF-E2 Assay on demand (Hs00232351_m1); Human AML1 Assay on demand (Hs00231079_m1); Human 18S Pre-Developed TaqMan Assay Reagents (4310893E); and Human β-2-Microglobulin Assay on demand (Hs00187842_m1).
The qPCR assays for the NF-E2 1A and 1F promoters were designed as follows for NF-E2 exon 1A, forward primer: 5′-CTCTCCTCACCCTGCTGTGA-3′ (Exon1A), reverse primer: 5′-GACATCCTACTGGGCCAGAGTC-3′ (Exon2); and probe: 5′-ACCACAGGTTTCTAGAGCC-3′; and for NF-E2 Exon 1F, forward primer: 5′-CTTTAGCCAGGAAAACAGTTTGG-3′ (Exon1F); reverse primer: 5′-GACATCCTACTGGGCCAGAGTC-3′ (Exon2); and probe: 5′-AAAGGTTTCTAGAGCCATC-3′.
Reverse transcription of 50 ng of total granulocyte RNA was performed by use of the TaqMan Reverse Transcription Kit (Applied Biosystems). Q-PCR assays were performed in duplicate in an ABI PRISM 7000 Cycler. A plasmid standard curve containing defined copy numbers was included in each experiment. With the use of this reference, gene expression was determined and is reported either in copy number per 1 000 000 copies of 18SrRNA or per 1000 copies of β-2-microglobulin mRNA.
Electrophoretic mobility shift assay analysis
HEL nuclear extracts were prepared as previously described.27 The AML1 electrophoretic mobility shift assays (EMSAs) were performed following the method previously described by Libermann et al.28 In brief, 1 to 5 μg of nuclear extracts were added to a binding reaction containing 2 μg of poly dI-dC, 2 μL of 10× binding buffer (10mM HEPES, pH 7.9; 5mM MgCl2; 30mM KCl; 1mM EDTA; 1mM dithiothreitol; and 12% glycerol), and 0.5 ng of 32P-labeled oligonucleotide. The AML1 consensus binding site oligonucleotide was purchased from Active Motif (cat. no. 37 300). All other oligonucleotides were purchased from Apara. When indicated, either a 100-fold excess of nonradioactive oligonucleotide or a AML1 antibody (Cell Signaling Technology) or NF-κB p65 antibody (Santa Cruz Biotechnology) were added. The reaction was incubated at 4°C for 15 minutes. For supershift assays, extracts and antibodies were preincubated for 15 minutes at 4°C before addition of the radioactive nucleotide. AML1 consensus wild-type and mutant oligonucleotides were obtained from Active Motif, and the NF-κB consensus oligonucleotide was purchased from Promega.
Retroviral transductions
The AML/ETO cDNA, a kind gift of Dr T. Berg and Prof Dr M. Luebbert (Department of Hematology-Oncology, University Hospital Freiburg), was cloned into the pSF91 retroviral vector. Retroviral pseudotypes were prepared as previously described by use of the RD114 envelope protein.10 UKE-1 and K562 cells were infected by 2 cycles of retroviral transduction performed during a 48-hour period by use of a multiplicity of infection between 3 and 11. After transduction, green fluorescent protein–positive cells were sorted by fluorescent-activated cell sorting.
Data analysis
The Student t test (2-sided or 1-sided) and the Mann-Whitney rank-sum test were used to determine whether a significant (P < .05) difference existed between 2 groups. To compare more than 2 groups, a Kruskal-Wallis one-way analysis of variance (ANOVA) on Ranks or a one-way ANOVA was used. These analyses were performed with the SigmaPlot 11.0 software (Systat). Correlation analyses were performed by use of the SigmaStat 3.1 software (Systat).
Results
NF-E2 overexpression in PV patients is mediated by both the 1A and the 1F promoters
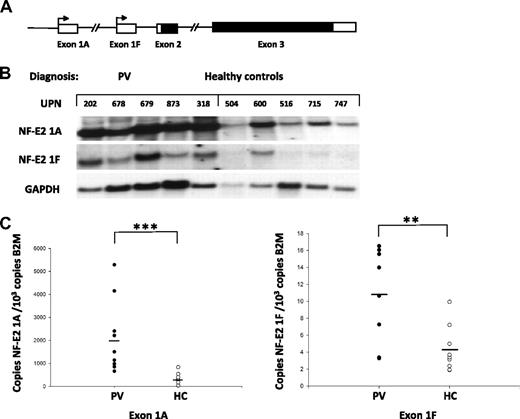
The NF-E2 mRNA is transcribed from 2 alternative promoters, NF-E2 1A and NF-E2 1F (Figure 1A).13,22 We therefore determined which promoter contributes to the observed NF-E2 overexpression in PV patients. The 2 promoters give rise to mRNA transcripts containing alternative first exons, thereby allowing the use of exon-specific probes to discriminate the transcripts. Two cDNA probes were generated, one encoding exon 1A, the other encoding exon 1F. A Northern blot containing RNA from purified granulocytes of 5 PV patients and 5 healthy controls was interrogated with the exon-specific probes (Figure 1B). These results indicate that NF-E2 overexpression is affected by both promoters, albeit to different extents.
Relative abundance of the alternatively transcribed NF-E2 mRNA isoforms 1A and 1F in PV patients and healthy controls. (A) Schematic diagram of the human NF-E2 genomic locus. Two alterative promoters, 1A and 1F, are present, transcribing noncoding exons 1A or 1F, respectively. Both NF-E2 mRNA isoforms share exons 2 and 3, which contain the ORF (indicated in black). (B) Northern blot analysis of exon 1A– or exon 1F–containing NF-E2 mRNA expression in PV patients and healthy controls. Total RNA from purified peripheral blood granulocytes was probed with cDNA fragments specific for Exon 1A (top) or 1F (middle), respectively. A cDNA fragment of the GAPDH gene was used a RNA loading control (bottom). (C) Quantitation of NF-E2 Exon 1A (left) and Exon 1F (right) expression in PV patients and healthy controls. RNA was isolated from purified granulocytes of 10 PV patients as well 9 healthy controls (HC) and subjected to quantitative RT-PCR analysis for NF-E2 exon 1A and exon 1F expression. A standard curve with known copy numbers of the 2 NF-E2 exons and β-2-microglobulin (B2M) was included on each plate. Sample copy numbers of each NF-E2 exon and β-2-microglobulin were determined from the standard curve and are expressed as relative ratios (copy number NF-E2 exon per 103 B2M molecules). The median is depicted by a horizontal line; **P < .01, ***P < .001 by t test.
Relative abundance of the alternatively transcribed NF-E2 mRNA isoforms 1A and 1F in PV patients and healthy controls. (A) Schematic diagram of the human NF-E2 genomic locus. Two alterative promoters, 1A and 1F, are present, transcribing noncoding exons 1A or 1F, respectively. Both NF-E2 mRNA isoforms share exons 2 and 3, which contain the ORF (indicated in black). (B) Northern blot analysis of exon 1A– or exon 1F–containing NF-E2 mRNA expression in PV patients and healthy controls. Total RNA from purified peripheral blood granulocytes was probed with cDNA fragments specific for Exon 1A (top) or 1F (middle), respectively. A cDNA fragment of the GAPDH gene was used a RNA loading control (bottom). (C) Quantitation of NF-E2 Exon 1A (left) and Exon 1F (right) expression in PV patients and healthy controls. RNA was isolated from purified granulocytes of 10 PV patients as well 9 healthy controls (HC) and subjected to quantitative RT-PCR analysis for NF-E2 exon 1A and exon 1F expression. A standard curve with known copy numbers of the 2 NF-E2 exons and β-2-microglobulin (B2M) was included on each plate. Sample copy numbers of each NF-E2 exon and β-2-microglobulin were determined from the standard curve and are expressed as relative ratios (copy number NF-E2 exon per 103 B2M molecules). The median is depicted by a horizontal line; **P < .01, ***P < .001 by t test.
To quantitate the contribution of each of the 2 transcripts to NF-E2 overexpression, we developed exon-specific quantitative RT-PCR assays. NF-E2 expression from each of the 2 promoters (1A and 1F) was quantitatied in 10 PV patients and 9 healthy controls (Figure 1C). These data revealed that both promoters contribute to NF-E2 overexpression in PV patients as expression of both mRNA transcripts is significantly increased. Because transcription of the NF-E2 1A promoter is several orders of magnitude greater than the 1F promoter (Figure 1C; note the differences in scales between the 2 panels) and the mean overexpression from the 1A promoter was 7-fold compared to the mean 2.7-fold overexpression from the 1F promoter, we investigated the molecular mechanism underlying increased NF-E2 transcription from the 1A promoter in PV patients.
AML1 binding sites in a conserved upstream element of the NF-E2 1A promoter are required for NF-E2 transcription
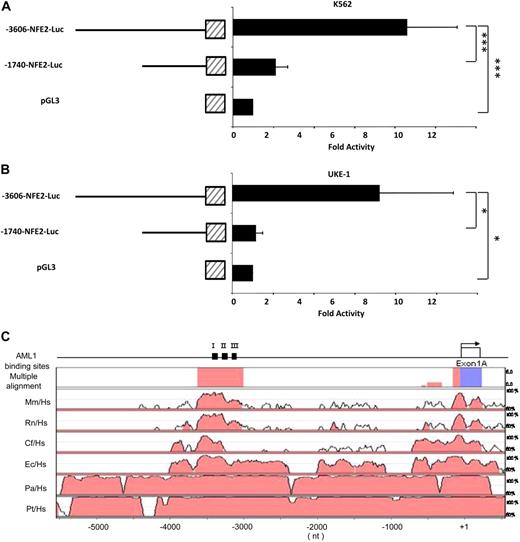
To determine the site of functional elements with the NF-E2 1A promoter, deletion analysis was conducted in 2 cell line models. Both cell lines express NF-E2, but K562 erythroleukemia cells contain wild-type JAK2, whereas UKE-1 cells, which stem from a patient with essential thrombocythemia (ET), are homozygous for the JAK2V617F mutation.29 The cells were transfected with reporter gene constructs containing either a 3.6-kb fragment of the NF-E2 1A promoter or a deletion construct retaining the proximal 1.7 kb (Figure 2A-B). In both models, reporter gene activity was drastically reduced by deletion of the segment between −3.6 kb and −1.7 kb upstream of the transcriptional start site, irrespective of the JAK2 mutational status. In silico analysis with multiple species alignment to determine phylogenetic conservation revealed a highly conserved 612-bp DNA fragment spanning bp −3561 to −2950 of the NF-E2 1A promoter (Figure 2C).
Deletion Analysis of the NF-E2 1A promoter in K562 and UKE-1 cells. A DNA fragment encoding bp −3606 to bp +1 upstream of the exon 1A transcriptional start site was cloned into the pGL3-Luciferase reporter vector. A deletion mutant encoding bp −1740 to +1 of the NF-E2 1A promoter was generated. Both constructs as well as the empty pGL 3 luciferase vector were transiently nucleofected into K562 cells (A) or UKE-1 cells (B). At 24 hours after transfection, luciferase activity was determined. Results were normalized for transfection efficiency by cotransfection of a Tk-driven Renilla luciferase reporter gene vector. Mean and standard deviation of 3 independent experiments, each measured in duplicate, are shown. ***P < .001, one-way ANOVA, *P < .05 Kruskal Wallis one-way ANOVA on Ranks. (C) Phylogenetic conservation of the NF-E2 1A promoter region. Top: schematic representation of the NF-E2 promoter, exon 1A, indicated by an open box, and the 3 predicted AML1 binding sites, indicated by black boxes and numbered I, II, and III. Top row: Multiple alignment of the NF-E2 promoter sequence across all indicated species by use of Genome Vista software. Promoter segments showing more than 75% identity are highlighted in pink, exonic sequences in cyan. Remaining rows: Pair-wise alignment of the NF-E2 promoter species across the indicated species. Hs indicates Homo sapiens; Mm, Mus musculus; Rn, Rattus norvegicus; cf, Canis familiaris; Ec, Equus caballus; Pa, Pongo pygmaeus abelii; and Pt, Pan troglodytes. Promoter segments showing more than 75% identity are highlighted in pink, exonic sequences in cyan.
Deletion Analysis of the NF-E2 1A promoter in K562 and UKE-1 cells. A DNA fragment encoding bp −3606 to bp +1 upstream of the exon 1A transcriptional start site was cloned into the pGL3-Luciferase reporter vector. A deletion mutant encoding bp −1740 to +1 of the NF-E2 1A promoter was generated. Both constructs as well as the empty pGL 3 luciferase vector were transiently nucleofected into K562 cells (A) or UKE-1 cells (B). At 24 hours after transfection, luciferase activity was determined. Results were normalized for transfection efficiency by cotransfection of a Tk-driven Renilla luciferase reporter gene vector. Mean and standard deviation of 3 independent experiments, each measured in duplicate, are shown. ***P < .001, one-way ANOVA, *P < .05 Kruskal Wallis one-way ANOVA on Ranks. (C) Phylogenetic conservation of the NF-E2 1A promoter region. Top: schematic representation of the NF-E2 promoter, exon 1A, indicated by an open box, and the 3 predicted AML1 binding sites, indicated by black boxes and numbered I, II, and III. Top row: Multiple alignment of the NF-E2 promoter sequence across all indicated species by use of Genome Vista software. Promoter segments showing more than 75% identity are highlighted in pink, exonic sequences in cyan. Remaining rows: Pair-wise alignment of the NF-E2 promoter species across the indicated species. Hs indicates Homo sapiens; Mm, Mus musculus; Rn, Rattus norvegicus; cf, Canis familiaris; Ec, Equus caballus; Pa, Pongo pygmaeus abelii; and Pt, Pan troglodytes. Promoter segments showing more than 75% identity are highlighted in pink, exonic sequences in cyan.
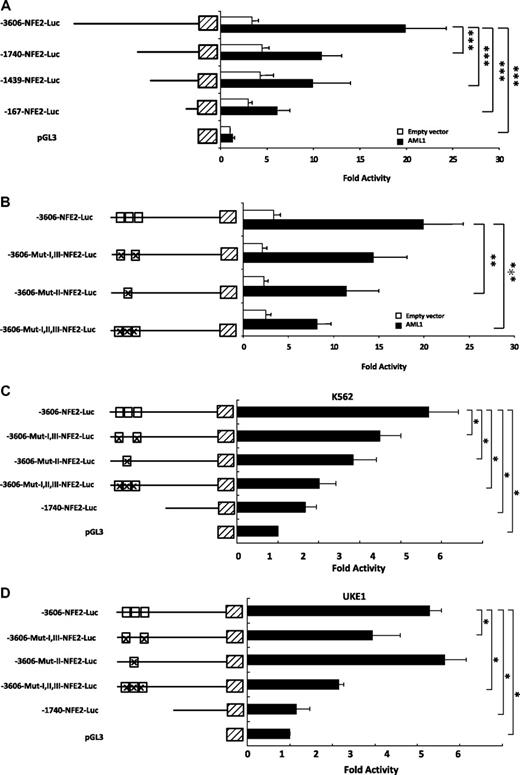
A search for candidate transcription factor binding sites did not reveal any STAT binding sites within this conserved fragment of the NF-E2 1A promoter. Rather, 3 potential Acute Myeloid Leukemia-1 (AML1/RUNX1) DNA motifs were predicted within the conserved segment at bp −3376, −3235, and −3085 of the NF-E2 1A promoter (Figure 2C). The functionality of these sites, termed I, II, and III (Figure 2C), was tested in γ-2A cells by reporter gene assays. This cell line was chosen because it does not express JAK2, and hence, the effect of AML1 can be analyzed in isolation. Upon cotransfection of an expression vector encoding AML1, reporter gene activity driven by the 3.6-kb NF-E2 1A promoter was induced 20-fold relative to the empty control vector (Figure 3A). Basal activity of the 3.6-kb NF-E2 1A promoter was 3.7-fold greater than the empty control, indicating that transcription factors besides AML1 contribute to NF-E2 expression. Deletion analysis using promoter constructs encoding 1.7 kb, 1.4 kb, and 167 bp of the NF-E2 1A promoter demonstrated that these constructs retained only an approximately 2-fold induction by AML1 and thus had lost the majority of the AML1 response (Figure 3A).
Figure 3. Effect of AML1 on NF-E2 1A promoter activity. (A) Cotransfection of AML1. Plasmids encoding the −3606 bp NF-E2-1A promoter-Luciferase construct or the −1740-bp fragment, a −1439-bp fragment, a −167-bp fragment, or the empty pGL3 vector were cotransfected into γ-2A cells either with an expression vector for AML1 (filled bars) or with an empty control vector (open bars). Luciferase activity was measured 16 hours after transfection and normalized for transfection efficiency by determination of Renilla luciferase activity from a cotransfected vector. Activity of the empty pGL3 vector was set at 1, and fold activity relative to this control is depicted. Bar graphs represent the mean ± SD of 4 independent experiments, each performed in duplicate, ***P < .001 by one-way ANOVA. (B) Cotransfection of AML1 and mutation of AML1 binding sites. Plasmids encoding the wild-type −3606 bp NF-E2-1A promoter-Luciferase construct or vectors in which one or several of the AML1 binding sites (open boxes) were mutated (indicated by crosses in the boxes) were cotransfected into γ-2A cells, either with an expression vector for AML1 (filled bars) or with an empty control vector (open bars). The introduced mutations have previously been shown to inactivate AML1 DNA binding.30 Luciferase activity was measured 16 hours after transfection and normalized for transfection efficiency by determination of Renilla luciferase activity from a cotransfected vector. Activity of the empty pGL3 vector was set at 1, and fold activity relative to this control is depicted. Bar graphs represent the mean ± SD of 4 independent experiments, each performed in duplicate ***P < .001, **P < .01 by one-way ANOVA. (C-D) Effect of mutating the AML1 binding sites on NF-E2 promoter activity in hematopoietic cells. Plasmids encoding the −3606-bp NF-E2-1A promoter-Luciferase construct, the −3606-bp NF-E2-1A construct in which one or several of the AML1 binding sites (open boxes) were mutated (indicated by crosses in the boxes) or the −1740-bp NF-E2 1A fragment were transiently nucleofected into K562 cells (C) or UKE-1 cells (D). At 24 hours after transfection, luciferase activity was determined. Results were normalized for transfection efficiency by cotransfection of a Tk-driven Renilla luciferase reporter gene vector. Mean and SD of 3 independent experiments, each measured in duplicate, are shown. *P < .05 by Kruskal Wallis one-way ANOVA on Ranks.
Figure 3. Effect of AML1 on NF-E2 1A promoter activity. (A) Cotransfection of AML1. Plasmids encoding the −3606 bp NF-E2-1A promoter-Luciferase construct or the −1740-bp fragment, a −1439-bp fragment, a −167-bp fragment, or the empty pGL3 vector were cotransfected into γ-2A cells either with an expression vector for AML1 (filled bars) or with an empty control vector (open bars). Luciferase activity was measured 16 hours after transfection and normalized for transfection efficiency by determination of Renilla luciferase activity from a cotransfected vector. Activity of the empty pGL3 vector was set at 1, and fold activity relative to this control is depicted. Bar graphs represent the mean ± SD of 4 independent experiments, each performed in duplicate, ***P < .001 by one-way ANOVA. (B) Cotransfection of AML1 and mutation of AML1 binding sites. Plasmids encoding the wild-type −3606 bp NF-E2-1A promoter-Luciferase construct or vectors in which one or several of the AML1 binding sites (open boxes) were mutated (indicated by crosses in the boxes) were cotransfected into γ-2A cells, either with an expression vector for AML1 (filled bars) or with an empty control vector (open bars). The introduced mutations have previously been shown to inactivate AML1 DNA binding.30 Luciferase activity was measured 16 hours after transfection and normalized for transfection efficiency by determination of Renilla luciferase activity from a cotransfected vector. Activity of the empty pGL3 vector was set at 1, and fold activity relative to this control is depicted. Bar graphs represent the mean ± SD of 4 independent experiments, each performed in duplicate ***P < .001, **P < .01 by one-way ANOVA. (C-D) Effect of mutating the AML1 binding sites on NF-E2 promoter activity in hematopoietic cells. Plasmids encoding the −3606-bp NF-E2-1A promoter-Luciferase construct, the −3606-bp NF-E2-1A construct in which one or several of the AML1 binding sites (open boxes) were mutated (indicated by crosses in the boxes) or the −1740-bp NF-E2 1A fragment were transiently nucleofected into K562 cells (C) or UKE-1 cells (D). At 24 hours after transfection, luciferase activity was determined. Results were normalized for transfection efficiency by cotransfection of a Tk-driven Renilla luciferase reporter gene vector. Mean and SD of 3 independent experiments, each measured in duplicate, are shown. *P < .05 by Kruskal Wallis one-way ANOVA on Ranks.
We therefore sought to verify the functional contribution of the 3 AML1 binding sites by site directed mutagenesis. Three basepair mutations, previously shown to abrogate AML1 binding,30 were introduced into the 3.6-kb NF-E2 1A promoter and tested for reporter gene activity by cotransfection of AML1 (Figure 3B). Although mutation of binding sites I and III alone had no effect, mutation of binding site II significantly reduced the ability of AML1 to stimulate NF-E2 reporter gene activity (Figure 3B). Mutation of all 3 binding sites concurrently had an even larger effect (Figure 3B). Nonetheless, a 3-fold induction remained, raising the possibility that additional, unidentified AML1 sites contribute to NF-E2 expression. Because the basal reporter gene expression was also slightly decreased by mutation of the 3 AML1 sites, it is possible that the binding of other transcription factors is affected.
Because the γ-2A fibrosarcoma cells previously mentioned do not contain JAK2, we investigated the effect of AML1 binding site mutations in hematopoietic cells containing either wild-type or mutant JAK2. Mutation of 1 or 2 AML1 sites already showed significant effects in one or both hematopoietic cell lines, whereas mutation of all 3 AML1 binding site reduced NF-E2 expression by 60% in K562 cells, which express wt JAK2 and by 50% in UKE-1 cells, which are homozygous for the JAK2V617F mutation (Figure 3C-D).29 As in γ-2A cells, the 3.6-kb NF-E2 1A promoter activity remained 2-fold greater than the empty vector control after mutation of the 3 AML1 binding sites, indicating that supplementary AML1 sites or additional trans-activating factors contribute to promoter activity. Because the 1.7-kb promoter had minimal activity above background in both cell lines (Figure 3C-D), most of the activity seems to reside in the 1.9-kb fragment extending from −3.6 to −1.7 kb upstream of the 1A transcriptional start site.
AML1 protein binds the NF-E2 promoter in vitro and in vivo
Although deletion analysis combined with site directed mutagenesis pinpoint the promoter sequences that confer activity, they do not unequivocally identify the protein bound at these sites. We used both EMSAs as well as ChIP to identify protein/DNA interactions on the NF-E2 promoter. By using nuclear extracts from HEL cells and a consensus AML1 binding site, we demonstrated the presence of an AML1 protein/DNA complex by EMSA (Figure 4A lane 1). This complex was competed by an excess of oligonucleotides encoding each of the 3 the AML1 binding sites in the NF-E2 1A promoter (Figure 4A lanes 4, 6, and 8) but not oligonucleotides of these binding sites containing the 3-bp mutations previously demonstrated to abrogate AML1 binding (Huang et al30 ; see Figure 3B), as seen in Figure 4A lanes 5, 7, and 9.
Protein/DNA interactions on the NF-E2 1A promoter. (A- B) EMSA of the putative AML1 binding sites in the NF-E2 promoter. Nuclear extracts from HEL cells were incubated with a 32P-labeled oligonucleotide containing either a consensus AML1 binding site (A) or an oligonucleotide spanning bp −3394 to −3360 of the NF-E2 1A promoter, which contains a predicted AML1 binding site (B). (A-B) In the indicated lanes, a 100× excess of the indicated, nonradioactive oligonucleotide was added. Numbers indicate the position of the oligonucleotides within the NF-E2 promoter; “cons” indicates the consensus sequence; “mut” indicates oligonucleotides containing 3-bp mutations in the AML1 binding sites (see Figure 3). Alternatively, an antibody to AML1 (B, lane 6) or a control antibody (B, lane 7) was added. A filled arrowhead indicates the specific AML1/DNA complex. The open circle shows nonspecific binding to the DNA probe, and the open arrowhead denotes unbound oligonucleotide. A filled circle shows the supershifted AML1-antibody complex. (C-D) ChIP analysis of AML1 binding sites on the NF-E2 1A promoter. (C) HEL cell lysates were chromatin immunoprecipitated (ChIPed) either with an antibody to AML1 or with an unrelated IgG control, as indicated. ChIPed DNA was amplified by PCR by the use of either primers covering the AML1 binding sites in the NF-E2-1A promoter or control primers from a distal region in the NF-E2 1A promoter or from the GAPDH promoter, as indicated. In lane 1, a 1:50 dilution of the input DNA was used; lane 4 shows control PCRs without DNA. (D) Lysates from purified peripheral blood granulocytes of 4 PV patients and 3 healthy controls were used for ChIP with an antibody to AML1 (top) or with an unrelated IgG control (middle). The ChIPed DNA was amplified by the use of primers spanning the AML1 binding sites between bp −3466 and −3192 in the NF-E2 1A promoter. In the bottom panel, a 1:50 dilution of the input DNA was used.
Protein/DNA interactions on the NF-E2 1A promoter. (A- B) EMSA of the putative AML1 binding sites in the NF-E2 promoter. Nuclear extracts from HEL cells were incubated with a 32P-labeled oligonucleotide containing either a consensus AML1 binding site (A) or an oligonucleotide spanning bp −3394 to −3360 of the NF-E2 1A promoter, which contains a predicted AML1 binding site (B). (A-B) In the indicated lanes, a 100× excess of the indicated, nonradioactive oligonucleotide was added. Numbers indicate the position of the oligonucleotides within the NF-E2 promoter; “cons” indicates the consensus sequence; “mut” indicates oligonucleotides containing 3-bp mutations in the AML1 binding sites (see Figure 3). Alternatively, an antibody to AML1 (B, lane 6) or a control antibody (B, lane 7) was added. A filled arrowhead indicates the specific AML1/DNA complex. The open circle shows nonspecific binding to the DNA probe, and the open arrowhead denotes unbound oligonucleotide. A filled circle shows the supershifted AML1-antibody complex. (C-D) ChIP analysis of AML1 binding sites on the NF-E2 1A promoter. (C) HEL cell lysates were chromatin immunoprecipitated (ChIPed) either with an antibody to AML1 or with an unrelated IgG control, as indicated. ChIPed DNA was amplified by PCR by the use of either primers covering the AML1 binding sites in the NF-E2-1A promoter or control primers from a distal region in the NF-E2 1A promoter or from the GAPDH promoter, as indicated. In lane 1, a 1:50 dilution of the input DNA was used; lane 4 shows control PCRs without DNA. (D) Lysates from purified peripheral blood granulocytes of 4 PV patients and 3 healthy controls were used for ChIP with an antibody to AML1 (top) or with an unrelated IgG control (middle). The ChIPed DNA was amplified by the use of primers spanning the AML1 binding sites between bp −3466 and −3192 in the NF-E2 1A promoter. In the bottom panel, a 1:50 dilution of the input DNA was used.
Similarly, when an oligonucleotide encoding bp −3394 to −3360 of the NF-E2 1A promoter, which comprises the AML1 binding site “I,” was used as a probe in EMSA, a protein/DNA complex was formed (Figure 4B lane 1). This protein DNA complex was competed by the consensus AML1 oligonucleotide (Figure 4B lane 4) but not by an oligonucleotide encoding a NF-kappaB binding site (Figure 4B lane 5). Moreover, the protein/DNA complex formed on the NF-E2 promoter bp −3394 to −3360 oligonucleotide was supershifted by an antibody against AML1 (Figure 4B lane 6) but not by an antibody against NF-κB (Figure 4B lane 7). Similar data were obtained with oligonucleotides derived from binding sites “II” and “III” of the NF-E2 1A promoter (data not shown). Thus, AML1 binds the NF-E2 1A promoter at the 3 predicted sites in vitro.
EMSAs are used to investigate in vitro protein/DNA binding, which may not reflect physiologic interactions on intact chromatin. Therefore, we used ChIP to investigate potential AML1 binding to the NF-E2 promoter in vivo. HEL cells as well as primary granulocytes from PV patients and healthy controls were treated with formaldehyde to crosslink existing protein/chromatin interactions. Subsequently, AML1 proteins were precipitated and the bound chromatin amplified by PCR. In HEL cells, AML1 binding to NF-E2 1A promoter sequences between bp −3466 and −3192, which comprise the identified binding sites “I” and “II,” as well as to sequences between bp −3076 and −2752, encompassing binding site “III,” was seen (Figure 4C). Control precipitations that used an unrelated IgG antibody, as well as PCRs amplifying a more upstream region of the NF-E2 promoter (bp −4589 and −4317) or a segment of the GAPDH promoter (−332 to −114) did not show AML1 binding (Figure 4C). More importantly, in primary cells from PV patients, AML1 binding to the NF-E2 1A promoter is visibly increased compared to healthy controls (bp −3466 to −3192 encompassing binding sites “I” and “II”; Figure 4D).
AML1 mRNA is overexpressed in granulocytes from patients with MPN
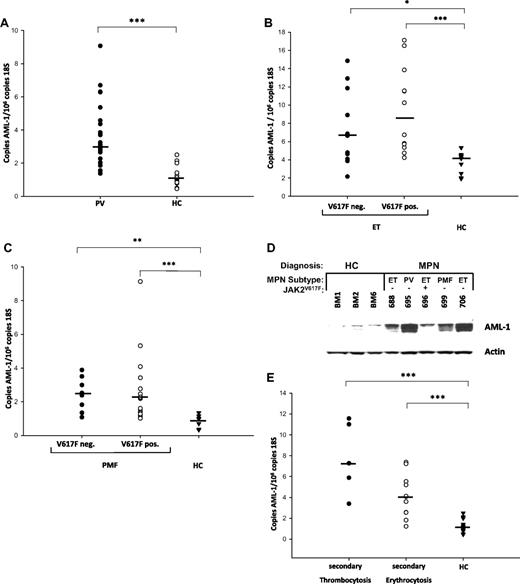
We therefore investigated whether AML1 mRNA expression is increased in MPN patients. Peripheral blood granulocytes were isolated from 31 PV patients, 23 ET patients, 25 primary myelofibrosis (PMF) patients, and 28 healthy controls. AML1 mRNA expression was quantitated by qRT-PCR and found to be statistically significantly elevated in PV patients (Figure 5A). Similarly, AML1 levels were elevated in ET and PMF patients compared with healthy controls, irrespective of the presence or absence of the JAK2V617F mutation (Figure 5B-C). Moreover, AML-1 protein levels were also increased in erythroid precursor cells of MPN patients (Figure 5D). AML1 expression is also elevated in patients with secondary erythrocytosis or secondary thrombocytosis (Figure 5E).
AML1 expression in MPN patients. (A) AML1 expression in PV patients and healthy controls. (B) AML1 expression in JAK2V617F-positive and -negative ET patients and healthy controls. (C) AML1 expression in JAK2V617F-positive and -negative PMF patients and healthy controls. (D) AML1 expression in erythroid progenitors of MPN patients and healthy controls. Erythroid progenitor cells were cultured from 5 MPN patients and 3 healthy controls as described24 and interrogated for AML-1 and beta-actin expression by Western blot. HC indicates healthy controls. (E) AML1 expression in secondary erythrocytosis and thrombocytosis. (A-C and E) RNA was isolated from purified granulocytes of 31 PV patients, 12 JAK2V617F-positive and 11 JAK2V617F-negative ET patients, 17 JAK2V617F-positive and 8 JAK2V617F-negative PMF patients, 5 patients with secondary thrombocytosis and 11 patients with secondary erythrocytosis, and 28 healthy controls as indicated and subjected to quantitative RT-PCR analysis for AML1 expression. A standard curve with known copy numbers of AML1 and 18S rRNA was included on each plate. Sample copy numbers of AML1 and 18S rRNA were determined from the standard curve and are expressed as relative ratios (copy number AML1 per 106 18S molecules). The median is depicted by a horizontal line; *P < .05, **P < .01, ***P < .001 by Kruskal Wallis one-way ANOVA on Ranks and Mann-Whitney Rank sum test.
AML1 expression in MPN patients. (A) AML1 expression in PV patients and healthy controls. (B) AML1 expression in JAK2V617F-positive and -negative ET patients and healthy controls. (C) AML1 expression in JAK2V617F-positive and -negative PMF patients and healthy controls. (D) AML1 expression in erythroid progenitors of MPN patients and healthy controls. Erythroid progenitor cells were cultured from 5 MPN patients and 3 healthy controls as described24 and interrogated for AML-1 and beta-actin expression by Western blot. HC indicates healthy controls. (E) AML1 expression in secondary erythrocytosis and thrombocytosis. (A-C and E) RNA was isolated from purified granulocytes of 31 PV patients, 12 JAK2V617F-positive and 11 JAK2V617F-negative ET patients, 17 JAK2V617F-positive and 8 JAK2V617F-negative PMF patients, 5 patients with secondary thrombocytosis and 11 patients with secondary erythrocytosis, and 28 healthy controls as indicated and subjected to quantitative RT-PCR analysis for AML1 expression. A standard curve with known copy numbers of AML1 and 18S rRNA was included on each plate. Sample copy numbers of AML1 and 18S rRNA were determined from the standard curve and are expressed as relative ratios (copy number AML1 per 106 18S molecules). The median is depicted by a horizontal line; *P < .05, **P < .01, ***P < .001 by Kruskal Wallis one-way ANOVA on Ranks and Mann-Whitney Rank sum test.
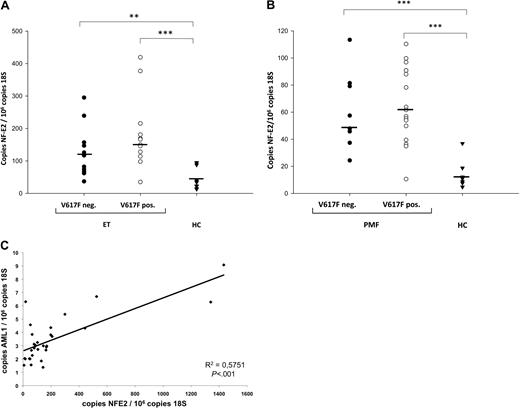
NF-E2, which had previously only been shown to be elevated in PV patients,8 was likewise found to be elevated in both JAK2V617F-positive and JAK2V617F-negative ET (Figure 6A) as well as in JAK2V617F-positive and JAK2V617F-negative PMF (Figure 6B). Because we observed JAK2-independent overexpression of both AML1 and NF-E2, we asked whether the degree of AML1 and NF-E2 expression correlated. Indeed, in the 31 PV patients assayed, the degree of NF-E2 and AML1 expression were highly correlated (P < .001; Figure 6C). A significant correlation between NF-E2 and AML1 expression was similarly seen in all 79 MPN patients (31 PV, 23 ET, and 25 PMF, P = .002).
NF-E2 expression in MPN patients. (A) NF-E2 expression in JAK2V617F-positive and -negative ET patients and healthy controls. (B) NF-E2 expression in JAK2V617F-positive and -negative PMF patients and healthy controls. (A-B) RNA was isolated from purified granulocytes of 12 JAK2V617F-positive and 11 JAK2V617F-negative ET patients, 17 JAK2V617F-positive and 8 JAK2V617F-negative PMF patients, and 28 healthy controls as indicated and subjected to quantitative RT-PCR analysis for NF-E2 expression. A standard curve with known copy numbers of NF-E2 and 18S rRNA was included on each plate. Sample copy numbers of NF-E2 and 18S rRNA were determined from the standard curve and are expressed as relative ratios (copy number NF-E2 per 106 18S molecules). The median is depicted by a horizontal line; *P < .05, **P < .01, ***P < .001 by Kruskal Wallis one-way ANOVA on Ranks and Mann-Whitney Rank sum test. HC indicates healthy controls. (C) Correlation of NF-E2 and AML1 mRNA expression in 31 PV patients. P < .001.
NF-E2 expression in MPN patients. (A) NF-E2 expression in JAK2V617F-positive and -negative ET patients and healthy controls. (B) NF-E2 expression in JAK2V617F-positive and -negative PMF patients and healthy controls. (A-B) RNA was isolated from purified granulocytes of 12 JAK2V617F-positive and 11 JAK2V617F-negative ET patients, 17 JAK2V617F-positive and 8 JAK2V617F-negative PMF patients, and 28 healthy controls as indicated and subjected to quantitative RT-PCR analysis for NF-E2 expression. A standard curve with known copy numbers of NF-E2 and 18S rRNA was included on each plate. Sample copy numbers of NF-E2 and 18S rRNA were determined from the standard curve and are expressed as relative ratios (copy number NF-E2 per 106 18S molecules). The median is depicted by a horizontal line; *P < .05, **P < .01, ***P < .001 by Kruskal Wallis one-way ANOVA on Ranks and Mann-Whitney Rank sum test. HC indicates healthy controls. (C) Correlation of NF-E2 and AML1 mRNA expression in 31 PV patients. P < .001.
Functional AML1 is required for NF-E2 protein expression
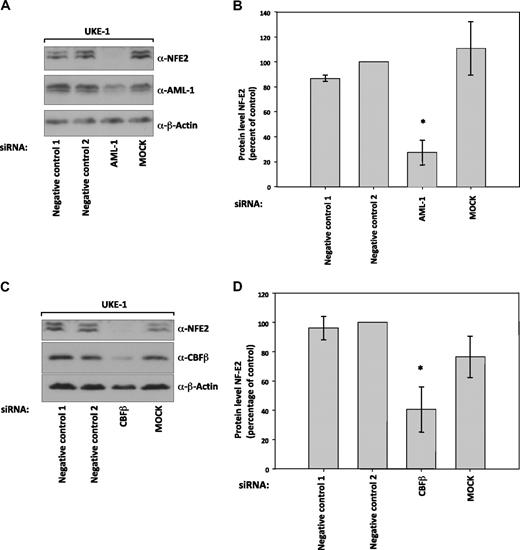
To determine whether AML1 activity is required for NF-E2 expression, even in the presence of JAK2V617F, we nucleofected UKE-1 cells with siRNA against AML1 as well as its binding partner CBF-β. Abrogation of either AML1 or CBF-β expression lead to a significant decrease in NF-E2 protein levels (Figure 7A-D).
Suppression of NF-E2 expression by siRNA-mediated inhibition of AML1 and CBF-β expression. UKE-1 cells were nucleofected with the indicated siRNAs or mock treated (MOCK). Seventy-two to 96 hours after nucleofection, protein extracts were prepared and analyzed by Western blotting for (A) NF-E2, AML1, and β-actin expression or (C) NF-E2, CBF-β, and β-actin expression. (B and D) Quantification and statistical analysis of NF-E2 protein expression in at least 3 independent experiments, representative examples of which are shown in panels A and C, respectively. NF-E2 expression in negative control 2 treated cells was set at 100%. *P < .05 by t test.
Suppression of NF-E2 expression by siRNA-mediated inhibition of AML1 and CBF-β expression. UKE-1 cells were nucleofected with the indicated siRNAs or mock treated (MOCK). Seventy-two to 96 hours after nucleofection, protein extracts were prepared and analyzed by Western blotting for (A) NF-E2, AML1, and β-actin expression or (C) NF-E2, CBF-β, and β-actin expression. (B and D) Quantification and statistical analysis of NF-E2 protein expression in at least 3 independent experiments, representative examples of which are shown in panels A and C, respectively. NF-E2 expression in negative control 2 treated cells was set at 100%. *P < .05 by t test.
The AML-1 locus is targeted by the t(8;21) translocation, detected in approximately 15% of adult acute myelogenous leukemias (AMLs). The translocation produces a fusion protein, AML/ETO, which has been shown to repress various AML-1 target genes.31,32 To investigate the effect of AML/ETO on NF-E2 expression, we transduced both UKE-1 and K562 cells with either an empty retrovirus (pSF91), or a retrovirus encoding AML/ETO (pSF91-AML/ETO; Figure 8A). Both cell lines express high levels of NF-E2, which were completely suppressed upon AML/ETO expression, whereas the empty vector control had no effect (Figure 8A), suggesting that functional AML1 is required for NF-E2 expression. NF-E2 is similarly not expressed in Kasumi cells, which carry the AML/ETO translocation (Figure 8B).
Effect of AML/ETO expression on NF-E2 protein levels. (A) Suppression of NF-E2 expression by the leukemic fusion protein AML/ETO. UKE-1 and K562 cells were retrovirally transduced either with the empty vector (pSF91) or with a vector expressing the AML/ETO fusion protein (pSF91-AML/ETO). At 72 hours after transduction, cells were sorted for green fluorescent protein expression, to yield a population of 100% transduced cells. Three days after the sort, protein extracts were prepared and analyzed by Western blotting for AML/ETO, NF-E2, and β-actin expression. A representative experiment is shown. (B) AML/ETO and NF-E2 expression in various hematopoietic cell lines. Total cell extracts from the indicated cell lines were subjected to Western blotting and interrogated with antibodies directed against AML/ETO, NF-E2, and beta-actin as indicated. A representative experiment is shown.
Effect of AML/ETO expression on NF-E2 protein levels. (A) Suppression of NF-E2 expression by the leukemic fusion protein AML/ETO. UKE-1 and K562 cells were retrovirally transduced either with the empty vector (pSF91) or with a vector expressing the AML/ETO fusion protein (pSF91-AML/ETO). At 72 hours after transduction, cells were sorted for green fluorescent protein expression, to yield a population of 100% transduced cells. Three days after the sort, protein extracts were prepared and analyzed by Western blotting for AML/ETO, NF-E2, and β-actin expression. A representative experiment is shown. (B) AML/ETO and NF-E2 expression in various hematopoietic cell lines. Total cell extracts from the indicated cell lines were subjected to Western blotting and interrogated with antibodies directed against AML/ETO, NF-E2, and beta-actin as indicated. A representative experiment is shown.
Discussion
We have identified NF-E2 as a novel AML1 target gene. AML1 binds the NF-E2 1A promoter at 3 sites within a highly conserved enhancer region. We demonstrate that both AML1 and NF-E2 are overexpressed in MPN patients, irrespective of the JAK2 mutational status. AML1 is required for NF-E2 expression in MPN cells and its binding to the NF-E2 1A promoter is increased in vivo in primary cells from PV patients.
The essential role of the transcription factor AML1 in hematopoiesis is manifested by the observation that AML1−/−-deficient animals die on day E12.5 and lack definitive hematopoiesis.33 To date, several AML1 target genes have been characterized, which themselves play critical roles in myeloid and lymphoid development.30,34,35 In this report, we have identified the megakaryocytic/erythroid transcription factor NF-E2 as a novel AML1 target.
A physiologic role for AML1 in platelet production has been implicated by a variety of previous findings. First, germline mutations in AML1 cause familial platelet disorder with a propensity to transform to AML (FDP/AML).36 These patients, which carry heterozygous AML1 mutations, present with thrombocytopenia and contain decreased numbers of CFU-Meg in the bone marrow.36,37 Second, AML1 transgenic mice display a transient, mild but consistent increase in the number of CD61-positive cells in the bone marrow at birth.38 Conversely, adult animals in which AML1 was conditionally deleted displayed defects in megakaryocytic maturation and platelet formation.39 The oncogenic fusion protein CBF-β/MHY11, which inactivates the AML1 binding partner CBF-β and acts as a dominant negative factor, likewise blocks megakaryocytic maturation.40 Our data suggest that these AML1-mediated effects on megakaryocyte development may occur by regulating expression of the transcription factor NF-E2.
We demonstrate here that the “adult” NF-E2 1A promoter is bound and regulated by AML1. Although 2 other factors, GATA-1 and Scl/Tal, have been shown to bind the “fetal” NF-E2 1F promoter,14,16 this is the first functional characterization of the “adult” NF-E2 1A promoter. RNA interference demonstrates that AML1 and CBF-β are required for NF-E2 expression in a cell line derived from an adult MPN patient (Figure 7). In contrast, Okada and colleagues41 have previously shown that AML1−/− yolk sacs and fetuses still express NF-E2 by RT-PCR analysis, albeit without quantification. However, this is not surprising because these fetuses also still express GATA-1 and Scl/Tal, which effect NF-E2 1F transcription, and the primers used did not distinguish between the NF-E2 1A or 1F transcript.
We show here that AML1 levels are increased in all 3 subtypes of MPN. Several molecular mechanisms can contribute to increased AML1 gene and protein expression. First, transcription of the AML1 gene may be increased. This could result from either increased or altered transcription factor activity on the AML1 promoter. Alternatively, AML1 mRNA or protein stability may be increased. We are currently investigating these possibilities to elucidate the molecular changes causing AML1 overexpression in MPN patients.
AML1 expression is also elevated in patients with secondary erythrocytosis or secondary thrombocytosis (Figure 5E). We propose that this does not diminish the role of increased AML1 expression in the pathophysiology of MPN. A precedent is set by the JAK2 kinase. JAK2 is physiologically phosphorylated by EPO, and hence the kinase is phosporylated and active in patients with secondary erythrocytosis, who have high EPO levels. Nonetheless, its aberrant activation and phosphorylation in the absence of an exogenous stimulus in MPN patients is pathologic.
We therefore propose that the elevated AML1 levels present in MPN patients are pathophysiologic and evoked by the molecular changes causing these diseases. In contrast, in secondary erythrocytosis and thrombocytosis patients, the bone marrow is stimulated to produce excess blood cells. This process hyperactivates the same signal transduction pathways physiologically involved in blood cell production. Hence, the observation of elevated AML1 levels in secondary erythrocytosis and thrombocytosis patients underscores the physiologic role of this transcription factor in blood cell development.
AML1 is one of the most frequently altered targets in hematologic disorders. The t(8;21) translocation, which gives rise to the AML/ETO fusion protein, is found in 10% of all AML cases and 40% of AML-M2.42 Elucidating the molecular consequences of AML/ETO expression is vital to understanding its contribution to leukemogenesis. The AML/ETO fusion protein in many cases acts as a dominant-negative repressor of AML1 target genes, depending on the promoter context.31,32 Several methods have been used to search for AML/ETO targets. The consequence of either heterologous AML/ETO expression in cell lines or of its repression in cell lines and primary cells that carry the t(8;21) translocation was investigated. In these experiments, NF-E2 was concordantly down-regulated by AML/ETO expression and induced by repression of AML/ETO.43,44 These finding were confirmed both in primary cells of AML patients and by transfection of primary CD34-positive HSCs with AML/ETO.43,44
In an alternative approach, gene expression profiling of different AML subtypes revealed clusters of patients with similar expression patterns.45 All samples carrying the t(8;21) translocation clustered together, and these patients displayed markedly decreased levels of NF-E2 expression (supplemental Figure 1). All of the aforementioned data were obtained at the RNA level by investigating NF-E2 mRNA expression. Here we have shown that NF-E2 protein levels are dramatically decreased upon expression of the AML/ETO fusion protein in 2 different cell lines (Figure 8A). Because AML/ETO has been shown to interact physically with other transcription factors, this oncogenic fusion protein may down-regulate NF-E2 levels either by decreasing transcription or by posttranscriptional and/or posttranslational mechanisms, such as direct protein-protein interaction.46
Interestingly, NF-E2 is not only repressed by the AML/ETO fusion protein but also by PML/RAR, the product of the t(15;17) translocation characteristic of acute promyelocytic leukemia, and by PZLF/RAR, the product of the t(11;17) translocation found in a small subset of acute promyelocytic leukemia patients.43 The observation that NF-E2 expression is repressed by 3 structurally diverse fusion proteins suggests that inactivation of NF-E2 is a crucial step in leukemogenesis. It appears that leukemogenic fusion proteins repress the expression of transcription factors involved in the commitment or control of lineage differentiation such as PU.1, C/EBP-α, and that this group includes NF-E2.43,47,48
It has been demonstrated that variations in the expression levels of hematopoietic transcription factors influence stem cell fate and differentiation and promote leukemic transformation.49 Here we show that 2 central hematopoietic transcription factors, AML1 and NF-E2, are dysregulated in MPN patients. Increased NF-E2 expression delays erythroid maturation, thereby increasing the number of mature erythroid elements generated from one progenitor cell.10 Increased AML1 levels promote leukemogenesis in a murine model38 and AML1 mutations were recently shown to occur during leukemic transformation of MPN.50 We therefore propose a role for dysregulated AML1 and NF-E2 expression in the pathogenesis of MPNs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We gratefully acknowledge Drs Martin Carroll (University of Pennsylvania Cancer Center) and Pawel Dobrzanski (Cephalon Inc, West Chester, PA) for many fruitful discussions, insightful comments, and critical review of the manuscript. Our sincere thanks go to Dr Tobias Berg and Prof Dr Michael Lübbert (University Medical Center, Freiburg, Germany) for the generous gift of the AML1/ETO retrovirus. We are very grateful to Dr Ruud Delwel (Erasmus MC, Rotterdam, The Netherlands) for sharing the data on NF-E2 expression in his AML panel and to Prof Dr W. Fiedler (Universitätsklinikum Hamburg, Eppendorf, Germany) for the generous gift of UKE-1 cells. We gratefully acknowledge support from the MPD Research Consortium (MPD-RC) Tissue Bank, which provided tissue samples.
E.O.H. is supported by an American Society of Hematology Scholar Award and by a National Heart, Lung, and Blood Institute Mentored Career Development Award (K23). W.W. and S.S. were funded by the Excellence Initiative of the German Federal and States Governments (GSC-4, Spemann Graduate School). This work was supported by grants from the National Cancer Institute (PO1 CA108671 to H.L.P.) and the Deutsche Forschungsgemeinschaft (DFG; Pa 611/5-1 to H.L.P.). H.L.P. and E.O.H. are members of the Myeloproliferative Disorders Research Consortium.
National Institutes of Health
Authorship
Contribution: W.W. and S.S. performed research and analyzed data; E.O.H. performed research and critically reviewed the paper; and H.L.P. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Heike L. Pahl, Department of Experimental Anaesthesiology, University Hospital Freiburg, Center for Clinical Research, Breisacher Str 66, 79106 Freiburg, Germany; e-mail: Heike.Pahl@klinikum.uni-freiburg.de.
References
Author notes
W.W. and S.S. contributed equally to this publication.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal