Abstract
The nonobese diabetic/severe combined immune deficiency (NOD-scid) xenotransplantation model is the “gold standard” for assaying human hematopoietic stem cell activity. Systematic advancements, such as depletion of natural killer cell activity with anti-CD122 antibody, direct intrafemoral injection, and deletion or truncation of IL2Rγ, have improved human cell engraftment; however, questions remain whether these mouse models are equivalent or, if not, which model is superior for assaying hematopoietic stem cell activity. To address this, we compared overall engraftment and multilineage differentiation of near-limiting doses of lineage-depleted human umbilical cord blood cells by direct intrafemoral injection into NOD/Lt-scid, NOD/Shi-scid, NOD/Lt-scid/IL2Rγnull (NSG), and NOD/Shi-scid/IL2Rγnull mice. Transplantation into NSG mice generated moderately higher human engraftment levels in bone marrow compared with other strains. At limiting doses, NSG mice of both sexes were 3.6-fold more sensitive in detecting SCID-repopulating cells compared with NOD/Lt-scid mice. However, NSG females exhibited higher engraftment at limiting cell doses, resulting in an overall increase in SCID-repopulating cell detection of 9-fold. Both NSG and NOD/Shi-scid/IL2Rγnull support significantly improved engraftment in peripheral tissues compared with NOD/Lt-scid and NOD/Shi-scid mice, whereas NSG mice provide greater human engraftment in bone marrow than all other strains, especially at limiting doses.
Introduction
Since the original SCID (severe combined immune deficiency, Prkdcscid) mutation on the C.B-17 strain was described1 and subsequently crossed onto the nonobese diabetic (NOD) strain,2 the NOD-scid xenotransplantation model has emerged as a “gold-standard” in vivo repopulation assay that enables characterization of human hematopoietic stem cells (HSCs).3 Immune barriers act to restrict human engraftment, and many improvements to the NOD-scid model continue to be made to overcome these limitations.4 One such barrier is natural killer (NK)-cell activity, which is one of the foremost impediments to HSC engraftment.5 Indeed, NOD mice have lower levels of NK-cell activity than C.B-17 mice in addition to other defects in innate immunity.2 Further depletion of NK-cell activity by treatment of NOD-scid mice with a monoclonal antibody to CD122 also improves HSC engraftment.6-8 NOD-scid-β2-microglobulin-null (NOD-scid-β2m−/−) mice, which are genetically depleted of NK cells, also demonstrate enhanced HSC engraftment.9 Yahata et al demonstrated that intratibia injection, compared with intravenous injection, improves engraftment and detection of HSC activity in NOD-scid mice.10 We also previously showed that direct injection of cells in the bone marrow (BM) cavity of the femur, termed intrafemoral injection, provided more robust engraftment than intravenous injection in NOD-scid mice.6 Using this technique, we identified a previously undetected rapidly engrafting cell population in NOD-scid mice.11 It is now known that the SIRPA allele expressed on the macrophages of NOD mice also provides a permissive environment for human HSC engraftment.12 Collectively, these data suggest that, in addition to immune background, the method of transplantation impacts HSC engraftment, perhaps through mitigation of barriers to HSC migration processes and enhanced immune evasion.
NOD mice were originally established in Japan in 1980 from the CTS strain after spontaneous development of insulin-dependent diabetes mellitus with insulitis.13,14 These NOD/ShiJic mice were shipped to Dr E. Leiter at The Jackson Laboratory in 1984 and termed NOD/ShiLt. Two groups independently crossed C.B-17-Prkdcscid mice with NOD/ShiLt2 and NOD/ShiJic15 to generate NOD/ShiLtSz-Prkdcscid (NOD/Lt-scid)2 and NOD/ShiJic-Prkdcscid (NOD/Shi-scid) mice, respectively.15 Subsequent crossing of these NOD-scid strains with 2 different IL2Rγnull mice, one a complete null mutation16 and the other a truncation of the intracellular signaling domain,17 generated NOD/ShiLtSz-scid/IL2Rγnull (NSG)18 and NOD/ShiJic-scid/IL2Rγnull (NOG)19 mice. The IL2Rγ-chain is required for signaling of IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 cytokines,20 and IL2Rγnull mice have severe defects in NK-cell activity in addition to T- and B-cell development. NSG and NOG mice provide improved engraftment of mobilized CD34+ human peripheral blood mononuclear cells and CD34+ umbilical cord blood (CB) cells, respectively.18,19
In recent years, the existence of normal and malignant stem cells in various human tissues, including the breast, brain, pancreas, and colon, has been established on the basis of tumor initiation assays using NOD-scid mice.21-24 Recent reports showing improved engraftment of human leukemia25 and single melanoma26 cells in NOD-scid–IL2Rγnull mice compared with NOD-scid mice point to increased use of NOD-scid–IL2Rγnull mice as a mouse model for basic and translational research. However, the applicability of these results to other tissues remains unknown. In addition, these strains have not been directly compared quantitatively for engraftment potential using the same source of hematopoietic cells, resulting in considerable uncertainty in the research community as to which model is superior.
Because of the widespread interest in the xenotransplantation of human cells, here we provide quantitative comparison of engraftment and multilineage differentiation of lineage-depleted (Lin−) CB cells in these 4 strains. To ensure that our comparison was made with the most optimized xenotransplantation system currently available, all mice received cells by intrafemoral injection and NOD/Lt-scid and NOD/Shi-scid mice received anti-CD122 treatment (NSG and NOG did not). This approach enabled direct comparison of the effect of host environment among the 4 strains. We demonstrate that NSG mice provide a moderate 1.5-fold engraftment improvement in the BM when evaluated against the other 3 strains. As reported previously, NSG and NOG mice support significantly improved engraftment in the spleen and thymus compared with NOD/Lt-scid and NOD/Shi-scid mice. In addition, we found that NSG mice generated a consistently higher human BM graft compared with NOG mice with no detectable difference in the spleen and thymus. Limiting dilution analysis in NSG mice demonstrated a 3.6-fold improved detection of SCID-repopulating cells (SRCs), as a functional definition of human HSCs.27 Finally, at limiting doses, NSG mice exhibited a marked sex difference in engraftment with females being superior to males.
Methods
Animals
NOD/ShiLtSz-Prkdcscid (NOD.CB17-Prkdcscid, termed NOD/Lt-scid)2 and NOD/ShiLtSz-scid/IL2Rγnull (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ, termed NSG)18,28 mice were generously provided by Dr Leonard Shultz (The Jackson Laboratory). NOD/ShiJic-Prkdcscid (termed NOD/Shi-scid)15,29,30 and NOD/ShiJic-scid/IL2Rγnull (NOD.Cg-PrkdcscidIl2rgtm1Sug/Jic; termed NOG)19,31,32 were generously provided by Dr Mamoru Ito (Central Institute for Experimental Animals, Kanagawa, Japan) and NOD/Shi-scid mice were rederived by Cesarean section into our facility. All mice were bred at the Ontario Cancer Institute and housed in ventilated micro-isolator cages with autoclaved water and irradiated food ad libitum in a high-barrier facility under specific pathogen–free conditions.
Human cells
CB samples were obtained according to procedures approved by the institutional review boards at University Health Network (Toronto, ON) and Trillium Hospital (Mississauga, ON). Samples were pooled and Lin− CB cells were purified by negative selection to remove CD2, CD3, CD14, CD16, CD19, CD24, CD56, CD66b, and GlyA-positive cells (StemSep Human Progenitor Enrichment Cocktail; StemCell Technologies), thereby enriching CD34+ cells to 42% to 75%. These cells were frozen viably. For each Lin− CB pool, a frozen aliquot was thawed and slowly diluted in phosphate-buffered saline (PBS)/1% fetal bovine serum (FBS)/0.2 mg/mL DNase I, counted, and resuspended at 25 μL per mouse in PBS/1% FBS for intrafemoral injection.
Intrafemoral injection
Mice were irradiated 24 hours previously with 275 cGy (NOD/Lt-scid and NOD/Shi-scid) or 225 cGy (NSG and NOG) from a cesium-137 source at 105 cGy/min (Gammacell 40; Theratronics). NOD/Lt-scid and NOD/Shi-scid mice received one intraperitoneal injection of 200 μg purified anti-CD122 antibody immediately after irradiation. The anti-CD122 monoclonal antibody generated from the hybridoma cell line, TM-β1 (gift of Dr T. Tanaka, Osaka University Medical Center, Osaka, Japan),8 was purified using the High Trap Protein G Column (GE Healthcare). Mice were maintained on Baytril in the drinking water at 25.5 mg/kg for 2 weeks after injection. Lin− CB cells (experiment 1, 2.3 × 104; experiment 2, 3.0 × 104; limiting dilution assay, 2.5 × 103, 5 × 103, 1 × 104, or 2 × 104) were injected into the right femur (RF) of 8- to 13-week-old female and male mice (5-7 per group), as described previously.11 Briefly, mice were anesthetized by isofluorane inhalation, the knee was flexed, and 25 μL of cells were injected through a 28.5-gauge needle through the joint into the RF. All animal studies were carried out according to the regulations of the Canadian Council on Animal Care and with the approval of the institutional review board at University Health Network (Toronto).
Human engraftment analysis by flow cytometry
Nine to 13 weeks after injection, surviving mice were killed and the injected RF, noninjected left femur (BM), spleen, and thymus were removed. Bones were flushed with 1 to 2 mL PBS/5% FBS, whereas spleens and thymi were homogenized in PBS/5% FBS through a 23-gauge needle and filtered through a 40-μm mesh. Cells were stained in 100 μL PBS/5% FBS for 30 to 60 minutes at room temperature, washed, and resuspended in PBS/5% FBS. Antibodies used included: CD3-fluorescein isothiocyanate (FITC; BD 349201), GlyA-FITC (Coulter IM2212U), CD7-FITC (BD 340737), GlyA-phycoerythrin (PE; Coulter IM2211U), CD19-PE (BD 349209), CD8-PE (BD 555635), CD38-PE (BD 347687), CD19-PE-Cy5 (Coulter IM2643U), IgM-PE-Cy5 (BD 551079), CD4-PE-Cy5 (Coulter IM2636U), CD33-PE-Cy7 (BD 333946), CD45-PE-Cy7 (BD 557748), CD34-allophycocyanin (APC; Coulter IM2472U or BD 340441), CD56-APC (BD 557711), CD3-APC (BD 555335), and CD45-APC-Cy7 (BD 557833 or BD 348795). Flow cytometric analysis was performed on a BD LSR-II equipped with 488-, 633-, 405-, and 355-nm (ultraviolet) lasers and appropriate filters. When possible, 1000 human CD45+ events were recorded; otherwise, between 30 000 and 100 000 events were acquired. Positive engraftment was defined using mice stained with isotype controls in addition to staining for lineage markers. Isotype controls ranged from 0.1% to 0.4% (experiment 1), 0.1% to 0.3% (experiment 2), 0.1% to 0.2% (supplemental Figure 3; supplemental Table 3; available on the Blood Web site; see the Supplemental Materials link at the top of the online article), and 0.05% to 0.1% (see Figure 5). Data analysis was performed using FlowJo Version 8.8.4 (TreeStar).
Statistical analysis
Data from experiments 1 and 2 were combined, presented as the mean plus or minus SEM, and graphed using Prism Version 5.0 (GraphPad). To test for differences in location, the nonparametric Wilcoxon rank-sum test was used (Mstat 5.10; Dr Norman Drinkwater, McArdle Laboratory, University of Wisconsin). Limiting dilution analysis was performed using L-Calc software Version 1.1 (StemCell Technologies).33
Results
Radiation sensitivity
NSG mice were previously reported to be more sensitive than NOD/Lt-scid mice to radiation18 ; however, comparative radiation sensitivity of NOG or NOD/Shi-scid mice has not been reported. To determine the effect of radiation, groups of 4 or 5 mice at 8 to 12 weeks of age were irradiated at doses from 200 to 400 cGy and monitored weekly until 12 weeks (Table 1). At 300 and 350 cGy, NOD/Lt-scid, NOD/Shi-scid, and NOG mice were comparable. Based on prior experience and these data, NOD/Lt-scid and NOD/Shi-scid mice were irradiated at 275 cGy, whereas NSG and NOG mice were irradiated at 225 cGy to minimize radiation toxicity in future experiments.
Radiation sensitivity of NOD-scid and NOD-scid IL2Rγnull mice
| Genotype . | Radiation dose, cGy . | ||||
|---|---|---|---|---|---|
| 200 . | 250 . | 300 . | 350 . | 400 . | |
| NOD/Lt-scid | ND | ND | 4/5* | 1/5 | 0/5 |
| NOD/Shi-scid | ND | ND | 4/4 | 0/5 | 0/5 |
| NSG | 3/4 | 4/4 | 2/4 | 3/5 | ND |
| NOG | 5/5 | 5/5 | 5/5 | 1/5 | ND |
| Genotype . | Radiation dose, cGy . | ||||
|---|---|---|---|---|---|
| 200 . | 250 . | 300 . | 350 . | 400 . | |
| NOD/Lt-scid | ND | ND | 4/5* | 1/5 | 0/5 |
| NOD/Shi-scid | ND | ND | 4/4 | 0/5 | 0/5 |
| NSG | 3/4 | 4/4 | 2/4 | 3/5 | ND |
| NOG | 5/5 | 5/5 | 5/5 | 1/5 | ND |
Eight- to 12-week-old mice were irradiated at the indicated dose and monitored weekly
ND indicates not done.
Number of surviving mice/total mice at 12 weeks.
Moderate improvement of engraftment in the RF and BM in NSG mice
Prior literature has reported that both NSG and NOG mice provided higher engraftment levels of CD34+ mobilized peripheral blood mononuclear cells (6-fold)18 or CD34+ CB cells (∼ 7-fold)19 than NOD/Lt-scid or NOD/Shi-scid mice, respectively. However, these studies used an intravenous administration route without anti-CD122 treatment, which we showed previously was not as efficient as direct intrafemoral injection.6,11 In addition, treatment of NOD/Lt-scid6,7 or NOD/Shi-scid30 mice with anti-CD122 antibody improves engraftment. Together, these 2 modifications result in an almost 10-fold increase in human engraftment and SRC detection. To directly compare the 4 strains using these optimized transplantation assay conditions, 2.3 × 104 (experiment 1) or 3.0 × 104 (experiment 2) Lin− CB cells were injected into the RF, and overall engraftment and multilineage differentiation was assayed 11 (experiment 1) or 13 (experiment 2) weeks after injection. NOD/Lt-scid and NOD/Shi-scid mice were treated once with anti-CD122 antibody before cell injection.
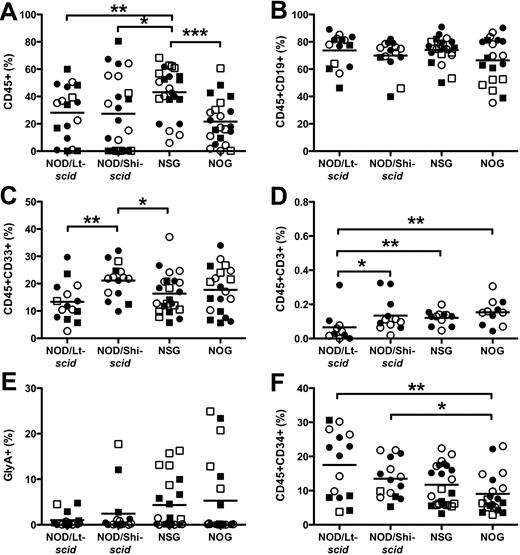
In contrast to the 6- to 7-fold engraftment improvement seen when using intravenous without anti-CD122 treatment,18,19 there was only a modest 1.5-fold engraftment improvement of CD45+ cells in the injected RF in NSG mice compared with NOD/Lt-scid mice (43.1% ± 3.7% vs 28.1% ± 4.7%; P < .01, Figure 1A). NOD/Lt-scid and NOD/Shi-scid mice engrafted at similar levels (P = .67). NSG mice were more efficiently engrafted than NOG mice (43.1% ± 3.7% vs 21.6% ± 3.5%), indicating that the presence of the extracellular domain of IL2Rγ-chain might negatively affect human engraftment in the BM. In all strains, the majority of human cells in the RF were CD19+ B cells (Figure 1B) with the remaining cells composing CD33+ myeloid (Figure 1C) or CD45−GlyA+ erythroid cells (Figure 1E). In all strains, we detected very low levels of CD3+ cells (Figure 1D); however, the functional significance of this finding is not known. NSG and NOG mice trended to support higher levels of CD45−GlyA+ erythroid cells (P > .08). NOG mice had significantly fewer CD34+ cells than NOD/Lt-scid and NOD/Shi-scid mice (Figure 1F; 9.0% ± 1.3%, 17.5% ± 2.5%, and 13.5% ± 1.4%, respectively). The majority of these CD34+ cells were also CD19+, which is indicative of pro-B cell phenotype. Cells from the second experiment were stained for primitive CD34+CD38− cells. Both NSG and NOG females had significantly fewer CD34+CD38− cells than males (supplemental Figure 2G).
Hematopoietic cell engraftment and multilineage development in the injected RF. The level of human CD45+ cells (A), CD19+ B cells (B), CD33+ myeloid cells (C), CD3+ T cells (D), CD45−GlyA+ erythroid cells (E), and CD34+ cells (F). Lineage engraftment (B-F) expressed as frequency of human CD45 cells. Bar represents mean. *P < .05, **P < .01, ***P < .001 by Wilcoxon rank-sum test. ● represents experiment 1 females; ○, experiment 1 males; ■, experiment 2 females; and □, experiment 2 males. N = 5 to 7 per group.
Hematopoietic cell engraftment and multilineage development in the injected RF. The level of human CD45+ cells (A), CD19+ B cells (B), CD33+ myeloid cells (C), CD3+ T cells (D), CD45−GlyA+ erythroid cells (E), and CD34+ cells (F). Lineage engraftment (B-F) expressed as frequency of human CD45 cells. Bar represents mean. *P < .05, **P < .01, ***P < .001 by Wilcoxon rank-sum test. ● represents experiment 1 females; ○, experiment 1 males; ■, experiment 2 females; and □, experiment 2 males. N = 5 to 7 per group.
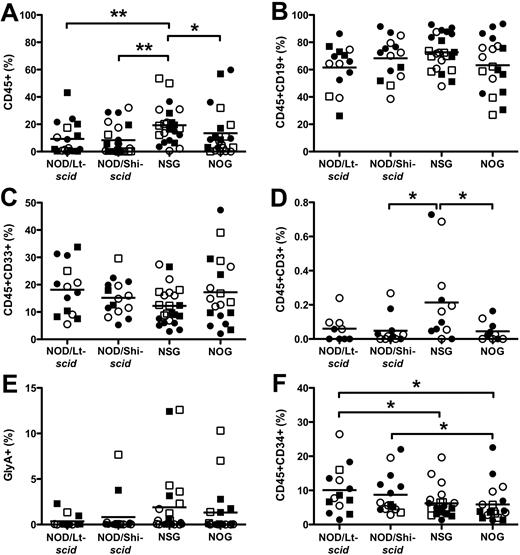
Repopulation of noninjected BM is an excellent measure of the migration and self-renewal capacity of the transplanted cells.6,11 We found that all strains had a similar pattern of overall engraftment compared with the RF (Figure 2). In each strain, BM engraftment was somewhat lower than the injected RF (P < .037). NSG mice (19.3% ± 2.9%) supported greater engraftment in the noninjected BM than NOD/Lt-scid mice (9.3% ± 2.8%) and NOG mice (13.4% ± 3.7%). In all strains, multilineage differentiation was similar for CD19+ B cells, CD33+ myeloid, and CD45−GlyA+ erythroid cells (Figure 2B,C,E). NSG mice produced superior engraftment of CD3+ T cells than NOG mice (Figure 2D; 0.21% ± 0.07% vs 0.04% ± 0.02%); however, levels were very low, indicating that additional time is needed for T-cell development and/or migration from the thymus. As was observed in the RF, NOG mice had fewer CD34+ cells than NOD/Lt-scid and NOD/Shi-scid mice (Figure 2F; 5.9% ± 1.2%, 10.1% ± 1.8%, and 8.7% ± 1.5%, respectively). Similarly, NSG mice (6.2% ± 0.88%) had fewer CD34+ cells than NOD/Lt-scid mice, and NSG females had fewer CD34+ cells than males (supplemental Figure 2D).
Hematopoietic cell engraftment and multilineage development in noninjected BM. The level of human CD45+ cells (A), CD19+ B cells (B), CD33+ myeloid cells (C), CD3+ T cells (D), CD45−GlyA+ erythroid cells (E), and CD34+ cells (F). Lineage engraftment (B-F) expressed as frequency of human CD45 cells. *P < .05, **P < .01 by Wilcoxon rank-sum test. ● represents experiment 1 females; ○, experiment 1 males; ■, experiment 2 females; and □, experiment 2 males. N = 5 to 7 per group.
Hematopoietic cell engraftment and multilineage development in noninjected BM. The level of human CD45+ cells (A), CD19+ B cells (B), CD33+ myeloid cells (C), CD3+ T cells (D), CD45−GlyA+ erythroid cells (E), and CD34+ cells (F). Lineage engraftment (B-F) expressed as frequency of human CD45 cells. *P < .05, **P < .01 by Wilcoxon rank-sum test. ● represents experiment 1 females; ○, experiment 1 males; ■, experiment 2 females; and □, experiment 2 males. N = 5 to 7 per group.
Improved engraftment in the spleen of NSG and NOG mice
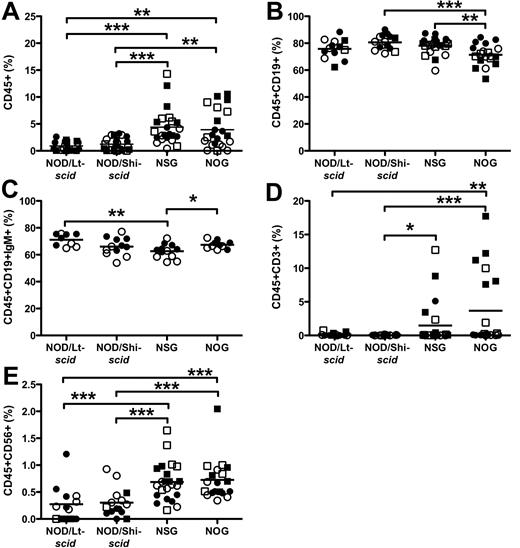
Previous studies observed that the spleens of NOD/Lt-scid11 and NOD/Shi-scid29 mice are not as efficiently engrafted as BM, whereas NSG and NOG spleens engraft at levels similar to BM.18,19 In our study, more NSG and NOG mice were engrafted in the spleen with CD45+ cells than NOD/Lt-scid and NOD/Shi-scid mice (Table 2). Concordantly, spleens in NSG (4.4% ± 0.70%) and NOG (3.9% ± 0.76%) mice had more CD45+ cells than NOD/Lt-scid (0.90% ± 0.20%) and NOD/Shi-scid (1.2% ± 0.23%) mice (Figure 3A). Unlike what has been previously reported,18,19 we found significantly lower engraftment in the spleen than BM in NSG and NOG mice. The majority of cells in the spleens were CD19+ B cells, including CD19+IgM+ mature B cells (Figure 3B-C). NOG mice had slightly fewer B cells than NSG and NOD/Shi-scid mice (71.4% ± 1.8%, 78.1% ± 1.3%, and 80.6% ± 1.4%, respectively). NSG mice had fewer CD19+IgM+ mature B cells than NOG and NOD/Lt-scid mice (62.6% ± 1.5%, 67.4% ± 0.95%, and 71.1% ± 1.5%, respectively). As reported previously,18,19,31 NSG and NOG supported CD3+ T-cell differentiation (1.5% ± 0.7%, 3.7% ± 1.3%), but NOD/Lt-scid and NOD/Shi-scid mice did not (Figure 3D; 0.15% ± 0.07%, 0.04% ± 0.01%). CD56+ NK cells were detected in NSG and NOG spleens (0.69% ± 0.07%, 0.73% ± 0.09%), and at lower levels in NOD/Lt-scid and NOD/Shi-scid mice (Figure 3E; 0.27% ± 0.09%, 0.30% ± 0.07%).
Engraftment efficiency of tissues and strains
| Tissue/genotype . | No. engrafted mice*/total . | |||
|---|---|---|---|---|
| Experiment 1 . | Experiment 2 . | Combined . | Percentage . | |
| RF | ||||
| NOD/Lt-scid | 10/11† | 5/7 | 15/18 | 83.3 |
| NOD/Shi-scid | 12/12 | 3/11 | 15/23 | 65.2 |
| NSG | 12/12 | 11/11 | 23/23 | 100 |
| NOG | 11/12 | 9/10 | 20/22 | 90.9 |
| BM | ||||
| NOD/Lt-scid | 9/11 | 5/7 | 14/18 | 77.8 |
| NOD/Shi-scid | 12/12 | 3/11 | 15/23 | 65.2 |
| NSG | 12/12 | 11/11 | 23/23 | 100 |
| NOG | 10/12 | 9/10 | 19/22 | 86.4 |
| Spleen | ||||
| NOD/Lt-scid | 9/11 | 4/7 | 13/18 | 72.2 |
| NOD/Shi-scid | 12/12 | 3/11 | 15/23 | 65.2 |
| NSG | 12/12 | 11/11 | 23/23 | 100 |
| NOG | 10/12 | 9/10 | 19/22 | 86.4 |
| Thymus | ||||
| NOD/Lt-scid | 0/9‡ | 0/7 | 0/16 | 0 |
| NOD/Shi-scid | 3/10‡ | 1/11 | 4/21 | 19.0 |
| NSG | 4/7‡ | 10/11 | 14/18 | 77.8 |
| NOG | 5/11‡ | 7/10 | 12/21 | 57.1 |
| Tissue/genotype . | No. engrafted mice*/total . | |||
|---|---|---|---|---|
| Experiment 1 . | Experiment 2 . | Combined . | Percentage . | |
| RF | ||||
| NOD/Lt-scid | 10/11† | 5/7 | 15/18 | 83.3 |
| NOD/Shi-scid | 12/12 | 3/11 | 15/23 | 65.2 |
| NSG | 12/12 | 11/11 | 23/23 | 100 |
| NOG | 11/12 | 9/10 | 20/22 | 90.9 |
| BM | ||||
| NOD/Lt-scid | 9/11 | 5/7 | 14/18 | 77.8 |
| NOD/Shi-scid | 12/12 | 3/11 | 15/23 | 65.2 |
| NSG | 12/12 | 11/11 | 23/23 | 100 |
| NOG | 10/12 | 9/10 | 19/22 | 86.4 |
| Spleen | ||||
| NOD/Lt-scid | 9/11 | 4/7 | 13/18 | 72.2 |
| NOD/Shi-scid | 12/12 | 3/11 | 15/23 | 65.2 |
| NSG | 12/12 | 11/11 | 23/23 | 100 |
| NOG | 10/12 | 9/10 | 19/22 | 86.4 |
| Thymus | ||||
| NOD/Lt-scid | 0/9‡ | 0/7 | 0/16 | 0 |
| NOD/Shi-scid | 3/10‡ | 1/11 | 4/21 | 19.0 |
| NSG | 4/7‡ | 10/11 | 14/18 | 77.8 |
| NOG | 5/11‡ | 7/10 | 12/21 | 57.1 |
Eight- to 13-week-old female and male mice were injected with 2.3 × 104 (experiment 1) or 3.0 × 104 (experiment 2) Lin− CB cells (n = 5-7 per sex).
Positive engraftment defined using isotype controls in addition to staining for lineage markers.
Number of mice engrafted with CD45+ cells/total mice.
Thymic tissue was not assayed because of limited tissue availability for some mice in experiment 1.
Hematopoietic cell engraftment and multilineage development in the spleen. The level of human CD45+ cells (A), CD19+ B cells (B), CD19+IgM+ B cells (C), CD3+ T cells (D), and CD56+ NK cells (E). Lineage engraftment (B-E) expressed as frequency of human CD45 cells. *P < .05, **P < .01, ***P < .001 by Wilcoxon rank-sum test. ● represents experiment 1 females; ○, experiment 1 males; ■, experiment 2 females; and □, experiment 2 males. N = 5 to 7 per group.
Hematopoietic cell engraftment and multilineage development in the spleen. The level of human CD45+ cells (A), CD19+ B cells (B), CD19+IgM+ B cells (C), CD3+ T cells (D), and CD56+ NK cells (E). Lineage engraftment (B-E) expressed as frequency of human CD45 cells. *P < .05, **P < .01, ***P < .001 by Wilcoxon rank-sum test. ● represents experiment 1 females; ○, experiment 1 males; ■, experiment 2 females; and □, experiment 2 males. N = 5 to 7 per group.
Improved engraftment in the thymus of NSG and NOG mice
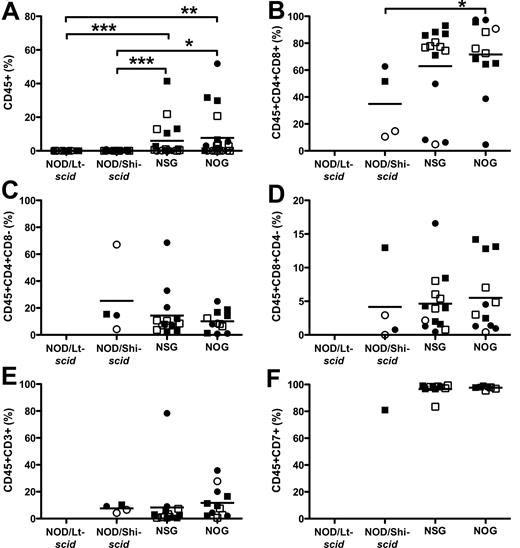
NSG and NOG mice were previously reported to efficiently engraft and produce functional T cells in the thymus.18,19,28,31,32 NOG mice have a truncated IL2Rγ-chain that precludes intracellular signaling,17 and it has been suggested that the remaining IL2Rγ-chain extracellular domain in NOG mice could bind various cytokines important for T-cell development, such as IL-7, thereby preventing binding on human cells and affect differentiation.4,18 In our studies, the thymi of NSG and NOG mice were similarly engrafted and more efficiently engrafted than NOD/Lt-scid and NOD/Shi-scid mice (Figure 4A; 6.0% ± 2.6%, 7.7% ± 3.0%, 0.02% ± 0.006%, and 0.10% ± 0.04%, respectively). Unexpectedly, in 4 of 21 NOD/Shi-scid mice, engraftment of T cells was observed (Table 2), albeit at low levels (0.823%, 0.458%, 0.128%, and 0.125%). CD4+CD8+ double-positive, CD4+ single-positive, and CD8+ single-positive cells were generated (Figure 4B-D) in NSG and NOG mice. Most thymocytes were CD4+CD8+ double-positive (63.0% ± 8.6%, 71.6% ± 7.9%). The CD4/CD8 T-cell ratio in NSG mice was higher than NOG mice and tended toward physiologic levels (supplemental Figure 1C; 2.27 ± 0.35 vs 1.32 ± 0.17, P > .044). CD3 staining was low (Figure 4E), but cells were approximately 95% CD7+ (Figure 4F).
Hematopoietic cell engraftment and T-cell differentiation in the thymus. The level of human CD45+ cells (A), CD4+CD8+ double-positive T cells (B), CD4+ single-positive T cells (C), CD8+ single-positive T cells (D), CD3+ T cells (E), and CD7+ T cells (F). *P < .05, **P < .01, ***P < .001 by Wilcoxon rank-sum test. ● represents experiment 1 females; ○, experiment 1 males; ■, experiment 2 females; and □, experiment 2 males. N = 5 to 7 per group.
Hematopoietic cell engraftment and T-cell differentiation in the thymus. The level of human CD45+ cells (A), CD4+CD8+ double-positive T cells (B), CD4+ single-positive T cells (C), CD8+ single-positive T cells (D), CD3+ T cells (E), and CD7+ T cells (F). *P < .05, **P < .01, ***P < .001 by Wilcoxon rank-sum test. ● represents experiment 1 females; ○, experiment 1 males; ■, experiment 2 females; and □, experiment 2 males. N = 5 to 7 per group.
Anti-CD122 antibody treatment does not improve engraftment in NSG mice
NK-cell activity is known to affect engraftment of human hematopoietic cells in mice,5 and NSG and NOG mice have very low levels of NK-cell activity.18,19 As a corollary, treatment of NOD/Lt-scid mice with an anti-CD122 antibody that inhibits NK-cell activity8 provided higher engraftment than NOD-scid-β2m−/− mice, which are depleted of NK cells, suggesting that other CD122+ cells, probably macrophages or monocytes, have a negative effect on human engraftment (Figure 5).6 We tested whether anti-CD122 antibody treatment of NSG mice would improve human engraftment but found no improvement in the RF, BM, spleen, or thymus in female or male mice (supplemental Figure 3). This indicates that residual CD122+ cells do not impede HSC engraftment in NSG mice.
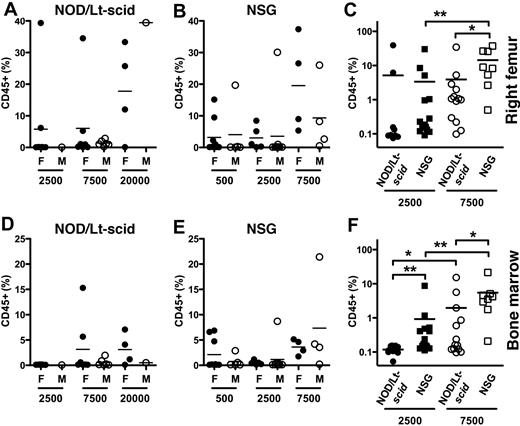
Increased engraftment in NSG mice at limiting doses. Eight- to 9-week-old female (F) and male (M) NOD/Lt-scid and NSG mice were injected in the RF at limiting doses with Lin− CB cells (500, 2500, 7500, or 20 000). The frequency of human CD45+ cells in the injected RF (A-C) and BM (D-F) of NOD/Lt-scid (A,D) and NSG (B,E) mice was measured 13 weeks later. Engraftment data at 2500 and 7500 cells for males and females were combined for comparison (C,F). Bar represents mean. *P < .05, **P < .01 by Wilcoxon rank-sum test.
Increased engraftment in NSG mice at limiting doses. Eight- to 9-week-old female (F) and male (M) NOD/Lt-scid and NSG mice were injected in the RF at limiting doses with Lin− CB cells (500, 2500, 7500, or 20 000). The frequency of human CD45+ cells in the injected RF (A-C) and BM (D-F) of NOD/Lt-scid (A,D) and NSG (B,E) mice was measured 13 weeks later. Engraftment data at 2500 and 7500 cells for males and females were combined for comparison (C,F). Bar represents mean. *P < .05, **P < .01 by Wilcoxon rank-sum test.
Improved HSC detection in NSG mice
A critical component of our study was a quantitative measure of HSC engraftment as assayed by the functional SRC assay.3 We and other groups have systematically enriched for SRC based on CD34, CD38, CD90, CD45RA, and rhodamine staining in CB cells10,11,19,34-38 (supplemental Table 1 for comparison of SRC frequencies among strains and fractions reported in the literature). Most SRC frequencies have been determined in NOD/Lt-scid mice; however, NSG mice have not been directly compared with NOD/Lt-scid mice in an SRC assay. To determine whether NSG mice detect SRCs more efficiently than NOD/Lt-scid mice, we performed limiting dilution analysis with 5 × 102, 2.5 × 103, 7.5 × 103, and 2 × 104 Lin− CB cells (Figure 5) and calculated the SRC frequency using a maximum likelihood estimator.6,11,33,37 The SRC frequency in the injected RF was approximately 3.6-fold higher in NSG than NOD/Lt-scid mice (1 in 1799 vs 1 in 6497; Table 3; supplemental Table 2). Similar improvements (4- to 7-fold) in SRC frequency were detected in the noninjected BM and spleens of NSG mice (supplemental Table 2). These data indicate that NSG are more sensitive for SRC detection compared with NOD/Lt-scid mice using optimized transplantation methods.
Limiting dilution analysis of NOD/Lt-scid and NSG mice
| Genotype/group . | Frequency of SRCs* . | Pearson P† . | |
|---|---|---|---|
| Mean . | 95% CI . | ||
| NOD/Lt-scid | |||
| Female | 1/8801 | 4424-17 509 | .472 |
| Male | 1/2545 | 891-7268 | .367 |
| Both | 1/6497 | 3781-11 166 | .097 |
| NSG | |||
| Female | 1/964 | 444-2095 | .209 |
| Male | 1/2767 | 1379-5553 | .371 |
| Both | 1/1799 | 1076-3008 | .023 |
| Genotype/group . | Frequency of SRCs* . | Pearson P† . | |
|---|---|---|---|
| Mean . | 95% CI . | ||
| NOD/Lt-scid | |||
| Female | 1/8801 | 4424-17 509 | .472 |
| Male | 1/2545 | 891-7268 | .367 |
| Both | 1/6497 | 3781-11 166 | .097 |
| NSG | |||
| Female | 1/964 | 444-2095 | .209 |
| Male | 1/2767 | 1379-5553 | .371 |
| Both | 1/1799 | 1076-3008 | .023 |
Eight- to 9-week-old female and male NOD/Lt-scid and NSG mice were injected in the RF at limiting doses with Lin− CB cells (500, 2500, 7500, or 20 000), and engraftment was measured 13 weeks later.
Frequency of SRCs in the RF was calculated using L-Calc software (StemCell Technologies) with the number of positively engrafted mice at each dose.
Pearson P value to test for inconsistency.
In a recent separate study, our laboratory has injected NSG mice with highly purified HSCs at very low (10-25 cells) and high (245-5000 cells) cell doses and found approximately 11-fold higher engraftment in female vs male NSG mice at low doses, whereas the advantage was only approximately 1.4-fold at higher cell doses.39 We confirmed the gender difference in a separate limiting dilution assay using Lin− CB cells (supplemental Table 3; supplemental Figure 3). In agreement with these results, we found female NSG mice were 9-fold more sensitive than female NOD/Lt-scid mice (1 in 964 vs 1 in 8801; Table 3). Based on these observations, the CD45+ engraftment data from experiments 1 and 2 where nonlimiting cell doses were used were reanalyzed for gender differences. In those experiments with 2.3 × 104 or 3 × 104 Lin− CB cells, only NOG mice had a gender difference in CD45+ engraftment in BM, spleen, and thymus (P = .021, data not shown). These data show that NSG females are more sensitive than NOD/Lt-scid mice in detecting SRCs; however, this was only seen at or near limiting doses, suggesting that the effect can be saturated or overcome if higher cell doses are used.
Discussion
The recent observations of increased leukemogenic engraftment and tumor initiation from melanoma cells in NOD-scid-IL2Rγnull mice25,26 compared with NOD-scid mice raise some uncertainty of how the stem cell field should interpret the large prior body of work that used NOD-scid mice.4,18 If the NOD-scid system dramatically underestimated the frequency of HSCs and also failed to support engraftment of whole classes of putative stem cells, then it follows that our understanding of the hierarchical relationship of the various cell types fractionated on the basis of cell surface phenotype might not be accurate. Hence, it is critical to compare human engraftment in NOD-scid and other more immune-deficient recipients. Here, we directly compared human multilineage hematopoietic engraftment in NOD-scid and NOD-scid-IL2Rγnull mice and found modest improvement in NOD-scid-IL2Rγnull mice at nonlimiting cell doses; but at near-limiting doses, NOD-scid-IL2Rγnull mice were superior at detecting HSC engraftment. Our results indicate that, in contrast to the massive 104 increase in cancer stem cell detection observed in melanoma, the engraftment of normal human HSCs was only improved by less than 10-fold when NSG or NOG mice were used. Whereas the entire cancer stem cell hypothesis for melanoma had to be revised when NSG was used,40 our studies indicate that the modest improvement of HSC detection in NSG will not change the core understanding of the human stem cell hierarchy as derived from prior NOD-scid studies.
Although prior studies did not perform quantitative SRC analysis, they reported a 6- to 7-fold improvement in overall human cell engraftment in the BM when the intravenous injection method was used18,19 ; we found only a modest 1.5-fold engraftment improvement in NSG mice compared with NOD/Lt-scid mice using the intrafemoral transplantation approach. Surprisingly, NOG mice had lower engraftment than NSG mice in both the RF and BM and showed no improvement over NOD/Shi-scid mice. Cells injected intravenously must home and intravasate into the BM, a process regulated in part by NK-cell activity and bypassed by intrafemoral injection.6 Our inability to show improved engraftment in NOG mice may be the result of improved detection of SRCs in NOD/Shi-scid mice by intrafemoral injection. We found no difference in CD45+ engraftment in NOD/Lt-scid or NOD/Shi-scid mice, the strains used to generate the NSG and NOG mice. Ito et al also reported no difference in CD45+ engraftment in the peripheral blood of NOD/Lt-scid and NOD/Shi-scid mice treated with anti–NK-cell antibody.19 NOD/Shi-scid mice have higher levels of NK-cell activity than NOD/Lt-scid mice19 ; however, treatment with anti-CD122 antibody probably minimized any differences in residual NK-cell activity. Thus, the differences in overall BM engraftment in NSG and NOG mice can be attributed to the presence of the IL2Rγ-chain extracellular domain and not subtle differences in NOD background.
Here, we found that anti-CD122 treatment of NSG mice did not improve engraftment when injected with nonlimiting cell doses. However, it remains to be determined whether anti-CD122 treatment would impact engraftment in NSG (or NOG) mice when limiting numbers of SRCs are injected. In addition, anti-CD122 treatment may improve engraftment in NOG mice, but we did not treat NOG mice with anti-CD122. NOG mice supported higher engraftment than NOD-scid-β2m−/− mice,19 which are depleted of NK cells, suggesting that improved engraftment is influenced by functional impairment of other immune cells in NOG mice, such as CD11c+ dendritic cells.19 Thus, anti-CD122 treatment of NOG mice may have an impact on human engraftment, although the NSG data would indicate it would be limited at best.
Clearly, NSG and NOG mice represent a significant advancement over NOD-scid mice because of increased survival and, more importantly, multilineage engraftment with efficient, sustained development of functional B and T cells in the spleen and thymus. The longer life span of NSG and NOG mice allows for studying HSC self-renewal because progenitors do not persist for more than 16 weeks.41 Although overall hematopoietic engraftment was higher in NSG mice, we found only minor differences among the strains in multilineage differentiation in the BM. The majority of cells were CD19+ B cells, with the remaining cells composed of CD33+ myeloid and CD45-GlyA+ erythroid cells. T-cell differentiation in the RF and BM was very low (< 1% CD3+) in our experiments compared with other reports. However, this was probably because of fewer injected SRCs compared with other studies and earlier assay time point because we have found that at longer times of engraftment higher T cell levels result, even at limiting cell doses.18,19,28,31,39 Multilineage differentiation also occurred in the spleen with the presence of CD19+ B cells, CD56+ NK cells, and CD3+ T cells in NSG and NOG mice. We confirmed the absence of CD3+ T cells in NOD-scid spleens.31 The majority of cells were CD19+IgM+ mature B cells, which was higher (∼ 65%) than previous reports for NOG31 and NSG18 mice (∼ 25%). NOG mice demonstrated a modest increase in CD19+IgM+ cells compared with NSG mice; however, serum immunoglobulin levels were not compared in this study. Even though B-cell differentiation was detected in NOD/Lt-scid and NOD/Shi-scid mice (CD19+IgM+ cells), human IgM and IgG were not detected in NOD/Shi-scid plasma.29 Further studies are needed to confirm whether NSG and NOG mice generate similar amounts of functionally competent B cells and serum IgM and IgG levels.
Shultz et al4,18 speculated that the IL2Rγ-chain extracellular domain in NOG mice might bind various cytokines that would affect differentiation. Exogenous IL-7 was shown to improve T-cell development in the thymus and peripheral blood of NSG mice.18 Although we were able to document lower BM CD45+ engraftment in NOG mice compared with NSG, we found minimal differences in lineage development in NSG and NOG mice (eg, CD3+ T cells in BM, Figure 2D; CD19+ and CD19+IgM+ B cells in spleen, Figure 3B-C). We found no differences in CD45+ engraftment in NSG or NOG thymi or differentiation into CD4+CD8+ double-positive cells. Even though there was no difference in CD4+ or CD8+ single-positive cells, NOG mice had a lower ratio of CD4 to CD8 single-positive cells (supplemental Figure 1C). The clinical relevance of this finding is unknown because both NSG and NOG mice are able to generate functional human B and T cells.18,19,28,31 In contrast to previous studies,18,19,28,31 we found lower T-cell engraftment, which may be the result of differences in injection route (intravenous vs intrafemoral), assay time point, or the number of injected SRCs. Indeed, CD45+ engraftment in the NSG thymus increased with more input cells (supplemental Figure 3D). Here, we measured engraftment at 11 to 13 weeks when T-cell development is still increasing.31 Examination of NSG mice 16 to 24 weeks after intrafemoral injection with nonlimiting doses of highly purified HSCs showed approximately 25% to 40% CD45+ engraftment in the thymus,39 suggesting that intrafemoral injection does not reduce the capacity of thymocyte progenitor cells to migrate from the BM to the thymus.
The relative efficiency of human HSC engraftment was the most important component of our comparison. Human HSCs are assayed by the functional SRC assay in which limiting numbers of cells are transplanted into NOD-scid mice and assayed for multilineage differentiation. Numerous studies from our group and others have enriched for SRCs based on sorting for CD34 and CD38 expression such that approximately 1 in 121 CD34+CD38−Lin− CB cells are SRCs in NOD/Lt-scid mice. In this study, we used a less enriched population, Lin− CB, and found approximately 1 in 6500 cells are SRCs in NOD/Lt-scid mice and 1 in 1800 cells in NSG mice. Thus, NSG mice were 3.6-fold more sensitive in detecting SRCs. Based on limiting dilution analysis in NSG mice, we found an approximate 9-fold improvement in SRC frequency in female mice compared with NOD/Lt-scid mice. Death of most male NOD/Lt-scid mice precluded accurate determination of SRC frequency (data not shown). The discovery of sex differences when limiting numbers of HSCs are transplanted is of great significance in efforts to purify HSCs. Using female NSG mice, in combination with a more purified population of HSCs, such as CD34+CD38−CD90+CD45RA−, our group39 has significantly enriched SRC frequency to levels approaching those reported for mouse HSCs.42 Even though we found lower levels of CD34+CD38− cells in the RF of female NSG and NOG mice (supplemental Figure 2G), suggesting that these cells may have reduced stem cell self-renewal, our group has shown that cells from female NSG mice engraft more efficiently in secondary transplantations compared with male NSG mice.39 Although we did not perform secondary transplantation in this study, the increased secondary transplantation from female NSG mice suggests that the reduction in CD34+CD38− cells in female NSG mice does not reflect the ability of these cells to self-renew.
These studies raise additional questions that remain to be resolved: (1) Are the B and T cells functionally equivalent in NSG and NOG mice? (2) What are the differences between male and female that affect human hematopoietic engraftment? (3) How does the IL2Rγ-chain extracellular domain in NOG affect differentiation? For many of these questions, studies must be done using both male and female mice injected intrafemorally at or near clonal levels with relatively pure cell populations. Given the advancements to the original NOD-scid model, there is no doubt that NSG and NOG mice will become the new “gold standard” for human HSCs and cancer stem cell research.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the laboratory of J.E.D. for critical input regarding this work and the obstetrics unit of Trillium Hospital (Mississauga, ON) for providing cord blood samples and co-op students for processing cord blood samples.
This work was supported by the Leukemia & Lymphoma Society (fellowship; K.E.), the Canadian Institutes for Health Research, the Stem Cell Network of Canadian National Centres of Excellence, the Canadian Cancer Society, the Terry Fox Foundation; Genome Canada through the Ontario Genomics Institute, Ontario Institute for Cancer Research (with funds from the province of Ontario), the Leukemia & Lymphoma Society, the Canadian Institutes for Health Research, a Canada Research Chair, and the Ontario Ministry of Health and Long Term Care.
The views expressed do not necessarily reflect those of the Ontario Ministry of Health and Long Term Care.
Authorship
Contribution: S.P.M., K.E., and E.L. designed experiments, performed research, analyzed data, and wrote the paper; M.D. designed experiments and performed research; and J.E.D. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John E. Dick, Division of Cell and Molecular Biology, University Health Network, Toronto Medical Discovery Tower, Rm 8-301, 101 College St, Toronto, ON, Canada M5G 1L7; e-mail: jdick@uhnres.utoronto.ca.