Abstract
Suppressor of cytokine signaling-3 (SOCS3) is the main intracellular regulator of signaling by granulocyte colony-stimulating factor, an immune-modulatory cytokine used to mobilize stem cells for transplantation. We have therefore studied the contribution of SOCS3 to the spectrum of graft-versus-host disease (GVHD) after allogeneic stem cell transplantation (SCT). Grafts from SOCS3−/Δvav donor mice in which SOCS3 deficiency is restricted to the hematopoietic compartment had an augmented capacity to induce acute GVHD. With the use of SOCS3−/ΔLysM and SOCS3−/Δlck donors in which SOCS3 deficiency was restricted to the myeloid or T-cell lineage, respectively, we confirmed SOCS3 deficiency promoted acute GVHD mortality and histopathology within the gastrointestinal tract by effects solely within the donor T cell. SOCS3−/Δlck donor T cells underwent enhanced alloantigen-dependent proliferation and generation of interleukin-10 (IL-10), IL-17, and interferon-γ (IFNγ) after SCT. The enhanced capacity of the SOCS3−/Δlck donor T cell to induce acute GVHD was dependent on IFNγ but independent of IL-10 or IL-17. Surprisingly, SOCS3−/Δlck donor T cells also induced severe, transforming growth factor β– and IFNγ-dependent, sclerodermatous GVHD. Thus, the delivery of small molecule SOCS3 mimetics may prove to be useful for the inhibition of both acute and chronic GVHD.
Introduction
Graft-versus-host disease (GVHD) is the main limitation of hematopoietic stem cell transplantation (SCT) and may be acute or chronic in nature. Acute GVHD occurs early after transplantation in the context of a T helper type 1 (Th1)–dominant “cytokine storm” that causes characteristic apoptosis in target tissues (gastrointestinal tract, liver, and skin). Later chronic forms of the disease are characterized by fibrosis and the development of scleroderma.1 The induction of GVHD depends on the presentation of host alloantigen by antigen-presenting cells to naive donor T cells.2,3 To date, most therapeutic strategies to prevent or treat GVHD involve the depletion of T cells or suppression of critical molecular pathways involved in T-cell activation and proliferation (eg, limiting the production or access to interleukin-2 [IL-2]). Indeed, the access of the donor T cell to exogenous cytokine cues is critical to their propensity to induce inflammation after transplantation and centers on activation of the relevant Janus kinase (JAK)–signal transducer and activator of transcription (STAT) proteins after the interaction of a cytokine with its surface receptor. Thus, the activation of STAT4 (by IL-12) induces interferon-γ (IFNγ) and Th1 differentiation that is perpetuated by subsequent STAT1 activation. In contrast, signaling by IL-4 characteristically activates STAT6 and promotes Th2 differentiation, whereas STAT3 activation (eg, by IL-6) invokes Th17 differentiation.4
Suppressor of cytokine signaling (SOCS) proteins are key regulators of immune responses and exert their effects in a classic negative feedback loop.5 SOCS3 is transiently expressed by multiple lineages of cells within the immune system and functions predominantly as a negative regulator of cytokines that activate the JAK-STAT3 pathway. The method of signal inhibition appears to differ in response to diverse stimuli. In the case of IL-6, SOCS3 binds with high affinity to the gp130 receptor (IL-6R/IL-11R) and granulocyte colony-stimulating factor receptor (G-CSFR), and subsequently inhibits JAK kinase activity.6,7 The SOCS proteins can also target bound proteins for proteasomal degradation8 and thus act to regulate excessive cytokine function by inhibition of both receptor stability and downstream signal transduction.
SOCS3 can suppress Toll-like receptor (TLR) and IL-1 signaling in myeloid cells (eg, macrophages) by inhibition of the tumor necrosis factor (TNF) receptor–associated factor 6–transforming growth factor-β (TGFβ)/activated kinase 1 transcription complex9 and modulates, in part, the negative regulation of IL-6 signaling induced by TLR signaling.10 It also has differential effects on IL-6 and IL-10 signaling (both STAT3-dependent) by virtue of its ability to bind to the IL-6 receptor (IL-6R) and to suppress STAT3 function.11 However, SOCS3 does not bind to the IL-10R, and ligation of the receptor by IL-10 results in prolonged STAT3 activation, which appears to inhibit inflammatory cytokine generation.12 The absence of SOCS3 and sustained action of STAT3 in T cells appears to result in increased secretion of TGFβ and IL-10 and the subsequent promotion of (induced or Tr1) regulatory T-cell function.13 However, it was suggested that naturally occurring (FoxP3+) regulatory T cells themselves lack SOCS3 protein14 and that, in the absence of STAT3, regulatory T cells are impaired in their ability to suppress Th17 responses.15 In contrast, Th2 cells contain high amounts of SOCS3 relative to Th1-differentiated effectors,16 and SOCS3 inhibits IL-12–induced STAT4 activation by binding to IL-12R.17 Importantly, the Th17 differentiation induced by IL-6 and IL-23 is mediated by STAT3 activation and is suppressed by SOCS3.18 Furthermore, TGFβ appears to drive Th17 differentiation by suppressing SOCS3.19 IL-1–induced SOCS3 also appears to inhibit Th17 differentiation,20 although this probably depends on inhibition of the TNF receptor-associated factor 6–TGFβ/activated kinase 1 transcription complex rather than STAT3.9
Therefore, SOCS3 appears an attractive therapeutic target for the modulation of immune-mediated pathology characterized by excessive cytokine responses. We have now studied the effect of SOCS3 on the outcome of transplantation in relation to GVHD. These studies used donor mice expressing a conditional allele of SOCS3 flanked by loxP sites that were intercrossed with mice expressing vav-cre, LysM-cre, or lck-cre transgenes to conditionally delete SOCS3 from the hematopoietic compartment, the myeloid compartment, or T cells, respectively, as previously described.21-23 Because SOCS3 is the main negative regulator of G-CSF signaling and this cytokine is routinely administered to donors to mobilize stem cells, the pathway is particularly relevant to allogeneic stem cell transplantation.
Methods
Mice
Female C57Bl/6j (H-2b) and B6D2F1 (H-2b/d) mice were purchased from the Animal Resources Center. LP/J (H-2b) mice were imported from The Jackson Laboratory. Socs3−/Δvav, Socs3−/Δlck, and Socs3−/ΔLysM SOCS3-deficient mice (all on a C57Bl/6j background, H-2b) were generated with a conditional gene targeting approach with the use of the Cre-loxP system as previously described.21 Littermate SOCS3loxP/loxP mice, without the Cre transgene, were used as controls and are hereafter referred to as wild-type (WT). Mice were housed in sterilized microisolator cages and received acidified autoclaved water (pH 2.5) after transplantation. All mouse experiments were performed under the authorization of and in accordance with the Queensland Institute of Medical Research's Animal Ethics Committee.
Cytokine treatment
Recombinant human G-CSF (Amgen) was diluted in saline (Baxter Healthcare) before injecting subcutaneously into mice (10 μg/animal/day) once daily from day −4 to day −1.
Cell preparation
Hematopoietic stem cell transplantation
Mice received a transplant as described previously.26 Briefly, on day −1, B6D2F1 mice received 1100 cGy total body irradiation (TBI; 137Cs source), split into 2 doses separated by 3 hours to minimize gastrointestinal toxicity. For GVHD test groups, unfractionated splenocytes containing 2 × 106 CD3 T cells from naive or G-CSF–treated donors were administered through the tail vein. For the B6 → LP/J model of scleroderma, LP/J recipients received 900 cGy TBI split into 2 doses, followed by intravenous transfer of 25 × 106 splenocytes 24 hours later. In both models, T cell–depleted spleen was administered to non-GVHD control animals. In bone marrow transplantation (BMT) studies 5 × 106 bone marrow cells were added to 2 × 106 splenic T cells from respective naive donor strains (purified by negative selection to > 85% purity as previously described27 ) and injected by tail vein injection the day after TBI. Animal procedures were undertaken with the use of protocols approved by the institutional animal ethics committee (Queensland Institute of Medical Research). In some experiments, recipient mice were given (mouse-specific) anti–IL-10R (1B1.3a), anti–IL-17 monoclonal antibody (mAb; M210; Amgen), anti-IFNγ (XMG1.2 or H22 in B6D2F1 and LP/J recipients, respectively), anti-TGFβ1 (1D11), or irrelevant rat/hamster isotype control mAb at 100 μg to 1000 μg/dose, by intraperitoneal injection on alternate days from day 3 to 30 after transplantation or as indicated. These antibodies have all been shown to be biologically active (as assessed by modification of cytokine responses) by us (data not shown) and other investigators.28-30
Assessment of GVHD
The degree of systemic GVHD was assessed by scoring as previously described (maximum index = 10).31 Scores were generated according to animal movement, posture, fur ruffling, skin integrity, and weight loss. Mice that received a transplant were monitored daily, and those with GVHD clinical scores of 6 or greater were killed, and the date of death was registered as the next day in accordance with institutional animal ethics committee guidelines.
Carboxyfluorescein succinimidyl ester labeling
After red cell lysis, splenocytes were resuspended in serum-free medium containing 5μM carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes) and incubated at 37°C for 10 minutes. Cells were washed 2 times in media containing 2% fetal calf serum and resuspended in L15 (Gibco) before transplantation. T-cell proliferation was measured by CFSE dilution and analyzed with the use of Modfit Software (Verity Software House) as previously described.32 Calculated proliferation index = Σ all cells/computed number of parent cells.
Antibodies
The following mAbs were purchased from BioLegend: fluorescein isothiocyanate–conjugated anti-Ly6C (AL-21); phycoerythrin-conjugated CD3 (145-2C11), CD4 (GK1.5), CD19 (6D5), anti-Ly6G (IA8), anti-TNF (MP6-XT22), anti–IL-17A (TC11-18H10.1), anti-IFNγ (XMG1.2), rat immunoglobulin G2b (IgG2b) isotype control; allophycocyanin-conjugated CD8 (53-6.7), anti–IL-10 and IgG2b isotype control; Alexa Fluor 647 anti–mouse FoxP3 antibody and IgG2b isotype control. Fluorescein isothiocyanate–conjugated CD4 (GK1.5) and IgG2b (Mac5) isotype control, purified CD3 (2C11), CD4 (RL172.4), CD8 (TIB211), Thy1.2 (HO-13-4), FcγR II/III (2.4G2), anti–IL-10R (1B13a), and TGFβ1 (1D11) were produced in house.
Cell culture
Unfractionated splenocytes were plated into 96-well tissue culture plates at 5 × 104 CD3 T-cell equivalents/well and stimulated with soluble CD3 (2C11; 2 μg/mL). Plates were incubated at 37°C for 72 hours before tissue culture supernatants were harvested and [3H]-thymidine (1 μCi/well [0.037 Bq/well]) was added. Twelve to 16 hours later, cultures were harvested onto glass fiber filter mats and [3H] thymidine incorporation determined with the use of a 1205 Betaplate reader (Wallac Turku).
Cell staining
For cell-surface labeling, cells were first incubated with anti-FcγR II/III for 15 minutes at 4°C, followed by the relevant conjugated mAb for 30 minutes at 4°C. Labeled cells were washed twice with phosphate-buffered saline/0.2% bovine serum albumin and analyzed with a FACSCalibur (BD Biosciences). 7-Amino-actinomycin D was added in the final wash to label dead cells. For intracellular cytokine staining, ex vivo donor splenocytes were stimulated at 2 × 106 splenocytes/mL with phorbol myristate acetate (50 ng/mL; Sigma-Aldrich) and ionomycin (500 ng/mL; Sigma) and Brefeldin A (1:1000 dilution; BioLegend) for 4 hours. Cells were processed for intracellular cytokine staining with the use of the BD Cytofix/Cytoperm Kit (BD Biosciences) as per the manufacturer's protocol and analyzed by flow cytometry (FACSCanto; BD Biosciences). Intracellular FoxP3 staining was performed on freshly isolated naive or ex vivo splenocytes with the use of a BioLegend FoxP3 fix/perm buffer set (BioLegend) per the manufacturer's protocol.
Cytokine analysis
Tissue culture supernatant IFN-γ, TNF, IL-2, IL-4, IL-5, IL-6, and IL-10 were determined with the use of the BD Flex Cytometric Bead Array system (BD PharMingen). Tissue culture supernatant IL-17A, IL-17F, and TGFβ were quantified by enzyme-linked immunoabsorbent assay using commercial kits from BioLegend, R&D Systems, and Invitrogen, respectively. All assays were performed according to the manufacturer's protocol.
Histology
At various times after transplantation, GVHD target tissue was harvested, formalin-preserved, embedded in paraffin, and processed to generate 5-μm-thick sections. Hematoxylin and eosin–stained sections of gut (small bowel), liver, and skin were examined in a blinded fashion by one investigator (A.D.C.) with the use of a semiquantitative scoring system for GVHD as previously published.27,33 Scores on tissue taken from animals with acute GVHD were added to provide a total score of 28 for gut, 40 for liver, and 24 for skin. For quantification of scleroderma in the B6 → LP/J model, blinded hematoxylin and eosin samples were scored from 0 to 4 for epidermal inflammation and dermal inflammation, epidermal fibrosis, dermal fibrosis, subcutaneous fibrosis, and loss of subcutaneous fat (total score = 24). Images of GVHD target tissue were acquired using an Olympus BX51 microscope, an Evolution MP 5.0 Camera, and Qcapture software (Qimaging).
Statistical analysis
Survival curves were plotted with Kaplan-Meier estimates and compared by log-rank analysis with the use of Prism 5 software (GraphPad Software). The Mann-Whitney U test was used for the statistical analysis of cytokine data, T-cell function, and clinical scores (Prism 5 software). P values less than .05 were considered statistically significant. Data are presented as mean plus or minus SEM.
Results
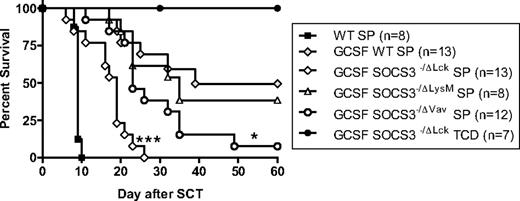
In initial experiments we examined the effects of G-CSF mobilization and SOCS3 deficiency on GVHD in the B6 → B6D2F1 model of acute GVHD directed to both major and minor histocompatibility antigens. GVHD induced in this model is severe with all recipients of control WT grafts dying within 10 days with characteristic features (diarrhea, weight loss, hunching) of GVHD (Figure 1). As previously reported, recipients of grafts from G-CSF–treated WT donors had significantly improved survival at day 60 compared with recipients of non–mobilized allogeneic WT grafts (50% vs 0%; P < .001). The absence of SOCS3 from all hematopoietic cells exacerbated acute GVHD because recipients of grafts from G-CSF–treated Socs3−/Δvav donors had reduced survival at day 60 (50% vs 8%; P < .05). To examine the lineage-specific role of SOCS3, these experiments also used Socs3−/ΔLysM mice in which SOCS3 deletion is restricted to the myeloid compartment in isolation and Socs3−/Δlck in which SOCS3 is absent only from the T-cell compartment. The expression of SOCS3 within the myeloid compartment did not contribute to acute GVHD because there was no significant difference in survival between recipients of G-CSF–treated WT or Socs3−/ΔLysM grafts. In contrast, a profound increase in acute GVHD mortality occurred when the T-cell compartment was SOCS3 deficient (GCSF WT vs G-CSF Socs3−/Δlck; 50% vs 0%; P < .01). The non-GVHD control group receiving T cell–depleted Socs3−/Δlck grafts all survived without features of GVHD. Thus, the protection against acute GVHD afforded by stem cell mobilization with G-CSF depends on SOCS3 activity within the donor T cell, whereas SOCS3 within the donor myeloid (ie, antigen-presenting cell [APC]) compartment is irrelevant.
SOCS3 within donor T cells attenuates GVHD after G-CSF–mobilized allogeneic SCT. Donor B6 mice were treated with G-CSF (10 μg/animal per day for 4 days) or were untreated. Unfractionated splenic grafts containing 2 million T cells were transplanted into lethally irradiated (1100 cGy) B6D2F1 recipient mice. Survival curves by Kaplan-Meier analysis, pooled from 2 experiments. ***P < .001 for recipients of G-CSF Socs3−/Δlck versus G-CSF WT spleen; *P < .05 for recipients of G-CSF SOCS3−/Δvav versus G-CSF WT spleen.
SOCS3 within donor T cells attenuates GVHD after G-CSF–mobilized allogeneic SCT. Donor B6 mice were treated with G-CSF (10 μg/animal per day for 4 days) or were untreated. Unfractionated splenic grafts containing 2 million T cells were transplanted into lethally irradiated (1100 cGy) B6D2F1 recipient mice. Survival curves by Kaplan-Meier analysis, pooled from 2 experiments. ***P < .001 for recipients of G-CSF Socs3−/Δlck versus G-CSF WT spleen; *P < .05 for recipients of G-CSF SOCS3−/Δvav versus G-CSF WT spleen.
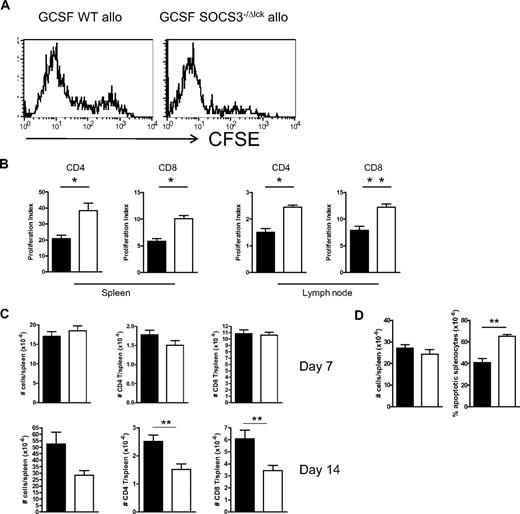
Because T cell–restricted SOCS3 modulated GVHD outcome, we next compared G-CSF–treated WT or Socs3−/Δlck grafts for cellular composition and T-cell function. After mobilization with G-CSF the total cell count and T-cell (CD3+), B-cell (CD19+), natural killer cell (NK1.1+), neutrophil (Ly6G+), and monocyte/dendritic cell (Ly6C+Ly6G−) numbers, and proportions (data not shown) did not significantly differ between spleens harvested from WT or Socs3−/Δlck donors (Figure 2A). In addition, there was no difference in the number or frequency of naive or memory populations within the CD4+ or CD8+ T-cell compartment according to CD44 expression (data not shown). However, in response to T-cell receptor ligation with a CD3 mAb, splenocytes from G-CSF–treated Socs3−/Δlck mice exhibited enhanced proliferation and cytokine production (IFNγ, IL-10, IL-17A, IL-17F, TGFβ, and TNF) compared with splenocytes from G-CSF–treated WT mice (Figure 2B-C). This was also true when purified T cells were stimulated on plate-bound CD3/CD28, regardless of donor mobilization with G-CSF (Figure 2D). Thus, there appeared to be an intrinsic defect in Socs3−/Δlck T cells that promoted IFNγ secretion. To compare the proliferation of SOCS3-replete and -deficient T cells induced by allogeneic transplantation we transferred CFSE-labeled G-CSF–treated WT or Socs3−/Δlck splenic grafts and monitored CFSE dilution in the spleens and lymph nodes 72 hours later. The extent of CFSE dilution of splenic CD4 T cells was greater in recipients of G-CSF–treated Socs3−/Δlck grafts than in recipients of G-CSF–treated WT grafts (Figure 3A). Furthermore, analysis of CFSE dilution with Modfit software to determine the proliferation index confirmed a significant increase in CD4+ and CD8+ T-cell proliferation in the spleens and lymph nodes of recipients of G-CSF–treated Socs3−/Δlck grafts (Figure 3B). However, by day 7 after transplantation, spleen size and CD4+ and CD8+ T-cell numbers were equivalent in recipients of either G-CSF–treated WT or Socs3−/Δlck splenic grafts, and by day 14 T-cell numbers were significantly reduced in recipients of Socs3−/Δlck splenic grafts (Figure 3C). This contraction within the T-cell compartment was a result of increased apoptosis in Socs3−/Δlck graft recipients as shown by increased proportion of Annexin V+7-amino-actinomycin D− cells within splenocytes after SCT (Figure 3D). Examination of T-cell cytokine production on day 7 after transplantation showed a significant increase in IL-10, IL-17, and IFNγ production by splenocytes from recipients of G-CSF–treated Socs3−/Δlck grafts compared with G-CSF–treated WT grafts. In contrast there was no difference in the levels of TNF, IL-6, or IL-2 (Figure 4A). The increase in IFNγ secretion after SCT was mainly in CD4 T cells, whereas the increase in IL-17 was restricted to T cells in either fraction that were also producing IFNγ (Figure 4B). Finally, we were unable to detect any defect of regulatory (FoxP3+) T cells in the absence of SOCS3, either before or after transplantation (data not shown), consistent with the described absence of SOCS3 protein in this cell population.14
SOCS3−/ΔLck T cells are hyperresponsive to T-cell receptor ligation after G-CSF mobilization. (A) Size and cell subset composition of spleens from G-CSF–treated WT or SOCS3−/ΔLck mice. Data are representative of 3 similar experiments (n = 3 animals/group). DN indicates double negative. (B) Splenocytes from G-CSF–treated WT or SOCS3−/ΔLck mice were cultured in the presence of soluble CD3, and proliferative responses were assessed by [3H] thymidine incorporation. (C) Supernatants from CD3 cultures were harvested at 72 hours and assayed for cytokines by cytometric bead array or enzyme-linked immunoabsorbent assay (IL-17A, IL-17F, and TGFβ). Data are pooled from 2 independent experiments, n = 4-6 animals/group. *P < .05; ** P < .01. (D) IFNγ secretion by purified CFSE-labeled T cells at the times indicated after stimulation with plate-bound CD3 and CD28. Data are representative of 2 experiments.
SOCS3−/ΔLck T cells are hyperresponsive to T-cell receptor ligation after G-CSF mobilization. (A) Size and cell subset composition of spleens from G-CSF–treated WT or SOCS3−/ΔLck mice. Data are representative of 3 similar experiments (n = 3 animals/group). DN indicates double negative. (B) Splenocytes from G-CSF–treated WT or SOCS3−/ΔLck mice were cultured in the presence of soluble CD3, and proliferative responses were assessed by [3H] thymidine incorporation. (C) Supernatants from CD3 cultures were harvested at 72 hours and assayed for cytokines by cytometric bead array or enzyme-linked immunoabsorbent assay (IL-17A, IL-17F, and TGFβ). Data are pooled from 2 independent experiments, n = 4-6 animals/group. *P < .05; ** P < .01. (D) IFNγ secretion by purified CFSE-labeled T cells at the times indicated after stimulation with plate-bound CD3 and CD28. Data are representative of 2 experiments.
SOCS3 within T cells limits T-cell proliferation and apoptosis after allogeneic SCT. Splenocytes from G-CSF–treated WT or SOCS3−/ΔLck mice were CFSE labeled, and grafts containing 2 million T cells were transplanted into lethally irradiated (1100 cGy) B6D2F1 recipient mice. (A) Spleens were harvested from recipients 3 days later, and CFSE dilution in the CD4+ T-cell compartment was examined by flow cytometry. (B) Modfit CFSE dilution analysis of splenic and lymph node T-cell proliferation. Data are representative of 2 similar experiments with 4 to 5 animals/group. (C) Splenocytes from G-CSF–treated WT or SOCS3−/ΔLck mice were transplanted into lethally irradiated (1100 cGy) B6D2F1 recipient mice. On days 7 and 14 after transplantation, spleens were harvested, total cellularity was determined with an automated cell counter, and CD4+ and CD8+ T cells were quantified by flow cytometry. (D) On day 7 after transplantation, spleens were stained with annexin V and 7-amino-actinomycin (7AAD), and the frequency of apoptotic splenocytes (annexin V+7AAD−) was determined by flow cytometric analysis. Black bars and white bars represent G-CS–treated WT or SOCS3−/ΔLck mice, respectively.
SOCS3 within T cells limits T-cell proliferation and apoptosis after allogeneic SCT. Splenocytes from G-CSF–treated WT or SOCS3−/ΔLck mice were CFSE labeled, and grafts containing 2 million T cells were transplanted into lethally irradiated (1100 cGy) B6D2F1 recipient mice. (A) Spleens were harvested from recipients 3 days later, and CFSE dilution in the CD4+ T-cell compartment was examined by flow cytometry. (B) Modfit CFSE dilution analysis of splenic and lymph node T-cell proliferation. Data are representative of 2 similar experiments with 4 to 5 animals/group. (C) Splenocytes from G-CSF–treated WT or SOCS3−/ΔLck mice were transplanted into lethally irradiated (1100 cGy) B6D2F1 recipient mice. On days 7 and 14 after transplantation, spleens were harvested, total cellularity was determined with an automated cell counter, and CD4+ and CD8+ T cells were quantified by flow cytometry. (D) On day 7 after transplantation, spleens were stained with annexin V and 7-amino-actinomycin (7AAD), and the frequency of apoptotic splenocytes (annexin V+7AAD−) was determined by flow cytometric analysis. Black bars and white bars represent G-CS–treated WT or SOCS3−/ΔLck mice, respectively.
SOCS3−/ΔLck T cells exhibit dysregulated cytokine production after allogeneic SCT. Splenocytes from G-CSF–treated WT or SOCS3−/ΔLck mice were transplanted into lethally irradiated (1100 cGy) B6D2F1 recipient mice, and at day 7 after transplantation splenocytes were examined for cytokine production. (A) Splenocytes were cultured for 24 hours with soluble CD3, and supernatants were collected and assayed for cytokines by cytometric bead array. Data represent mean ± SEM of pooled results from 2 similar experiments (n = 10 animals/group). Black bars and white bars represent G-CSF–treated WT or SOCS3−/ΔLck mice, respectively. (B) After 4-hour culture with phorbol myristate acetate and ionomycin, cell cytokine production was analyzed by intracellular cytokine staining with 4-color flow cytometry. Numbers in quadrants represent the percentage of gated CD4 or CD8 T cells as indicated.
SOCS3−/ΔLck T cells exhibit dysregulated cytokine production after allogeneic SCT. Splenocytes from G-CSF–treated WT or SOCS3−/ΔLck mice were transplanted into lethally irradiated (1100 cGy) B6D2F1 recipient mice, and at day 7 after transplantation splenocytes were examined for cytokine production. (A) Splenocytes were cultured for 24 hours with soluble CD3, and supernatants were collected and assayed for cytokines by cytometric bead array. Data represent mean ± SEM of pooled results from 2 similar experiments (n = 10 animals/group). Black bars and white bars represent G-CSF–treated WT or SOCS3−/ΔLck mice, respectively. (B) After 4-hour culture with phorbol myristate acetate and ionomycin, cell cytokine production was analyzed by intracellular cytokine staining with 4-color flow cytometry. Numbers in quadrants represent the percentage of gated CD4 or CD8 T cells as indicated.
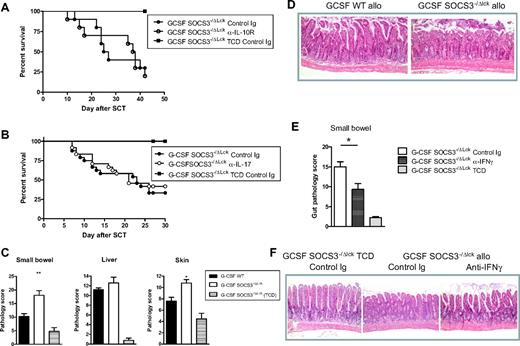
We next tested whether the dysregulation of IL-10, IL-17, or IFNγ production by donor T cells after transplantation contributed to the enhanced GVHD induced in the absence of SOCS3. Blockade of IL-10 signaling with an IL-10R-blocking mAb from day 7 after transplantation onward did not significantly affect survival (Figure 5A). The delayed administration of the anti–IL-10R blocking mAb was used because IL-10R blockade within the first week clearly enhanced GVHD regardless of SOCS3, consistent with the known protective effect of IL-10 early after BMT (data not shown).34 However, in neither setting did IL-10R blockade result in the attenuation of GVHD, as would be expected if the overproduction of this cytokine in the absence of SOCS3 was contributing to GVHD after transplantation. Similarly, IL-17 overexpression was not responsible, because the treatment of transplant recipients with an IL-17A–blocking mAb had no effect on GVHD outcome, whether administered from day 0 onward (Figure 5B) or from day 7 onward (data not shown). Histologic assessment of GVHD target tissues 7 days after transplantation showed a significant increase in the pathology scores in the gut and skin of recipients of G-CSF Socs3−/Δlck grafts compared with that of recipients of G-CSF WT grafts (Figure 5C-D). We have reported that IFNγ production after transplantation promotes Th1 differentiation and induces acute GVHD of the gastrointestinal tract.33 We next sought to study whether exaggerated generation of IFNγ from Socs3−/Δlck T cells was causally related to the augmentation of acute GVHD. However, because the absence of IFNγ after transplantation results in rapid induction of fatal idiopathic pneumonia syndrome,33 we used gastrointestinal tract histopathology (which is exquisitely IFNγ dependent33 ) as a read out rather than mortality. As shown in Figure 5E and F, blockade of IFNγ after SCT significantly reduced the severe gastrointestinal GVHD seen after transplantation of Socs3−/Δlck grafts, confirming this as the pathogenic pathway. It should be noted that this does not imply that SOCS3 controls gastrointestinal GVHD exclusively, merely that disease is further exacerbated by enhanced IFNγ production in the absence of regulation by SOCS3. We next studied whether the escalation of acute GVHD induced by Socs3−/Δlck T cells depended on signaling by G-CSF by performing BMT in the absence of cytokine mobilization. As shown in Figure 6, this was not the case because acute GVHD was also enhanced in recipients of steady-state bone marrow and T-cell grafts, and this effect again was due to the absence of SOCS3 within the T-cell compartment.
Enhanced IFNγ generation by SOCS3−/ΔLck T cells drives gastrointestinal tissue destruction after allogeneic SCT. SOCS3−/ΔLck donors were treated with G-CSF, and unfractionated splenocytes containing 2 million T cells or T cell–depleted grafts were transplanted into lethally irradiated B6D2F1 recipient mice. Survival curves by Kaplan-Meier analysis. (A) Transplant recipients received isotype control mAb or anti–IL-10R thrice/week from day 7 onward. Data represent a single experiment with 10 animals/GVHD group, and 4 animals/TCD group. (B) Transplant recipients received isotype control mAb or anti–IL-17 mAb thrice/week from day 0 onward. Data are pooled from 3 similar experiments (n = 24 animals/GVHD group, n = 12 animals/TCD group). (C) Transplant recipients received T cell–replete or T cell–depleted grafts from G-CSF–treated WT or SOCS3−/ΔLck donors, and small bowel, liver, and skin histopathology were assessed at day 7 after transplantation as described in “Histology.” (D) Images of hematoxylin and eosin–stained sections of small bowel taken day 7 after transplantation (magnification ×100). (E) Transplant recipients received isotype control mAb or anti-IFNγ on day 0, 2, and 6, and small bowel histopathology was assessed at day 7. Data are pooled from 2 similar experiments (n = 12-13 animals/GVHD group, n = 4 animals/TCD group). *P = .017 for recipients of G-CSF WT versus G-CSF SOCS3−/ΔLck spleen. (F) Images of hematoxylin and eosin–stained sections of small bowel taken day 7 after transplantation (magnification ×100).
Enhanced IFNγ generation by SOCS3−/ΔLck T cells drives gastrointestinal tissue destruction after allogeneic SCT. SOCS3−/ΔLck donors were treated with G-CSF, and unfractionated splenocytes containing 2 million T cells or T cell–depleted grafts were transplanted into lethally irradiated B6D2F1 recipient mice. Survival curves by Kaplan-Meier analysis. (A) Transplant recipients received isotype control mAb or anti–IL-10R thrice/week from day 7 onward. Data represent a single experiment with 10 animals/GVHD group, and 4 animals/TCD group. (B) Transplant recipients received isotype control mAb or anti–IL-17 mAb thrice/week from day 0 onward. Data are pooled from 3 similar experiments (n = 24 animals/GVHD group, n = 12 animals/TCD group). (C) Transplant recipients received T cell–replete or T cell–depleted grafts from G-CSF–treated WT or SOCS3−/ΔLck donors, and small bowel, liver, and skin histopathology were assessed at day 7 after transplantation as described in “Histology.” (D) Images of hematoxylin and eosin–stained sections of small bowel taken day 7 after transplantation (magnification ×100). (E) Transplant recipients received isotype control mAb or anti-IFNγ on day 0, 2, and 6, and small bowel histopathology was assessed at day 7. Data are pooled from 2 similar experiments (n = 12-13 animals/GVHD group, n = 4 animals/TCD group). *P = .017 for recipients of G-CSF WT versus G-CSF SOCS3−/ΔLck spleen. (F) Images of hematoxylin and eosin–stained sections of small bowel taken day 7 after transplantation (magnification ×100).
SOCS3 within donor T cells attenuates GVHD after allogeneic BMT. Survival by Kaplan-Meier analysis. Irradiated B6D2F1 mice received a transplant with BM and T cells from WT or SOCS3−/Δvav mice (WT, n = 16; SOCS3−/Δvav, n = 16), WT BM and SOCS3−/Δvav T cells (n = 12), or T cell–depleted SOCS3−/Δvav BM (n = 10) as described in “Hematopoietic stem cell transplantation.” Data were pooled from 3 similar experiments. *P = .045, WT versus SOCS3−/Δvav BM and T cells.
SOCS3 within donor T cells attenuates GVHD after allogeneic BMT. Survival by Kaplan-Meier analysis. Irradiated B6D2F1 mice received a transplant with BM and T cells from WT or SOCS3−/Δvav mice (WT, n = 16; SOCS3−/Δvav, n = 16), WT BM and SOCS3−/Δvav T cells (n = 12), or T cell–depleted SOCS3−/Δvav BM (n = 10) as described in “Hematopoietic stem cell transplantation.” Data were pooled from 3 similar experiments. *P = .045, WT versus SOCS3−/Δvav BM and T cells.
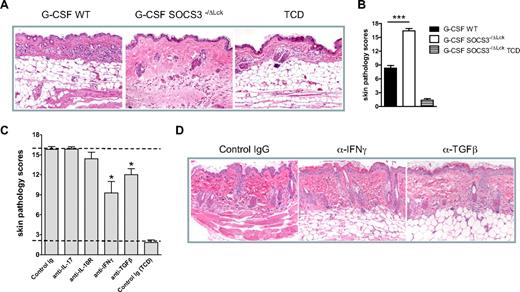
Finally, we used a second model of sclerodermatous GVHD directed to multiple minor histocompatibility antigens (B6 → LP/J). In this system the transplantation of Socs3−/Δlck grafts resulted in rapid and severe scleroderma that was only mild after transplantation of WT grafts (Figure 7A-B). Blockade of the excess IL-17A or IL-10 produced from Socs3−/Δlck grafts in this setting had no effect on reducing the severity of scleroderma (Figure 7C). In contrast, the neutralization of IFNγ or TGFβ inhibited the development of scleroderma generated in the absence of SOCS3 within donor T cells (Figure 7C-D). The parameters reduced after neutralization of IFNγ or TGFβ were dermal fibrosis, subcutaneous fibrosis, and loss of subcutaneous fat (P < .05). In contrast, parameters of inflammation were not affected. Thus, SOCS3 contributes to the control of both acute and sclerodermatous forms of GVHD after allogeneic SCT.
Socs3−/ΔLck T cells exacerbate scleroderma in an IFNγ- and TGFβ-dependent manner. WT or SOCS3−/ΔLck donors were treated with G-CSF, and unfractionated splenocytes containing 3.5 million T cells or T cell–depleted grafts were transplanted into lethally irradiated LP/J recipient mice. (A) Representative images of hematoxylin and eosin–stained skin taken day 14 after transplantation (magnification ×250). (B) Transplant recipients received T cell replete or T cell–depleted grafts from G-CSF–treated WT or SOCS3−/ΔLck donors, and skin histopathology was assessed at day 14 after transplantation as described in “Histology” (n = 12 animals per GVHD group, n = 3 animals per TCD group from 2 replicate experiments). *P < .001, WT versus SOCS3−/ΔLck. (C) Irradiated LP/J transplant recipients of SOCS3−/ΔLck grafts received isotype control mAb or anti–IL-17, anti–IL-10R, anti-IFNγ, or anti-TGFβ mAb thrice/week from day 0 onward. Skin histopathology was assessed at days 14 to 19. Data were pooled from 3 similar experiments (n = 4-17 animals per GVHD group, n = 6 animals per TCD group). Top and bottom dotted lines delineate pathology scores for control GVHD and non-GVHD groups, respectively. (D) Representative images of hematoxylin and eosin–stained skin taken day 14 after transplantation in animals receiving control IgG or anti-IFNγ or anti-TGFβ (magnification ×250). *P < .01, control versus blocking antibody.
Socs3−/ΔLck T cells exacerbate scleroderma in an IFNγ- and TGFβ-dependent manner. WT or SOCS3−/ΔLck donors were treated with G-CSF, and unfractionated splenocytes containing 3.5 million T cells or T cell–depleted grafts were transplanted into lethally irradiated LP/J recipient mice. (A) Representative images of hematoxylin and eosin–stained skin taken day 14 after transplantation (magnification ×250). (B) Transplant recipients received T cell replete or T cell–depleted grafts from G-CSF–treated WT or SOCS3−/ΔLck donors, and skin histopathology was assessed at day 14 after transplantation as described in “Histology” (n = 12 animals per GVHD group, n = 3 animals per TCD group from 2 replicate experiments). *P < .001, WT versus SOCS3−/ΔLck. (C) Irradiated LP/J transplant recipients of SOCS3−/ΔLck grafts received isotype control mAb or anti–IL-17, anti–IL-10R, anti-IFNγ, or anti-TGFβ mAb thrice/week from day 0 onward. Skin histopathology was assessed at days 14 to 19. Data were pooled from 3 similar experiments (n = 4-17 animals per GVHD group, n = 6 animals per TCD group). Top and bottom dotted lines delineate pathology scores for control GVHD and non-GVHD groups, respectively. (D) Representative images of hematoxylin and eosin–stained skin taken day 14 after transplantation in animals receiving control IgG or anti-IFNγ or anti-TGFβ (magnification ×250). *P < .01, control versus blocking antibody.
Discussion
The induction of GVHD is a complex process in which the disease process is amplified by the initial conditioning regimen and attendant gastrointestinal tract damage. This permits the systemic release of inflammatory cytokines and TLR ligands that act as an immune adjuvant on the subsequent critical host APC-donor T-cell interaction. The activation of the donor T cell after transplantation in this setting permissive of high levels of IL-12 characteristically leads to Th1 differentiation and the generation of a cytokine storm, leading to high levels of IL-6, TNF, and IFNγ that, together with cytotoxic T cells, damage recipient target tissue. Here, we show that SOCS3, the endogenous regulator of cytokine signaling through JAK-STAT3, is an important regulator of GVHD pathology. Importantly, it is SOCS3 within the donor T cell that plays this role, implicating the function of SOCS3 in donor T cells as a critical checkpoint in disease pathophysiology.
The key activators of JAK-STAT3 include IL-6, IL-11, G-CSF, IL-10, IL-21, and IL-23. In turn, the target genes include those involved in Th17 differentiation (e, IL-17, IL-23), proliferation (eg, cyclin D1), and the inhibition of apoptosis (eg, bcl-X and bcl-2).4 Thus, in the absence of SOCS3 within donor T cells, the persistent phosphorylation of STAT3 would be expected to result in enhanced Th17 differentiation and IL-10 secretion and T-cell activation and proliferation, as seen in this study. The increased apoptosis also shown here after transplantation most probably reflects enhanced activation-induced cell death in the absence of SOCS3 and thus an indirect downstream effect. Although SOCS3 does not control the signaling of IFNγ,22 the enhancement of IFNγ secretion in the absence of SOCS3 was unexpected and represents a novel finding that to our knowledge has not been previously described. Clearly, the control of signaling by any given cytokine can be completely independent of factors controlling its secretion. Thus, the absence of SOCS3 within the donor T cell has additional intrinsic effects in relation to IFNγ that are currently under further examination. It was shown that CD8+ T cells have an excessive proliferative response to T-cell receptor ligation that is IL-27 dependent.23 IL-27 appears critical for the initial commitment of Th1 differentiation and T-bet induction, and the IL-27R includes a gp139 subunit that signals by STAT1 and STAT3.35 The Th1 differentiation observed in GVHD is directed predominantly by recipient WT APCs, in conjunction with additional accessory cytokines (eg, IL-12) that may originate from donor or recipient hematopoiesis.2 This suggests that the absence of SOCS3 within the donor T cell in turn promotes the generation of externally derived Th1 cofactors (such as IL-12, IL-18, and IL-27) or conversely is more sensitive to these factors (eg, IL-27). The generation of these factors from an APC depends on the strength of T-cell costimulation (through major histocompatibility complex class II and CD40L),36 IFNγ signaling,37 and stimulation by TLR ligands.38 Thus, the enhanced activation of the donor T cell by alloantigen in the absence of SOCS3 would be predicted to enhance the secretion of, and the sensitivity to, Th1 cofactors extrinsically, while also enhancing the generation of IL-17 and IL-10 by intrinsic (SOCS3/STAT3-dependent) pathways.
Although IL-17 and IL-10 have little or no pathogenic effect on acute GVHD in these systems (and as shown previously34,39,40 ), the central role of Th1 differentiation and IFNγ in particular in acute GVHD is well described.33,41,42 Of note, the IL-17 produced after transplantation in these systems is concurrent with other Th1 cytokines (rather than in isolation of) and is predominantly from CD8+ T cells, suggesting that acute GVHD is associated with T-cell differentiation that is not exclusive to Th1 or Th17 pathways, even at a cellular level. In contrast, the role of specific T-cell differentiation pathways on fibrosis after BMT (ie, scleroderma) is not well defined. These data show that SOCS3 within donor T cells plays an important role in inhibiting this process. The predominant cell type producing TGFβ and subsequently invoking cutaneous fibrosis after BMT are those of the monocyte/macrophage lineage.43 The ability of this cytokine to induce fibrotic responses within fibroblasts and scleroderma in the absence of SOCS3 is thus not surprising. In contrast IFNγ generation after BMT is almost entirely derived from the donor T cell and, although the mechanism by which this cytokine may promote fibrosis is unclear, the association has been made with clinical chronic GVHD44 and systemic sclerosis.45 Although animal studies have shown that autoimmune “chronic” GVHD may occur in a Th1-dependent fashion,46 these studies do not induce the fibrosis that is characteristic of clinical chronic GVHD. Furthermore, the very rapid induction of sclerosis by IFNγ in the absence of SOCS3 does not imply that the Th1 pathway is causative of chronic GVHD when donor T cells are SOCS3 replete. This is particularly so when scleroderma develops over a more conventional and delayed time frame. In this setting we have recently shown that IL-17A is an important causative cytokine.47
The important effect of SOCS3 in GVHD described here is consistent with the recent description of JAK-STAT3 as a pathogenic transcription factor in the disease process.47 That study showed the presence of phosphorylated STAT3 in dividing cells after BMT.48 Furthermore, the preincubation of donor T cells with a small molecule inhibitor of STAT3 could reduce donor CD8 T-cell expansion and GVHD severity,47 although the mechanisms were not further assessed. Our study suggests that in the absence of SOCS3, the endogenous inhibitor of STAT3, GVHD is enhanced by means that are predominantly extrinsic to STAT3 within the T cell itself and consistent with STAT4-dependent T-cell differentiation (ie, IFNγ secretion). Thus, SOCS3 has a central and far greater regulatory effect on GVHD than would be anticipated on the basis of its “intrinsic” cellular effects. These studies highlight SOCS3 as an attractive target for therapeutic modulation in transplantation (ie, the administration of mimetics as previously described49 ) and suggest that functional augmentation of this suppressor molecule may preferentially inhibit alloreactive T-cell proliferation and effectively differentiate cells away from pathogenic Th17/Th1 pathways.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grants from the Queensland Cancer Fund, the National Institutes of Health (CA022556), and National Health and Medical Research Council (programs 461291 and 351439). K.P.A.M. is a National Health and Medical Research Council R.D. Wright Fellow. W.S.A., A.W.R., and G.R.H. hold fellowships from the National Health and Medical Research Council (Australia).
National Institutes of Health
Authorship
Contribution: G.R.H. designed studies and helped write the manuscript; R.D.K., N.C.R., S.D.O., K.A.M., and Y.A.W. performed experiments; A.L.J.D. performed experiments and generated reagents; J.T. and W.S.A. provided vital reagents; A.D.C. performed all blinded histologic analyses; A.W.R. helped design experiments and provided vital reagents; and K.P.A.M. designed studies and helped write the manuscript.
Conflict-of-interest disclosure: W.S.A. and A.W.R. have applied for a patent related to the work that is described in the present study. The remaining authors declare no competing financial interests.
Correspondence: Kelli MacDonald, Bone Marrow Transplantation Laboratory, Queensland Institute of Medical Research, 300 Herston Rd, Brisbane, Queensland 4006, Australia; e-mail: kelli.macdonald@qimr.edu.au.

![Figure 2. SOCS3−/ΔLck T cells are hyperresponsive to T-cell receptor ligation after G-CSF mobilization. (A) Size and cell subset composition of spleens from G-CSF–treated WT or SOCS3−/ΔLck mice. Data are representative of 3 similar experiments (n = 3 animals/group). DN indicates double negative. (B) Splenocytes from G-CSF–treated WT or SOCS3−/ΔLck mice were cultured in the presence of soluble CD3, and proliferative responses were assessed by [3H] thymidine incorporation. (C) Supernatants from CD3 cultures were harvested at 72 hours and assayed for cytokines by cytometric bead array or enzyme-linked immunoabsorbent assay (IL-17A, IL-17F, and TGFβ). Data are pooled from 2 independent experiments, n = 4-6 animals/group. *P < .05; ** P < .01. (D) IFNγ secretion by purified CFSE-labeled T cells at the times indicated after stimulation with plate-bound CD3 and CD28. Data are representative of 2 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/2/10.1182_blood-2009-12-259598/4/m_zh89991055190002.jpeg?Expires=1767709677&Signature=kmjW5q8GuC5BNCVUbD64-VowmPPM4yATFszMSqy9K16VnESMx4ER7eB~tPgde~qiyYxlEnFH6EswwD1adl8yfW6ednvAx2osF-O-QthVDBLSgYPjQNdLoOxvfpWXKTr6GHYJsoppjyxe5hqRfBBDxyO0fSmrixHAC0luRQg6C594fs3sQsb-fagNdLIq9Wym7MKps~Hw6Mcd6Iyey7QkJ4BE3oBN-NvIsi2M0u2YPSy~iYrrZTLIgwMt8sV0huGDRkJW~d1lqruga0uEi4FXjUplX2LYjpI0UEwbsB5eoHTLRnJ2q-08Bbb8grprQRZ9T-oHmVWs5cBlTQQaV0zUyA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)