Abstract
Axl is an oncogenic receptor tyrosine kinase that plays multiple roles in tumorigenesis and metastasis of many cancers. This study is the first to demonstrate that Axl is induced in Kaposi sarcoma and Kaposi sarcoma herpesvirus (KSHV) transformed endothelial cells. Conditionally, expression of one KSHV latency protein vFLIP induces Axl expression in endothelial cells. This induction can be blocked by nuclear factor-κB inhibitor, consistent with the known vFLIP mechanism of action. KS cell lines lacking KSHV also have elevated Axl expression, which probably resulted from hypomethylation of AXL promoter. Axl activation activates downstream phosphoinositol-3 kinase signaling, and Axl knockdown by siRNA impairs phosphoinositol-3 kinase signaling. Furthermore, Axl knockdown inhibits KS cell growth and invasion. To explore the potential for translation of these findings, we generated monoclonal antibodies to block the biologic functions of Axl. MAb173, which induces receptor degradation, showed activity in vitro to inhibit KS cell invasion. Moreover, in vivo xenograft studies with KS cells with or without KSHV infection showed that MAb173 reduced tumor growth, increased tumor cell apoptosis, and markedly decreased Axl protein level in tumors. Axl thus has a potential role in KS pathogenesis and is a candidate for prognostic and therapeutic investigations.
Introduction
Kaposi sarcoma (KS) is a common cancer in HIV-infected persons. It originates from endothelial cells transformed by KS-associated herpesvirus (KSHV).1 KS tumor cells have spindle cell morphology and characteristically produce excessive and abnormal vascular structures, which contain red blood cells and pigment from their lysis.1 Nearly all KS cells carry KSHV with viral gene expression profile of latency.2 It is thus assumed that latency proteins play an important role in KS pathogenesis.
Gene expression analysis in KS cells versus endothelial cells by subtractive hybridization showed induction of Axl receptor kinase in KS cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Axl is a member of TAM receptor tyrosine kinases family that also includes Tyro3 and Mer.3,4 Axl is composed of 2 immunoglobin-like domains and dual fibronectin type III repeats in the extracellular region, a single transmembrane and a cytoplasmic domain with kinase activity.4 Protein S and growth arrest–specific 6 (Gas6) are the ligands for Axl, whereas only the latter has high affinity to Axl.5,6 Axl activation and signaling have been implicated in multiple cellular responses, including cell survival, proliferation, migration, and adhesion.7 In vascular biology, Axl receptor signaling has been shown to regulate vascular smooth muscle homeostasis, endothelial cell migration, and vascular network formation.8-10 The primary downstream Axl signaling pathway appears to be phosphatidylinositol 3-kinase (PI3K) pathway.9,11 However, the Janus kinase-signal transducers and activator of transcription (STAT) pathway12 or p38 mitogen-activated protein kinase pathway13 is induced in some circumstances. In addition, cooperative interaction between Axl receptor and cytokine receptor signaling network is required for many Axl-regulated biologic functions.12,14,15
The role of Axl in cancer is highlighted by the fact that Axl was first cloned from myeloid leukemia cells as a transforming gene.4 Significance of Axl in cancer was reinforced by its ability to transform cells independent of its ligand.4,16 Subsequent studies showed that Axl is overexpressed in several human cancers.17-20 Furthermore, Axl is associated with metastasis in lung,21 prostate,22 breast,23 gastric,24 renal cell carcinoma,25 and glioblastoma.26 Axl knockdown in breast and lung cancer cells results in reduced invasion.21,27 Axl is also induced during evolution of resistance to therapy including imatinib in gastrointestinal stromal tumors,28 Herceptin therapy in breast cancer,29 and after chemotherapy in acute myeloid leukemia.30 In addition, Axl has been implicated to regulate tumor angiogenesis.27,31 These findings suggest that Axl may be involved in the regulation of multiple aspects of tumorigenesis.
The current study shows that Axl is induced in KSHV-transformed endothelial cells, KS cell lines, and KS tumor tissue. KSHV latency is sufficient to induce Axl, whereas lytic cycle induction had no effect. Examination of latency genes showed that vFLIP is responsible for Axl induction and that nuclear factor-κB (NF-κB) inhibitor abrogates vFLIP effect. The role of Axl in KS cells was further determined using siRNA- and Axl-specific antibodies. Loss of Axl inhibited KS cell growth and invasion in vitro and induced KS tumor cell death and hence tumor regression in vivo, indicating Axl is a potential therapeutic target in KS.
Methods
Antibodies and other reagents
Antibodies against human Mer, Axl (rabbit monoclonal antibody for Western blot after immunoprecipitation), phosphorylated Akt (Ser 473), and phosphorylated p38 MAPK (Thr180/Tyr182) were from Cell Signaling. Antibodies against human Axl (goat polyclonal, for immunostaining), Tyro3 (goat polyclonal), and Gas6 (mouse monoclonal) were from R&D Systems. β-Actin antibody was from Sigma-Aldrich. Biotinylated platinum phosphotyrosine antibody (4G10 platinum) was from Millipore. CD31 antibody was from BD Biosciences. Ki67 antibody was from Abcam. Horseradish peroxidase (HRP)-conjugated secondary antibodies were from Rockland Immunochemicals. TdT-mediated dUTP nick-end labeling (TUNEL) assay kit was from Promega. Inhibitors Bay 11-7085, U0126, and LY294002 were from EMD Biosciences. 4-Hydroxytamoxifen (4-OHT) and phorbol ester tetradecanoyl phorbol acetate were from Sigma-Aldrich. Protease inhibitor cocktail was from Thermo Scientific.
Cell culture
Human vein endothelial cell (HUVEC) line immortalized by telomerase and subsequently infected by KSHV (LTC) was obtained from Dr Renne.32 Cell lines 293T, HT29, and A549 were obtained from ATCC. KS-IMM and KS-SLK cells were obtained from Dr Albini33 and Dr Rubinstein,34 respectively. All these cells were propagated in RPMI 1640 supplemented with 10% fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin from Cellgro. HUVECs were purchased from Lonza Walkersville and maintained in medium provided by Lonza Walkersville. Telomerase-immortalized HUVEC stably expressing vFLIP-ERTAM (HUVEC/vFLIP-ER) or a control vector (HUVEC/vector) were generated as described previously and were obtained from Dr Preet M. Chaudhary.35 Cells were treated with 4-OHT (100nM) for induction of gene expression as described previously.35
Constructs, siRNA, and transfection
The expression vectors for KSHV genes were obtained from Dr Jae Jung (University of Southern California). They include pcDNA3/LANA, pcDNA4/vFLIP, pcDNA4/KaposinB, pCDNA4/vCyclin, pcDNA4/vGPCR, and pcDNA3.1/vIL6. Transfection was performed as described previously.36
Prevalidated Axl siRNA was purchased from QIAGEN. The sense strand sequence is: 5′-CAAGAUUCUAGAUGAUUAATT-3′. Nontargeting control siRNA was also purchased from QIAGEN (sequence is not disclosed by the vendor). vFLIP siRNA was synthesized at the University of Southern California core facility, and the sense strand sequence was previously described by Judge et al (5′-GUGGUAUUGUUCCUCCUAATT-3′).37 Transfection was performed with HiPerfect (QIAGEN) following the manufacturer's protocol. Cells were subjected to RNA isolation, Western blot, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays approximately 72 hours after transfection.
AXL copy number analysis with quantitative PCR
Genomic DNA was extracted with Promega Wizard kit. Serial dilution of DNA was used to amplify AXL, GAS6, and β-ACTIN gene. GAS6 and β-ACTIN were used as reference gene. The primers for amplification were: Axl-F, 5′-tggaggagcccgaagacaggact-3′; Axl-R 5′-gagaggccggggtgaatggag-3′; Gas6-F, 5′-agaacctgcccggctcctactcct-3′; Gas6-R, 5′-agccccactgttcccatcactg-3′; beta-actin-F, 5′-tctgaagtcggcaagggggagtga-3′; and β-actin-R, 5′-cgtcgcgccgctgggttttat-3′. Quantitative polymerase chain reaction (PCR) was performed with SYBR Green II mastermix on Stratagene MX3000P (Agilent Technologies). Each amplification reaction was checked for the absence of nonspecific PCR products by melting curve analysis and electrophoresis. The threshold cycle numbers obtained from quantitative PCR were compared to generate the relative copy number as Livak and Schmittgen described.38
Axl promoter methylation analysis
The genomic DNA (1.5 μg) of KS-SLK, KS-IMM, LTC, HT29, and A549 cells was used for bisulfite conversion using the EpiTect Bisulfite Kit (QIAGEN) following the manufacturer's instructions. Bisulfite-converted DNA was then used as template to amplify AXL promoter (−669 to −357). The primers were: sense 5′-TGTTTTAGTTTGTGTGTGTTAGTGA-3′ and antisense 5′-AAACCCTAATAACTATACCCCCTATC-3′. PCR was performed under the following conditions: 94°C for 2 minutes; 30 cycles of 94°C for 30 seconds, annealing at 62°C for 30 seconds, and extension at 72°C for 45 seconds; and final extension at 72°C for 5 minutes. Purified PCR products were cloned into pCR2.1-TOPO vector (Invitrogen), and 8 clones from each genomic DNA were subjected to DNA sequencing.
Recombinant proteins
Human Gas6 (amino acids 118-678, with Axl secretion signal in N-terminus) and Axl (see Figure 5F) were cloned into pCDNA3.1 with C-terminal fusion of hexahistidine or human IgG1 Fc. Plasmids were transiently transfected into 293T cells and conditioned medium were collected. His and Fc fused recombinant proteins were purified by Nickel-NTA agarose affinity chromatography (Bio-Rad) and protein A-Sepharose affinity chromatography (GE Healthcare), respectively.
Immunoprecipitation and Western blot
Cells grown on 100-mm dish were lysed on ice with 1 mL lysis buffer (phosphate-buffered saline [PBS], 2mM NaF, 1mM Na3VO4, 1× protease inhibitor cocktail, and 1% Triton X-100) for 30 minutes. Lysate was cleared by centrifugation at 20 000g for 30 minutes at 4°C. A total of 200 μL of the supernatant was incubated with 10 μL of protein A + G beads (Santa Cruz Biotechnology) and 4 μg of Axl antibody MAb173 overnight at 4°C. The beads were washed once with wash buffer (PBS, 2mM NaF, 1mM Na3VO4, 1× protease inhibitor cocktail, and 0.1% Triton X-100) and then resuspended in Laemmli buffer (Bio-Rad) supplemented with 50mM dithiothreitol, followed by 95°C incubation for 5 minutes.
For Western blot, typically 20 μg of whole-cell lysates was run on 4% to 20% Tris-glycine gradient gel (Bio-Rad) and transferred onto nitrocellulose membrane (Bio-Rad). The membrane was blocked with 5% nonfat dry milk in Tris-buffered saline and 0.05% Tris-buffered saline–Tween-20 for 40 minutes, and then incubated with 1 μg/mL primary antibody at 4°C overnight. Membrane was washed 3 times for 10 minutes each and incubated with secondary HRP-labeled secondary antibody for 40 minutes. Finally, the membrane was washed 3 times with Tris-buffered saline–Tween-20, and HRP signal was detected using Femto Maximum Sensitivity chemiluminescent substrate from Thermo Scientific.
Cell viability assay
Cells were seeded in 24-well plates at a density of 104 cells/well in a total volume of 500 μL. The medium was changed after cells were attached, and triplicate samples were treated. After treatment, cell viability was assessed using MTT as described previously.39
Invasion assay
The 24-transwell plate (BD Bioscience) was incubated at room temperature for 30 minutes. A total of 750 μL of culture medium (cell line specific) without serum was added in the lower chamber, and 500 μL of the same medium was added in the upper chamber, followed by 2-hour incubation at 37°C. Media were then removed from both chambers. Cells were harvested by trypsinization and (5 × 104 cells/well) seeded in 500 μL of serum-free medium in the upper chamber of the transwell plate. Migration was induced by adding 750 μL of medium with 10% fetal bovine serum in the bottom chamber. After overnight incubation at 37°C, medium was removed, and cells were removed from the inner side of upper chamber using cotton swab presoaked in serum-free medium. The cells on the outer surface of the membrane were fixed and stained with Diff Quick (Dade Behring). Matrigel membrane was cut off with a scalpel and mounted on the slides with 100% glycerol. The cells were counted in 10 individual high-powered fields for each membrane under a light microscope.
Generation of monoclonal antibodies
The extracellular domain of Axl (amino acids 33-442) (sAxl) with hexahistidine tag on C-terminus was transiently expressed in 293T cells and purified through nickel-NTA column (Bio-Rad). Female Swiss Webster mice were immunized 3 times (every second week) intraperitoneally with 50 μg of sAxl per mouse. Antigen was injected as 1:1 mixture with Complete Freund's Adjuvant (Sigma-Aldrich) in the first immunization, and with incomplete Freund's Adjuvant (Sigma-Aldrich) in the second and third doses. Mice were given a final boost with 20 μg of sAxl through tail-vein injection, and splenocytes were harvested 4 days later for fusion with myeloma cell line NS0 from ATCC. Hybridoma supernatants were screened for antibodies that immunoprecipitate sAxl fused to alkaline phosphatase. Selected monoclonal antibodies were produced in BD CELLine cultivation system (BD Biosciences) following the manufacturer's protocol. Monoclonal antibodies were purified using a 3-step protocol: ammonium sulfate precipitation, hydroxyapatite chromatography, followed by purification with anion-exchange resin. Estimated purity of monoclonal antibodies was higher than 95% based on high performance liquid chromatography analysis.
ELISA
Monoclonal antibodies against Axl (1 μg/well) were coated on an enzyme-linked immunosorbent assay (ELISA) plate (Thermo Scientific) in PBS overnight at 4°C. Wells were then blocked with 0.5% bovine serum albumin in PBS for 40 minutes, followed by application of recombinant human Axl proteins (with human IgG1 Fc fusion on the C-terminus as illustrated in Figure 5F). The bound Axl proteins were detected using HRP-conjugated anti–human Fc antibody (Rockland Immunochemicals). Wells coated with an unrelated mouse IgG1 monoclonal antibody were used as negative control to Axl MAb173. A human IgG1 Fc fragment (Rockland Immunochemicals) was used as a negative control to recombinant Fc-fused Axl proteins.
Antibody endocytosis
MAb173 was biotinylated with EZ-link biotin hydrazide from Thermo Scientific following the manufacturer's procedure. LTC cells were treated with 10 μg/mL biotinylated MAb173 for 15 minutes or 1 hour at 37°C or 1 hour at 4°C. The cells were then fixed with 4% paraformaldehyde for 20 minutes and washed with PBS 3 times. Cells were permeabilized with 0.1% Triton X-100 and washed with PBS 3 times. Subsequently, cells were stained with streptavidin-fluorescein isothiocyanate (Invitrogen) for 30 minutes at room temperature. Images were taken with a 100× objective on a Carl Zeiss LSM 510 confocal microscope with Carl Zeiss LSM software.
Immunofluorescence
Fresh frozen tissue embedded in OCT was sectioned at 5 μm and fixed in phosphate-buffered 4% paraformaldehyde and washed in PBS. Sections were then incubated with primary antibodies overnight at 4°C. After washing with PBS, antibody binding was localized with Alexa Fluor-conjugated appropriate secondary antibodies (Invitrogen). Nuclei were counterstained with 6-diamidino-2-phenylindole dihydrochloride hydrate (DAPI). Images were obtained with an Olympus AX70 fluorescence microscope and Spot Version 2.2.2 digital imaging system.
Murine tumor xenograft models
KS-SLK and LTC cells were propagated, collected after trypsin digestion, and resuspended in serum-free medium. Cells (2 × 106) were injected subcutaneously bilaterally in the flanks of 8-week-old Balb/C nu/nu mice. Tumor growth was measured 3 times a week, and volume was estimated as 0.52 × a × b2, where a and b are the largest and smallest lengths of the tumor, respectively. Once tumors were approximately 200 mm3 for KS-SLK and 100 mm3 for LTC (day 0), animals were distributed into treatment and control groups (n = 10 tumors per group) such that the mean tumor volume of each group was comparable and the SE between groups was minimal. Each group was treated by intraperitoneal injection of antibody 3 times a week at a dose of 10 mg/kg. At the end of the experiment, mice were killed for tissue analysis. All procedures were approved by Institutional Animal Care and Use Committee and performed in accordance with the Animal Welfare Act regulations.
Labeling of functional tumor vasculature in vivo
Rhodamine conjugated Ricinus communis agglutinin I (RCA) from Vector Laboratories (0.5 mg in 100 μL) was injected into the tail vein and allowed to circulate for 7 minutes before the mice were killed. The tumors were harvested, frozen on dry ice, and stored at −80°C until analysis.
Results
Axl overexpression in KS and KSHV tranformed endothelial cell lines
Gene expression analysis in KS cells versus endothelial cells by subtractive hybridization revealed the induction of Axl receptor kinase in KS cells (supplemental Figure 1). Subsequently, Axl expression was analyzed by Western blot in KS cell lines, KS tumors, and the KSHV-infected endothelial cell line LTC. Lung cancer cell line A549 was included as a positive control.31 Negative controls included 293T cell and colon cancer cell line HT2940 (Figure 1A). Compared with the HUVECs immortalized by telomerase (HUVEC/ER), Axl expression was significantly elevated in KS cell lines KS-SLK and KS-IMM and KSHV-infected endothelial cells (Figure 1A). The TAM ligand Gas6 was expressed in all tested cell types, consistent with its widespread expression (Figure 1A). It is notable that the molecular weight of Gas6 was approximately 300 kDa because it was in oliogomer form under nonreduced condition. (The Gas6 antibody used here only recognized nonreduced Gas6.) We further examined the expression of TAM receptors and ligand in primary human KS tumor tissue. Axl and Gas6 were highly expressed and colocalized in tumor tissues (Figure 1B-C). However, no expression of Mer and Tyro3 was observed (Figure 1B).
Axl and Gas6 expression in KS cells and KS tissue. (A) Expression of Axl and Gas6 in KS cell lines and endothelial cells infected by KSHV was analyzed by Western blot of whole-cell lysates. Cell lines 293T and HT29 were used as a negative control for Axl expression. A549 cell was used as a positive control. HUVEC/vector is a HUVEC line immortalized by telomerase, as described in “Cell culture.” (B) Axl, Mer, Tyro3, and Gas6 expression in human KS tissue was analyzed by immunostaining. Nuclei were counterstained with DAPI. The KS tumor regions are indicated by arrows. Images were taken with an Olympus AX70 fluorescence microscope (20× objective) and Spot Version 2.2.2 digital imaging system. (C) Confocal imaging of Axl and Gas6 colocalization in human KS tumor. (Bottom panel) Merged staining of Axl, Gas6, and nuclei. Enlarged view of the white-bordered insert in the lower left picture was shown on the right. Images were taken with a Carl Zeiss confocal microscope (100× objective) and LSM software.
Axl and Gas6 expression in KS cells and KS tissue. (A) Expression of Axl and Gas6 in KS cell lines and endothelial cells infected by KSHV was analyzed by Western blot of whole-cell lysates. Cell lines 293T and HT29 were used as a negative control for Axl expression. A549 cell was used as a positive control. HUVEC/vector is a HUVEC line immortalized by telomerase, as described in “Cell culture.” (B) Axl, Mer, Tyro3, and Gas6 expression in human KS tissue was analyzed by immunostaining. Nuclei were counterstained with DAPI. The KS tumor regions are indicated by arrows. Images were taken with an Olympus AX70 fluorescence microscope (20× objective) and Spot Version 2.2.2 digital imaging system. (C) Confocal imaging of Axl and Gas6 colocalization in human KS tumor. (Bottom panel) Merged staining of Axl, Gas6, and nuclei. Enlarged view of the white-bordered insert in the lower left picture was shown on the right. Images were taken with a Carl Zeiss confocal microscope (100× objective) and LSM software.
Axl expression is induced by viral protein vFLIP
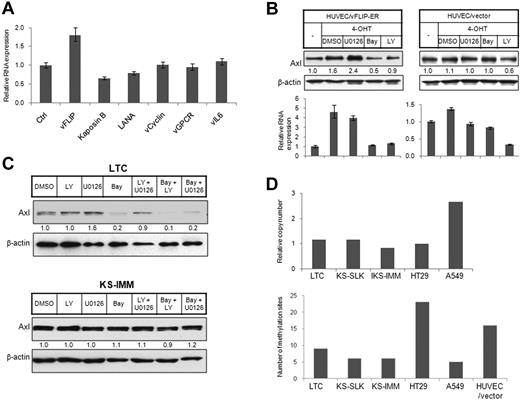
KSHV-transformed endothelial cells primarily express latency genes, including LANA, vFLIP, vCyclin, and Kaposins. Only rare cells express lytic phase viral proteins.2 KS tumor cells also primarily express latency genes. We observed induction of Axl in KSHV-infected endothelial cells and KS tumor cells, although we failed to see further induction in KSHV-infected endothelial cells undergoing tetradecanoyl phorbol acetate-induced lytic cycle41 (Figure 1A). We thus sought to determine whether latency proteins induce Axl expression. We ectopically expressed KSHV latency genes in 293T cells and examined Axl expression in RNA level by quantitative reverse-transcribed (RT)-PCR. Among 4 latency genes, only vFLIP induced Axl expression (Figure 2A). Expression of lytic genes vGPCR and vIL6 also did not induce Axl expression (Figure 2A). We thus used an immortalized endothelial cell line with 4-OHT-inducible vFLIP expression35 for further study. In this cell line, the vFLIP cDNA is fused in frame to the ligand-binding domain of a mutated estrogen receptor, which allows regulation of vFLIP activity by treatment with 4-OHT.35 Axl was up-regulated after induction of vFLIP activity with 4-OHT treatment (Figure 2B). In contrast, Axl expression was not changed in cells carrying empty control vector (HUVEC/vector) and treated with 4-OHT (Figure 2B). vFLIP regulates downstream events via activating the NK-κB pathway.42 We wished to determine whether Axl induction was also mediated by NF-κB activation. Axl induction in HUVECs expressing vFLIP (HUVEC/vFLIP-ER) was abolished by the NF-κB inhibitor Bay11-7085 but not the extracellular signal-regulated kinase (ERK) inhibitor U0126 (Figure 2B), indicating that vFLIP induces Axl activation via the NF-κB pathway. The PI3K inhibitor LY294002 can also reduce Axl level in both HUVECs expressing vFLIP and HUVEC harboring empty vector, suggesting that the basal Axl level in these cells might be regulated by PI3K signaling.
Elevated Axl expression in KS cells resulted from vFLIP expression or hypomethylation in AXL promoter. (A) The 293T cells were transiently transfected with expression vectors for KSHV latency genes (vFLIP, Kaposin B, LANA, and vCyclin) and lytic genes (vGPCR and vIL6). Empty pcDNA3 vector was used as control. Expression of Axl was analyzed by quantitative RT-PCR 72 hours after transfection and normalized to β-actin expression. (B) HUVECs stably transfected with a vFLIP-ERTAM retroviral vector (HUVEC/vFLIP-ER) or a control vector35 (HUVEC/vector) were grown on a 6-well plate and induced by 4-OHT (100nM) for 48 hours. The cells were treated with PI3K inhibitor LY (LY294002, 15μM, 5 hours), ERK inhibitor U0126 (15μM, 5 hours), and NF-κB inhibitor Bay (Bay 11-7085, 10μM, 2 hours), or vehicle dimethyl sulfoxide (DMSO). Cells were lysed, and expression of Axl was analyzed by Western blot (top panel) or quantitative RT-PCR (bottom panel). The relative protein level was quantitated by ImageJ (National Institutes of Health, Bethesda, MD), normalized to β-actin, and is shown below the Western blot. (C) LTC (top panel) and KS-IMM (bottom panel) were treated with PI3K inhibitor LY (LY294002, 15μM, 5 hours), ERK inhibitor U0126 (15μM, 5 hours), and NF-κB inhibitor Bay (Bay 11-7085, 10μM, 2 hours), or combinations of 2 of these 3. The whole-cell lysates were then subjected to Western blot analysis. (D) (Top panel) AXL copy number in LTC, KS-SLK, KS-IMM, HT29, and A549 cells was determined by quantitative PCR, as described in “AXL copy number analysis with quantitative PCR.” GAS6 was used as a reference gene. (Bottom panel) Genomic DNA isolated from 5 cell lines along with HUVEC/vector were converted by bisulfate, and the AXL promoter region was subsequently amplified, followed by cloning and sequencing. Total number of methylated sites from 8 clones was shown.
Elevated Axl expression in KS cells resulted from vFLIP expression or hypomethylation in AXL promoter. (A) The 293T cells were transiently transfected with expression vectors for KSHV latency genes (vFLIP, Kaposin B, LANA, and vCyclin) and lytic genes (vGPCR and vIL6). Empty pcDNA3 vector was used as control. Expression of Axl was analyzed by quantitative RT-PCR 72 hours after transfection and normalized to β-actin expression. (B) HUVECs stably transfected with a vFLIP-ERTAM retroviral vector (HUVEC/vFLIP-ER) or a control vector35 (HUVEC/vector) were grown on a 6-well plate and induced by 4-OHT (100nM) for 48 hours. The cells were treated with PI3K inhibitor LY (LY294002, 15μM, 5 hours), ERK inhibitor U0126 (15μM, 5 hours), and NF-κB inhibitor Bay (Bay 11-7085, 10μM, 2 hours), or vehicle dimethyl sulfoxide (DMSO). Cells were lysed, and expression of Axl was analyzed by Western blot (top panel) or quantitative RT-PCR (bottom panel). The relative protein level was quantitated by ImageJ (National Institutes of Health, Bethesda, MD), normalized to β-actin, and is shown below the Western blot. (C) LTC (top panel) and KS-IMM (bottom panel) were treated with PI3K inhibitor LY (LY294002, 15μM, 5 hours), ERK inhibitor U0126 (15μM, 5 hours), and NF-κB inhibitor Bay (Bay 11-7085, 10μM, 2 hours), or combinations of 2 of these 3. The whole-cell lysates were then subjected to Western blot analysis. (D) (Top panel) AXL copy number in LTC, KS-SLK, KS-IMM, HT29, and A549 cells was determined by quantitative PCR, as described in “AXL copy number analysis with quantitative PCR.” GAS6 was used as a reference gene. (Bottom panel) Genomic DNA isolated from 5 cell lines along with HUVEC/vector were converted by bisulfate, and the AXL promoter region was subsequently amplified, followed by cloning and sequencing. Total number of methylated sites from 8 clones was shown.
LTC is a cell line with latent infection of KSHV. Using siRNA targeting vFLIP, we down-regulated vFLIP in LTC cells, which also reduced Axl levels at both RNA and protein levels (supplemental Figure 2A-B). In addition, reduced vFLIP expression impaired the viability of LTC cells (supplemental Figure 2C), consistent with the transforming activity of vFLIP.43 Axl expression in LTC cells was also regulated by the NF-κB pathway, not the ERK or PI3K pathway (Figure 2C top panel). However, KS cell lines lacking KSHV, such as KS-IMM, were not responsive to the NF-κB pathway inhibitor (Figure 2C bottom panel). Therefore, induction of Axl in KS cell lines was maintained in the absence of KSHV viral proteins and even independent of the signaling pathway that viral proteins normally function through. How Axl induction is fulfilled in these cell lines became very intriguing.
AXL promoter hypomethylation, but not gene amplification, accounts for Axl up-regulation
In many cases, increased expression level results from gene amplification (ie, increased gene copy number). We thus isolated genomic DNA from KS cell lines, LTC, as well as HT29 and A549 cells, and then used quantitative PCR to compare AXL copy number. GAS6 and β-ACTIN genes were used as reference genes. We found that the AXL gene was amplified 2 times in A549 cells but was not changed in KS cells (Figure 2D top panel).
Hypomethylation of the AXL promoter was previously reported to regulate Axl expression,30,40 which inspired us to examine the DNA methylation status of the AXL promoter in KS cells. Genomic DNA was isolated and subjected to bisulfite conversion. One CpG island (−669 to −357) was amplified by PCR following the methods described by Mudduluru et al.40 The PCR product was subsequently cloned into pCR2.1 TOPO cloning vector, and 8 individual clones were sequenced to identify methylated sites (Figure 2D bottom panel). In total, 23 methylation sites were identified from clones derived from HT29, a cancer cell line with low Axl expression. Immortalized endothelial cell HUVEC/vector also had a large number (16) of methylation sites. In contrast, only 5 or 6 methylation sites were identified from KS cell lines KS-SLK and KS-IMM and lung carcinoma cell line A549, all of which have a high Axl expression level. Interestingly, LTC also has a relatively low level of methylation, indicating that the methylation profile already has been changed after KSHV infection.
Axl activation and PI3K/Akt pathway in KS cells
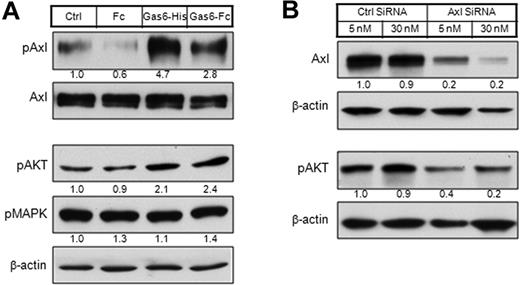
To determine whether Axl signaling can be activated in KSHV-infected endothelial cells, we treated LTC with recombinant Gas6, the high-affinity ligand for Axl (Figure 3A top panel). Activation was analyzed by kinase phosphorylation. Axl phosphorylation was induced within 15 minutes of ligand treatment. Similar results were obtained in KSHV-negative KS cell lines (data not shown).
PI3K is the major signaling pathway downstream to Axl activation. (A) LTC cells were starved overnight in serum-free medium and then stimulated with Gas6-His (1 μg/mL) or Gas6-Fc (1 μg/mL) for 20 minutes. Human Fc fragment (1 μg/mL) was used as a negative control. The whole-cell lysates were either subjected to immunoprecipitation by Axl antibody MAb173, followed by Western blot with Axl antibody (rabbit monoclonal) or 4G10 (biotinylated), or directly subjected to Western blot with antibodies against pAKT or pMAPK. The relative protein level was quantitated by ImageJ, normalized (phosphorylated Axl level was normalized to Axl level; pAKT and pMAPK levels were normalized to β-actin level), and is shown below each panel. (B) LTC cells were transfected by 5nM or 30nM of Axl siRNA and control siRNA. At 72 hours after transfection, whole-cell lysates were subjected to Western blot with antibodies against Axl or pAKT.
PI3K is the major signaling pathway downstream to Axl activation. (A) LTC cells were starved overnight in serum-free medium and then stimulated with Gas6-His (1 μg/mL) or Gas6-Fc (1 μg/mL) for 20 minutes. Human Fc fragment (1 μg/mL) was used as a negative control. The whole-cell lysates were either subjected to immunoprecipitation by Axl antibody MAb173, followed by Western blot with Axl antibody (rabbit monoclonal) or 4G10 (biotinylated), or directly subjected to Western blot with antibodies against pAKT or pMAPK. The relative protein level was quantitated by ImageJ, normalized (phosphorylated Axl level was normalized to Axl level; pAKT and pMAPK levels were normalized to β-actin level), and is shown below each panel. (B) LTC cells were transfected by 5nM or 30nM of Axl siRNA and control siRNA. At 72 hours after transfection, whole-cell lysates were subjected to Western blot with antibodies against Axl or pAKT.
We next examined the signaling downstream to Axl activation. Gas6 stimulation increased the level of phosphorylated AKT (pAKT) in LTC, but not phosphorylated p38 mitogen-activated protein kinase (pMAPK; Figure 3A bottom panel). These results suggest AKT, but not MAPK, is the signaling pathway downstream to Axl activation in KS. Consistently, knocking down of Axl expression by a prevalidated siRNA in both LTC (Figure 3B) and KS tumor cells (data not shown) led to a decrease of basal pAKT level.
Inhibition of KS cell growth and invasion by Axl knockdown
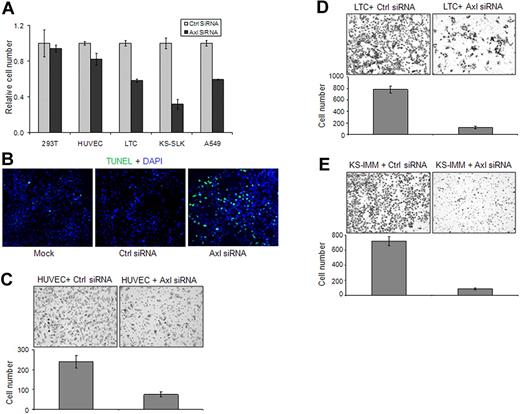
We next investigated the role of Axl in the growth of KS cells by knocking down Axl expression by siRNA. After transfection with Axl siRNA or control siRNA for 48 hours, the growth of 293T, HUVEC, LTC, and KS-IMM cells was analyzed by MTT assay. Axl knockdown significantly inhibited the growth of A549, LTC, and KS-IMM cells, mildly inhibited the growth of HUVECs, and had no effect on 293T cells (Figure 4A). Further analysis indicated that the apoptosis of LTC cells after Axl knockdown was significantly increased (Figure 4B).
Axl knockdown in KS cells inhibited cell growth and invasion. (A) The 293T, KS-SLK, KS-IMM, LTC, and A549 cells were transfected with Axl siRNA or control siRNA (50nM) for 72 hours. Cell numbers were determined at the end of the experiment with the MTT method. (B) Apoptosis of LTC cells 72 hours after 50nM siRNA transfection was analyzed with TUNEL assay (green). Nuclei were counterstained with DAPI. Apoptosis was significantly increased in Axl siRNA-transfected cells. Images were taken with an Olympus AX70 fluorescence microscope (20× objective) and Spot Version 2.2.2 digital imaging system. (C-E) Effect of Axl knockdown on the invasion of HUVEC (C), LTC (D), and KS-IMM (E) was analyzed by transwell invasion assay. The experiment was performed in triplicate. The cells that accomplished invasion were counted in 10 individual high-powered fields for each membrane under a light microscope, and the numbers were shown below each representative picture.
Axl knockdown in KS cells inhibited cell growth and invasion. (A) The 293T, KS-SLK, KS-IMM, LTC, and A549 cells were transfected with Axl siRNA or control siRNA (50nM) for 72 hours. Cell numbers were determined at the end of the experiment with the MTT method. (B) Apoptosis of LTC cells 72 hours after 50nM siRNA transfection was analyzed with TUNEL assay (green). Nuclei were counterstained with DAPI. Apoptosis was significantly increased in Axl siRNA-transfected cells. Images were taken with an Olympus AX70 fluorescence microscope (20× objective) and Spot Version 2.2.2 digital imaging system. (C-E) Effect of Axl knockdown on the invasion of HUVEC (C), LTC (D), and KS-IMM (E) was analyzed by transwell invasion assay. The experiment was performed in triplicate. The cells that accomplished invasion were counted in 10 individual high-powered fields for each membrane under a light microscope, and the numbers were shown below each representative picture.
Axl has been shown to be important for the migration of endothelial cells and metastasis of many cancers.7,27 To assess the role of Axl in the invasion of KS cells, we used a BD Matrigel transwell system. Cells were transfected for 48 hours and then trypsinized and seeded on the top of transwell in serum-free medium. After overnight incubation under normal culture conditions, the cells migrated through the Matrigel-coated membrane to the serum-containing bottom chamber. Compared with cells transfected with control siRNA, the invasion ability of HUVEC and KS cells transfected with Axl siRNA was significantly impaired (Figure 4C-E).
Generation of monoclonal antibodies that block Axl function
Our validation data using siRNA suggested that Axl is involved in KS tumor cell growth and invasion. We therefore set out to develop monoclonal antibodies against Axl. We immunized mice with human Axl extracellular domain and screened a panel of monoclonal antibodies to select ones capable of inhibiting KS cell growth or invasion. One antibody, designated as MAb173, was selected. As shown in Figure 5A-C, MAb173 significantly inhibited invasion of HUVEC, LTC, and KS-IMM cells. When MAb173 was preincubated with a soluble extracellular fragment of Axl and then applied to cells, its effect on invasion of these cells was reversed (supplemental Figure 3B), indicating the specificity of MAb173. However, MAb173 had no effect on the growth of these cells (data not shown).
Characterization of Axl MAb173. (A-C) Effect of MAb173 treatment on the invasion of HUVEC (A), LTC (B), and KS-IMM (C) was analyzed by transwell invasion assay. The experiment was performed in triplicate. An unrelated isotype control antibody (Ctrl IgG) was used as a negative control. The cells that accomplished invasion were counted in 10 individual high-powered fields for each membrane under a light microscope, and the numbers were shown below each representative picture. (D) LTC was treated with Ctrl IgG and MAb173 for the indicated time. The whole-cell lysates were subjected to Western blot with antibodies against Axl and pAKT. The relative expression level was quantitated by ImageJ, normalized to β-actin and is shown below each panel. (E) LTC cells were incubated with 10 μg/mL biotinylated MAb173 at 37°C for 15 minutes or 1 hour, or 4°C for 1 hour. The cells were fixed and MAb173 was localized with streptavidin-fluorescein isothiocyanate (green). Nuclei were counterstained with DAPI (blue). Images were taken with a Carl Zeiss confocal microscope (100× objective) and LSM software. (F) The scheme of Axl truncation variants (Fc fusion for all) is shown on the top left. The C-terminal amino acid of Axl in each construct was indicated. The expression of these constructs was analyzed by Western blot using antibody against Fc and was shown on the top right. The epitope of MAb173 was determined by ELISA (bottom panel), as described in “ELISA.” The experiment was performed in duplicate.
Characterization of Axl MAb173. (A-C) Effect of MAb173 treatment on the invasion of HUVEC (A), LTC (B), and KS-IMM (C) was analyzed by transwell invasion assay. The experiment was performed in triplicate. An unrelated isotype control antibody (Ctrl IgG) was used as a negative control. The cells that accomplished invasion were counted in 10 individual high-powered fields for each membrane under a light microscope, and the numbers were shown below each representative picture. (D) LTC was treated with Ctrl IgG and MAb173 for the indicated time. The whole-cell lysates were subjected to Western blot with antibodies against Axl and pAKT. The relative expression level was quantitated by ImageJ, normalized to β-actin and is shown below each panel. (E) LTC cells were incubated with 10 μg/mL biotinylated MAb173 at 37°C for 15 minutes or 1 hour, or 4°C for 1 hour. The cells were fixed and MAb173 was localized with streptavidin-fluorescein isothiocyanate (green). Nuclei were counterstained with DAPI (blue). Images were taken with a Carl Zeiss confocal microscope (100× objective) and LSM software. (F) The scheme of Axl truncation variants (Fc fusion for all) is shown on the top left. The C-terminal amino acid of Axl in each construct was indicated. The expression of these constructs was analyzed by Western blot using antibody against Fc and was shown on the top right. The epitope of MAb173 was determined by ELISA (bottom panel), as described in “ELISA.” The experiment was performed in duplicate.
We further investigated the mechanism of action of MAb173. LTC and KS-IMM cells were treated by MAb173 for 30 minutes, 8 hours, 24 hours, and 48 hours. Axl protein level was reduced slightly by MAb173 at 8 hours and dramatically at 24 hours and 48 hours (Figure 5D). This suggests that this antibody could degrade the Axl receptor. Endocytosis is a common pathway for cell surface proteins to be degraded or recycled. To determine whether MAb173 triggers endocytosis of Axl, we treated LTC cells with biotinylated MAb173 at 37°C or 4°C. The localization of MAb173 was then visualized using green fluorescence dye-conjugated streptavidin. After 15 minutes of treatment at 37°C, MAb173 began to induce endocytosis, although a fraction of antibody was still on the cell surface (Figure 5E). One hour after treatment at 37°C, nearly all MAb173 was translocated in the cytoplasm (Figure 5E). In contrast, after treatment of the cells with the antibody for a period of 1 hour at 4°C, no endocytosis was observed and MAb173 continued to localize to the cell surface (Figure 5E). We also examined the effect of MAb173 on the downstream signaling of Axl. As shown in Figure 5D, pAKT level was reduced at 24 hours and 48 hours, accompanying reduction of the Axl level. Moreover, the soluble extracellular fragment of Axl could efficiently block the MAb173-induced Axl degradation and pAKT down-regulation (supplemental Figure 3C), supporting that the effect of MAb173 is specific to Axl targeting.
To determine the binding epitope of MAb173, various truncation versions of recombinant Axl extracellular domain were produced as Fc fusion protein (Figure 5F). Epitope was mapped by ELISA as described in “ELISA.” The epitope for MAb173 appeared to be located within the first fibronectin domain (Figure 5F). We also determined that MAb173 did not cross-react with murine Axl or other members of TAM receptor members Tyro3 and Mer (supplemental Figure 3A).
Inhibition of KS-SLK and LTC xenograft tumor growth by MAb173
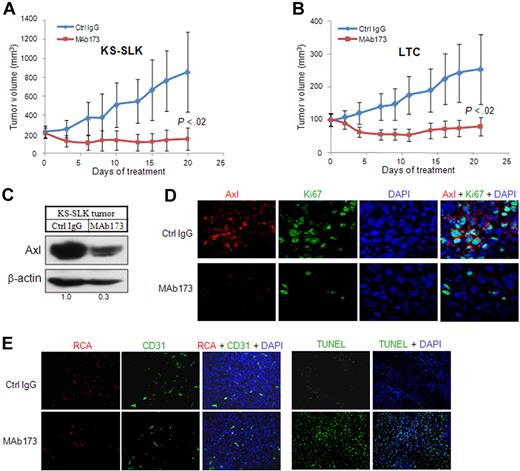
To evaluate whether mAb inhibition of Axl affects tumor cell growth in vivo, KS-SLK and KSHV-transformed endothelial cells (LTC) were implanted subcutaneously in nude mice. When the tumor size reached 200 mm3 for KS-SLK and 100 mm3 for LTC (day 0, Figure 6A), animals were randomized and treated with either Axl MAb173 or a control IgG at 10 mg/kg, 3 times a week. MAb173 significantly induced tumor regression in KS-SLK and LTC tumor growth compared with control. On day 20, KS-SLK tumor volume was reduced by 50% from the initial volume and was only 12% of the tumor volume of the control group (Figure 6A). LTC tumors grew slower. On day 7, tumor volume of those treated by MAb173 was 50% of the initial volume. On day 20, tumor volume was reduced by 20% from the initial volume in the MAb173-treated group (Figure 6B). At the end of the experiments, tumors were harvested for various analyses. Degradation of Axl receptor in MAb173-treated KS-SLK tumor was confirmed by Western blot (Figure 6C) and immunostaining (Figure 6D). Proliferation index measured by Ki67 staining showed marked reduction in the MAb173-treated group (Figure 6D). Apoptosis was measured by TUNEL assay, which showed a significant increase in the MAb173-treated group (Figure 6E). In addition, vessel perfusion (RCA, Figure 6E) was moderately impaired after MAb173 treatment, accompanied by slightly reduced vessel density (CD31 staining, Figure 6E). Similar results were obtained from the analysis of LTC tumors (data not shown).
MAb173 inhibits KS tumor growth in vivo. (A) Athymic mice were implanted with 2 × 106 KS-SLK cells. When tumor sizes reached approximately 200 mm3, mice were randomly assigned to treatment groups (5 per group) (day 0). Mice were then treated by intraperitoneal injection of MAb173 (10 mg/kg) or Ctrl IgG, 3 times a week for 20 days. Tumor volume was measured 3 times a week. The P value was calculated by Student t test. (B) LTC xenograft study was performed as in panel A, except that the treatment was started when the tumor sizes were approximately 100 mm3. (C) KS-SLK tumor tissues harvested at the end of the experiment were subjected to Western blot analysis with Axl antibody (rabbit monoclonal). (D) Immunostaining of KS-SLK tumors with Axl antibody (red) and Ki67 antibody (green). Images were taken with a Carl Zeiss confocal microscope (100× objective) and LSM software. (E) Just before tissue harvest, mice were infused with RCA-Lectin. Perfused vessels were localized by RCA-Lectin, and microvascular endothelial cells were localized by CD31 staining. Apoptosis was examined with TUNEL assay. Nuclei were counterstained with DAPI. Images were taken with an Olympus AX70 fluorescence microscope (20× objective) and Spot Version 2.2.2 digital imaging system.
MAb173 inhibits KS tumor growth in vivo. (A) Athymic mice were implanted with 2 × 106 KS-SLK cells. When tumor sizes reached approximately 200 mm3, mice were randomly assigned to treatment groups (5 per group) (day 0). Mice were then treated by intraperitoneal injection of MAb173 (10 mg/kg) or Ctrl IgG, 3 times a week for 20 days. Tumor volume was measured 3 times a week. The P value was calculated by Student t test. (B) LTC xenograft study was performed as in panel A, except that the treatment was started when the tumor sizes were approximately 100 mm3. (C) KS-SLK tumor tissues harvested at the end of the experiment were subjected to Western blot analysis with Axl antibody (rabbit monoclonal). (D) Immunostaining of KS-SLK tumors with Axl antibody (red) and Ki67 antibody (green). Images were taken with a Carl Zeiss confocal microscope (100× objective) and LSM software. (E) Just before tissue harvest, mice were infused with RCA-Lectin. Perfused vessels were localized by RCA-Lectin, and microvascular endothelial cells were localized by CD31 staining. Apoptosis was examined with TUNEL assay. Nuclei were counterstained with DAPI. Images were taken with an Olympus AX70 fluorescence microscope (20× objective) and Spot Version 2.2.2 digital imaging system.
Discussion
Axl is a receptor tyrosine kinase with transforming activity4 and has been shown to play a key role in the survival and metastasis of many tumors.27,29,31 We are the first to demonstrate the expression of Axl and its ligand Gas6 in KS tumor samples, KS cell lines, and endothelial cells latently infected by KSHV (LTC). However, Axl expression could not be further induced in the KSHV lytic phase; and consistent with this observation, lytic proteins, including vGPCR and vIL6, could not induce Axl expression. Among 4 major latency proteins LANA, vFLIP, vCyclin, and Kaposin B, we found that only vFLIP could induce Axl expression. Axl expression was also elevated in HUVEC cells on vFLIP induction. In addition, only the NF-κB inhibitor abolished Axl induction in HUVECs expressing vFLIP or latently infected by KSHV, whereas inhibitors of PI3K and ERK had no effect. This is consistent with the fact that vFLIP functions through the NF-κB pathway. How NF-κB regulates Axl expression needs further investigation.
These findings led us to investigate the mechanism of Axl induction in KS cell lines that no longer carry KSHV and also lack vFLIP expression. The NF-κB inhibitor failed to reduce Axl level in KS-IMM cells, indicating that KS cells use alternative mechanisms to maintain elevated Axl levels after the loss of KSHV. We first checked the gene copy number of AXL in KS cells and did not find any change. We then examined the methylation status of AXL promoter and found much less extent of methylation in KS cell lines. Hence, this epigenetic regulation probably accounts for up-regulation of Axl in KS cells. We also found hypomethylation in a cancer cell line A549, which has elevated Axl expression and is sensitive to Axl knockdown based on our data and previous report.31 Combined with hypomethylation of AXL promoter and elevated Axl expression in the human colorectal carcinoma cell line RKO40 and acute myeloid leukemia cell U937,30 hypomethylation may be a universal phenomenon in tumor cells with elevated Axl expression.
Other TAM members Mer and Tyro3 were not found in KS tumors. These 2 proteins are distinct from Axl both in function and expression profile. Tyro3 is mainly expressed in the neural system and probably functions as a neurotrophic factor receptor, whereas Mer is mainly expressed in blood cells and functions in phagocytosis.7 Recent studies have revealed that all TAM receptors are involved in innate immunity.44 However, unlike Axl, there is no consistent evidence showing expression of Mer and Tyro3 in cancer.7
We next determined the role of Axl in KS cell lines and KSHV-infected endothelial cell line by knocking down Axl expression using siRNA. Axl knockdown inhibited growth and invasion of these cells, suggesting that Axl may be one of the critical oncogenes in KS pathogenesis. Axl-Gas6 signaling has been well established in vascular biology, specifically its role in survival of endothelial and pericytes/vascular smooth muscle cells.10 Axl expression is particularly induced in injured arteries both in endothelial and pericytes.10 Consistently, in this study, we show that Axl knockdown inhibited the growth and invasion of HUVECs. The activation and role of Axl in KS cells are also consistent with their origin from endothelial cells and coexpression of smooth muscle/pericyte specific markers, such as smooth muscle actin, calponin, and desmin.36 Axl activation by Gas6 in KS cells induces the PI3K/AKT pathway, whereas Axl knockdown impaired basal PI3K signaling, suggesting that Axl-Gas6 signaling constitutes a major fraction of PI3K signaling in these cells. Axl-Gas6 also regulates scavenger receptors in macrophages and phagocytic function.44 Thus, Axl-Gas6 may be induced in KS tissues in response to extravasation of blood components in large vascular spaces within the tumor. Axl-Gas6 may also be induced in tumor vessels by factors secreted by KS tumor cell to both promote vascular proliferation and prevent cell apoptosis. Targeting Axl may thus have dual functions: targeting KS tumor cells and targeting tumor vasculature. Besides, Axl expression can also be induced in stromal cells27 ; thus, additional functions of Axl in KS remain to be investigated.
The multiple roles that Axl may play in KS tumorigenesis make it an attractive therapeutic target. We have generated monoclonal antibodies and screened antibodies that could inhibit tumor cell growth/invasion in vitro and reduce tumor growth in vivo. We selected one monoclonal antibody MAb173, which significantly inhibited tumor growth in KS-SLK and LTC xenograft models. Biochemical studies suggested that this antibody might function through degradation of the Axl receptor. Persistent MAb173 treatment degraded Axl receptor and eventually impaired PI3K signaling. Neutralizing antibody that could block Gas6-Axl interaction was thought to be another category of therapeutic antibodies. However, the neutralizing antibodies we obtained failed to show any activity in in vitro proliferation/invasion assay (data not shown). It is known that Axl can be activated by mechanisms other than Gas6 interaction. For example, Axl can be trans-activated by IL-15 through the interaction of Axl with the IL-15 receptor.14 Axl can also be activated by reactive oxygen species45 and ligand-independent dimerization of Axl intracellular domain.46 Therefore, blocking the interaction of Gas6 and Axl may not be enough to abolish the signaling mediated by Axl.
Analysis of tumor tissue revealed that the tumors treated with MAb173 had more cells undergoing apoptosis and less cells proliferating, compared with tumors treated with control antibody. MAb173 reduced Axl level in tumor cells, and this reduction was retained until the end of 3 weeks of treatment. Even though Axl knockdown in KS tumor cells in vitro only resulted in moderate growth inhibition, tumor growth was significantly inhibited in vivo by reduction of Axl, suggesting that the response occurs in the context of other cell types in the tumor microenvironment. Considering that MAb173 inhibits tumor cell invasion in vitro, inhibition of tumor metastases may significantly contribute to its biologic activity in vivo. In the future, we will consider using KS cell lines that have more metastasis potential, including KS-Y1. The KS-Y1 cell line was isolated from AIDS-associated KS lesion, which promotes angiogenesis and metastasis in immunodeficient mice.47
MAb173 could inhibit endothelial cell (HUVEC) invasion in vitro. However, because it does not recognize murine Axl, it can only target human tumor cells, not murine vasculature in our KS xenograft models. We did observe some effect on vasculature with MAb173 treatment, which may have resulted from the impaired interaction between tumor cells and endothelial/mural cells. For example, the angiogenic cytokine profile of tumor cells might be altered. Thus, we predict it will be more efficient in inhibiting tumor growth in humans, targeting directly both tumor cells and tumor vasculature. Moreover, MAb173 may have potential beyond treating KS. It is probably effective in the treatment of other cancers in which Axl plays a critical role in tumorigenesis and metastasis.48
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the National Cancer Institute (RO1 CA 079218-07, P.S.G.; and RO1CA085177, P.M.C.) and AIDS Malignancy Consortium (CA082057 and CA115284, J.J.).
National Institutes of Health
Authorship
Contribution: P.S.G., R.L., M.G., J.J., and P.M.C. designed the studies; P.S.G., R.L., and M.G. wrote the paper; and R.L., M.G., X.L., Y.Z., W.G., A.T., P.S.G., and J.J. performed research and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Parkash S. Gill, University of Southern California, Norris Hospital, NOR 6332, 1441 Eastlake Ave, Los Angeles, CA 90033; e-mail: parkashg@usc.edu.
References
Author notes
R.L. and M.G. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal