Abstract
Zosuquidar, which modulates P-glycoprotein (P-gp) with minimal delay of anthracycline clearance, may reverse P-gp–mediated resistance in acute myeloid leukemia without increased toxicity. A total of 449 adults older than 60 years with acute myeloid leukemia or high-risk myelodysplastic syndrome enrolled in a randomized placebo-controlled double-blind trial (Eastern Cooperative Oncology Group 3999). Overall survival was compared between patients receiving conventional-dose cytarabine and daunorubicin and either zosuquidar (550 mg; 212 patients) or placebo (221 patients). Median and 2-year overall survival values were 7.2 months and 20% on zosuquidar and 9.4 months and 23% on placebo, respectively (P = .281). Remission rate was 51.9% on zosuquidar and 48.9% on placebo. All cause mortality to day 42 was not different (zosuquidar 22.2% vs placebo 16.3%; P = .158). In vitro modulation of P-gp activity by zosuquidar and expression of P-gp, multidrug resistance-related protein 1, lung resistance protein, and breast cancer resistance protein, were comparable in the 2 arms. Poor-risk cytogenetics were more common in P-gp+ patients. P-gp expression and cytogenetics were correlated, though independent prognostic factors. We conclude that zosuquidar did not improve outcome in older acute myeloid leukemia, in part, because of the presence P-gp independent mechanisms of resistance. This trial is registered at www.clinicaltrials.gov as #NCT00046930.
Introduction
The expression of P-glycoprotein (P-gp), a member of the adenosine triphosphate-binding cassette family of transmembrane proteins is one factor responsible for multidrug resistance in acute myeloid leukemia (AML). Expression of P-gp correlates with a reduced complete remission (CR) rate and shorter durations of overall survival (OS) or disease-free survival (DFS) and may, in part, account for the poorer outcome of older adults with AML.1–5 A potential benefit to pharmacologic modulation of P-gp–mediated efflux was reported by the Southwest Oncology Group (SWOG 9126) trial in which patients with relapsed or refractory AML who received cyclosporine A (CSA), a competitive modulator of P-gp, had a superior event-free survival compared with patients who received placebo.6 However, subsequent randomized trials of CSA or PSC-833, a nonimmunosuppressive and nonnephrotoxic analog of CSA, failed to demonstrate an improvement in outcome.7–11 Potential explanations for the lack of benefit of P-gp modulation with PSC-833 in AML include suboptimal modulation of efflux and increased treatment toxicity because of inhibition of clearance of anthracyclines via interference with P-gp–mediated hepatobiliary excretion or metabolism. Thus, a more potent and specific modulator that does not prolong the clearance of daunorubicin may demonstrate an improved therapeutic index.
Zosquidar is a potent (Ki = 59nM) and highly selective modulator of P-gp that restored the sensitivity of cell lines selected for resistance based on P-gp expression.12,13 Preclinical studies demonstrated that zosuquidar had minimal effect on the pharmacokinetic profile of coadministered P-gp substrates. Zosuquidar does not inhibit other members of the adenosine triphosphate-binding drug transporter family, such as the multidrug resistance-related protein (MRP1) or the breast cancer resistance protein BCRP) or affect P450 isozymes at concentrations below the micromolar range.14 Phase I trials of intravenous or oral zosuquidar with doxorubicin in patients with solid tumors demonstrated only a modest decrease in clearance and increase in the area under the curve for doxorubicin.15,16 Increasing plasma concentrations of zosuquidar resulted in greater inhibition of rhodamine-123 efflux in CD56+ natural killer cells isolated from the peripheral blood of treated subjects.17 Zosuquidar was generally well tolerated, with reversible grade 1 or 2 neurologic toxicity the most common side effect.15,16 A phase 1 trial of zosuquidar administered with conventional doses of daunorubicin and cytarabine to patients with newly diagnosed AML confirmed an acceptable safety profile.18 Therefore, the Leukemia Committee of Eastern Cooperative Oncology Group (ECOG) conducted a double-blind, placebo-controlled trial of zosuquidar administered with conventional-dose induction chemotherapy in newly diagnosed AML or high-risk myelodysplastic syndromes (MDSs) who were older than 60 years.
Methods
Eligibility criteria
The protocol was reviewed and approved at the participating institutions' human subject review boards, and all patients signed informed consent in accordance with the Declaration of Helsinki. Patients older than 60 years with newly diagnosed refractory anemia with excess blasts in transformation (RAEB-t), high-risk RAEB, and de novo or secondary AML were eligible for enrollment. Secondary AML was defined as a history of an antecedent hematologic disorder or a history of prior chemotherapy or radiation therapy. Patients were considered to have high-risk RAEB if the bone marrow blast percentage was 11% to 20% or cytogenetics were poor risk.19 Eligible patients were required to have an ECOG performance status ≤ 3, a serum total bilirubin less than 3 mg/dL, and a serum creatinine less than 2 mg/dL. A resting left ventricular cardiac ejection fraction of greater than 45% by either a gated blood pool study or echocardiogram was required.
Registration and randomization procedures
Patients were registered and randomized to either placebo or zosuquidar. There were 2 stratification criteria at randomization, age (< 70 years or ≥ 70 years) and leukemia type ([1] de novo AML or RAEB-t, [3] RAEB, or [3] secondary AML). The original stratification level for leukemia type had been de novo versus secondary AML or RAEB-t (reflecting the modification of the definition of AML by the World Health Organization) and AML or RAEB-t versus high-risk RAEB, but after randomization of the first 11 patients, the stratification level was modified to be more easily understood.
Study design and protocol therapy
This was a randomized, double-blind trial of zosuquidar or placebo in addition to cytarabine (100 mg/m2 per day) by continuous intravenous infusion on days 1 to 7 and daunorubicin (45 mg/m2 per day over 10-15 minutes by intravenous infusion) on days 1, 2, and 3. There was no dose modification of daunorubicin during the initial cycle of induction. The zosuquidar or placebo infusion was initiated through a central venous catheter one hour before each dose of daunorubicin and continued for an additional 5 hours. Patients underwent a bone marrow aspirate and biopsy to assess for aplasia on days 10 to 14. Patients who achieved aplasia were allowed to receive granulocyte-macrophage colony-stimulating factor (250 μg/m2 per day) or granulocyte colony-stimulating factor (5 μg/kg per day) through recovery of the absolute neutrophil based on the institution's standard of care.
Patients with persistent AML in the bone marrow (20% cellularity with > 5% blasts) after the first induction were eligible to receive an identical second induction.
Postremission therapy
Patients with a CR or CRp (CR with incomplete recovery of platelet count) for at least 4 weeks and in whom all induction-related toxicity had resolved were eligible to receive 2 cycles of consolidation chemotherapy. The first cycle of consolidation consisted of cytarabine 1500 mg/m2 as a 1-hour intravenous infusion every 12 hours for a total of 12 doses (days 1-6) for patients younger than 70 years and 6 doses (once daily) for patients 70 years of age or older. The second cycle of consolidation was identical to the induction regimen, including either zosuquidar or placebo.
Outcome measures and definition of end points
The trial was designed to determine whether the addition of zosuquidar to conventional induction and consolidation therapy improved outcome of older adults with newly diagnosed AML or high-risk MDS. Response criteria were consistent with the revised recommendations of the International Working Group.20 The primary efficacy outcome was OS, defined as the time from randomization to death from any cause with censoring at the date last known to be alive. The secondary efficacy outcomes were rates of CR and CR plus CRp, treatment-related mortality, and progression-free survival (PFS). A CR required recovery of peripheral blood counts to an absolute neutrophil count more than 1000/μL, platelet count more than 100 000/μL, no circulating blasts, and an adequately cellular marrow with less than 5% myeloblasts. A CRp required identical findings with the exception of a platelet count between 50 000 and 99 000/μL. Treatment-related mortality was defined as death from any cause within 6 weeks of enrollment. PFS was defined as the time from randomization to documented disease progression or the date when nonprotocol therapy was administered. Death more than 3 months after the last disease evaluation was not counted as an event for PFS. Patients who were inevaluable for induction response were excluded from the PFS analysis if they neither had documented progression nor died within 3 months from registration. The date of progression was defined as the date of relapse for patients who achieved a CR or CRp. For patients with refractory disease, PFS was defined as the date when either the bone marrow aspirate was performed or the patient was removed from study. Patients without documented progression or death reported were censored at the time of the last disease evaluation.
Laboratory and nonlaboratory toxicities were graded using Version 2.0 of the Common Toxicity Criteria. To assess excess hematologic toxicity, a protocol specified bone marrow examination was performed on day 42 in the event of delayed recovery of peripheral blood counts.
Central review of pathology, cytogenetics, and immunophenotypic analyses
Fresh anticoagulated marrow aspirate or peripheral blood was submitted to the ECOG Leukemia Laboratory for 431 patients (96%). The qualifying diagnosis was confirmed by central pathology review. An informative immunophenotype was obtained by multiparameter flow cytometry in 408 patients (95%). Results were consistent with AML or the presence of leukemic myeloblasts (in high-risk MDS) in all but 2 patients (one T-lineage and one B-lineage ALL). In 23 patients, no immunophenotype was obtained because of the lack of blast cells in the submitted specimens. Conventional cytogenetic studies were performed by the institution's local cytogenetics laboratories; results and karyotypes were centrally reviewed by ECOG's Cytogenetics Committee. Each case was evaluated independently by 3 cytogeneticists and assigned to a cytogenetic risk category as defined by SWOG and ECOG.21 In the 36 patients with MDS, a normal karyotype was considered favorable.19 There was no evidence that this categorization affected the subgroup analysis.
Detection of P-gp and non-P-gp protein expression and modulation of P-gp function by zosuquidar in vitro
A secondary objective was to compare OS between treatment arms for patients who were positive for P-gp by immunologic and/or functional criteria. The detection of P-gp protein by surface binding of antibody MRK-16 (Kamiya Biomedical) was restricted to blasts by gating with CD34 or other leukemia-associated antigen(s), for instance, CD117 and HLA-DR. Expression of non-P-gp efflux proteins was studied using antibodies against MRP1, the lung resistance protein (LRP), and 2 epitopes of BCRP (Bxp-34 and Bxp-21).22–24 Patients' cells were first surface-stained with phycoerythrin-conjugated CD34 or CD117 antibody to allow gating on myeloblasts and then fixed and permeabilized with the Fix&Perm reagent (Invitrogen), incubated with MRP1, LRP-56, or the BCRP antibodies, and counterstained with goat antimouse antibody conjugated with fluorescein isothiocyanate (BD Biosciences).
Modulation of P-gp function in vitro was assessed by the flow cytometric rhodamine-123 efflux assay.9,25,26 Myeloblasts were stained with phycoerythrin-conjugated CD34, CD117, CD33, or HLA-DR (all from BD Biosciences). Maximal rhodamine-123 uptake was accomplished during a 45-minute incubation of mononuclear cells with 40μM rhodamine-123 at room temperature. Two-color flow cytometry was performed on a FACSCalibur using CellQuest Pro software Version 5.2.1 (BD Biosciences) to monitor dye efflux from blast cells during subsequent 90-minute incubation at 37°C. The extent of P-gp function was reflected in the difference of the rhodamine-123 mean fluorescence intensity (MFI) channel between baseline (before the 90-minute incubation) and maximal dye efflux (at the end of the 90-minute incubation) and expressed as percentage of rhodamine-123 shift. Modulation of P-gp function was assessed by adding either zosuquidar (100nM) or cyclosporine A (15μM, Sandoz) as P-gp modulators during the 37°C incubation.
In earlier publications, P-gp function was defined as a shift of ≥ 40% of rhodamine-123 MFI from baseline.9,25,26 In ECOG 3999, we included the extent of zosuquidar inhibition in the equation A ratio was calculated between rhodamine-123 MFI after the 37°C incubation in the presence of zosuquidar and that after incubation without zosuquidar. A ratio of 1.3 was used as threshold to define P-gp positivity, based on the finding that in 119 patients with ratios of ≤ 1.3 (median ratio = 1.09), the median shift of rhodamine-123 MFI from baseline was 22%, whereas in 256 patients with ratios more than 1.3 (median ratio = 2.52), the median shift was 71%. This interpretation of P-gp function is analogous to prior studies.11
Pharmacokinetic analysis
To evaluate the effect of zosuquidar on the pharmacokinetics of daunorubicin, blood samples were obtained from the first 100 patients enrolled from 9 ECOG sites. Samples were drawn immediately before and at 6 specified time intervals after the third dose of daunorubicin during the first course of induction and analyzed for daunorubicin and daunorubicinol concentrations using a validated high-performance liquid chromatography method. The effect of zosuquidar was assessed graphically. Scatter plots of concentration-by-time were analyzed with the median concentration-by-time curves in semilogarithmic scale for each treatment group.
Statistical design and analysis
The study was designed to have 80% power to detect a nonproportional hazards difference in OS between the 2 treatment arms at the one-sided .025 significance level. The alternative had little difference early, and 1-year, 2-year, and long-term survival rates of 30.2%, 12.8%, and 7.0% on placebo versus 39.6%, 27.1%, and 14.0% on zosuquidar. The total accrual was set at 450 to give full information of 354 deaths allowing for a 9% ineligibility rate. The design incorporated 2 interim analyses at approximately 33% and 67% information time. A truncated O'Brien and Fleming boundary was used for early stopping in favor of the alternative, and conditional power of 0.10 under the alternative was used for the boundary of futility stop.
Comparisons of baseline characteristics were performed using Fisher exact test for a 2 × 2 contingency table, the 2-sample Wilcoxon test for ordered categorical or continuous variables, and χ2 test for the others. The OS curves were estimated by the Kaplan-Meier method. Stratified log-rank tests and stratified Cox regression models were used for inference of treatment effect on the time-to-event data. The Mantel-Haenszel method was used for response rate comparison. For the stratified analyses, age and disease type were used as the stratification factors. Subgroup analyses were also conducted to investigate the consistency of treatment effect across subgroups. P values are all 2-sided, and confidence intervals are at the .95 level.
To evaluate effects of P-gp and non-P-gp proteins on OS, optimal cutoff points for P-gp ratio, rhodamine-123 shift, and protein expression levels were used. An optimal cutoff point was determined as the value that provided the most significant result in univariate Cox regression models.
Results
Patient and disease characteristics
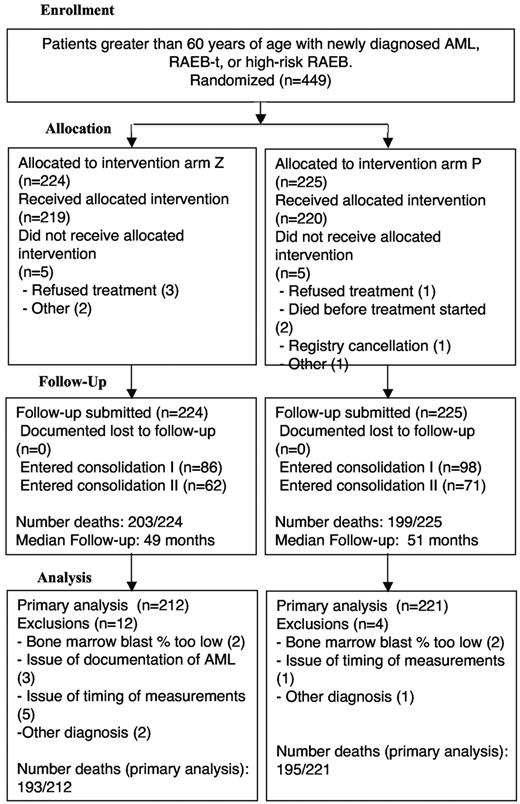
A total of 449 patients were enrolled and randomized from 30 ECOG centers between July 2002 and September 2005 (Figure 1). Sixteen patients were ineligible: 10 without confirmed AML or MDS and 6 resulting from inappropriate timing of eligibility tests. Thus, the current report summarizes the results of 433 eligible patients. A total of 212 and 221 patients were randomized to receive either zosuquidar or placebo, respectively. Of eligible patients, 5 in each arm never started treatment. A total of 330 (76.2%) patients received only one cycle of induction therapy, whereas 92 (21.2%) required a second cycle: 171 (39.5% of eligible patients and 78.4% of 218 patients in CR or CRp) received induction and consolidation cycle I, and 123 (28.4%) received induction and consolidation cycles I and II.
Selected demographic and disease characteristics of the enrolled patients are summarized in Tables 1 and 2. The median age was 69 years with an interquartile range (IQR) of 65 to 73 years. Overall, 43.3% of the patients were women. The majority (91.6%) had a diagnosis of AML; 133 (30.7%) had secondary AML or MDS, of whom 130 (97.7%) had an antecedent hematologic disorder. There were 337 evaluable diagnostic karyotypes submitted for review. The distribution of patients into risk categories is summarized in Table 2. As expected for this age group, there were few patients with favorable risk AML and nearly half of the patients had unfavorable risk (49.3% placebo; 47.0% zosuquidar). There was equal distribution of cytogenetic risk between the treatment groups. Rhodamine-123 efflux was successfully assessed and a ratio calculated in 379 patients (88% of the samples submitted before treatment). In 37 patients, the efflux assay could not be performed because of insufficient blasts. A total of 68% of the samples had a ratio more than 1.3 and were designated as P-gp+. There was no difference in the proportion of positive samples in patients randomized to zosuquidar or placebo (65.8% vs 70.4%, P = .377; Table 2). Expression of P-gp was significantly associated with the rhodamine-efflux ratio (P < .0001); cases with a ratio of less than or equal to 1.3 expressed P-gp on a median of 18% of blasts (IQR = 8.0%-38.5%), whereas cases with ratios more than 1.3 expressed P-gp on a median of 50% of blasts (IQR = 24.8%-90.0%). Using the 18% MRK-16+ blasts as threshold, 70% of patients were positive for P-gp protein expression. The proportion of samples with a ratio more than 1.3 in patients with unfavorable, intermediate, and favorable risk cytogenetics were 78.6%, 59.8%, and 36.4%, respectively (P < .001).
Baseline demographics
| Characteristic . | Placebo . | Zosuquidar . | All patients . |
|---|---|---|---|
| No. of patients | 221 | 212 | 433 |
| Age, y | |||
| Median (IQR) | 69 (65-73) | 69 (65-73) | 69 (65-73) |
| < 70, no. (%) | 123 (55.7) | 113 (53.3) | 236 (54.5) |
| 70-79, no. (%) | 92 (41.6) | 96 (45.5) | 188 (43.4) |
| ≥ 80, no. (%) | 6 (2.7) | 3 (1.4) | 9 (2.1) |
| Female sex | 85 (38.5) | 103 (48.6) | 188 (43.4) |
| ECOG performance status | |||
| 0, no. (%) | 82 (37.1) | 48 (22.9) | 130 (30.2) |
| 1, no. (%) | 110 (49.8) | 103 (49.0) | 213 (49.4) |
| 2, no. (%) | 22 (10.0) | 45 (21.4) | 67 (15.5) |
| 3, no. (%) | 7 (3.2) | 14 (6.7) | 21 (4.9) |
| Not available | 0 | 2 | 2 |
| Median LVEF, % (IQR) | |||
| Pretreatment (no.) | 64 (60-68) | 63 (57-68) | 64 (58-68) |
| Characteristic . | Placebo . | Zosuquidar . | All patients . |
|---|---|---|---|
| No. of patients | 221 | 212 | 433 |
| Age, y | |||
| Median (IQR) | 69 (65-73) | 69 (65-73) | 69 (65-73) |
| < 70, no. (%) | 123 (55.7) | 113 (53.3) | 236 (54.5) |
| 70-79, no. (%) | 92 (41.6) | 96 (45.5) | 188 (43.4) |
| ≥ 80, no. (%) | 6 (2.7) | 3 (1.4) | 9 (2.1) |
| Female sex | 85 (38.5) | 103 (48.6) | 188 (43.4) |
| ECOG performance status | |||
| 0, no. (%) | 82 (37.1) | 48 (22.9) | 130 (30.2) |
| 1, no. (%) | 110 (49.8) | 103 (49.0) | 213 (49.4) |
| 2, no. (%) | 22 (10.0) | 45 (21.4) | 67 (15.5) |
| 3, no. (%) | 7 (3.2) | 14 (6.7) | 21 (4.9) |
| Not available | 0 | 2 | 2 |
| Median LVEF, % (IQR) | |||
| Pretreatment (no.) | 64 (60-68) | 63 (57-68) | 64 (58-68) |
The differences in sex and performance status between patients randomized to zosuquidar or placebo are statistically significant at P ≤ .042 and .0001, respectively.
LVEF indicates left ventricular ejection fraction.
Baseline disease characteristics
| Characteristic . | Placebo . | Zosuquidar . | All patients . |
|---|---|---|---|
| Total | 221 | 212 | 433 |
| Disease, no. (%) | |||
| De novo AML | 137 (62) | 123 (59) | 260 (61%) |
| RAEB-t (WHO-AML) | 7 (3) | 15 (7) | 22 (5%) |
| HR-RAEB (IPSS) | 6 (3) | 8 (4) | 14 (3%) |
| Secondary AML or MDS | 70 (32) | 63 (30) | 133 (31%) |
| Unknown/missing | 1 | 3 | 4 |
| Diagnostic karyotype, no. (%) | |||
| Evaluable | 173 (78.3) | 164 (77.0) | 337 (77.6) |
| Not evaluable/not submitted | 48 (21.7) | 48 (23.0) | 96 (22.4) |
| Cytogenetic risk classification, no. (%) | |||
| Favorable | 8 (5.3) | 4 (2.7) | 12 (4.0) |
| Intermediate | 68 (45.3) | 75 (50.3) | 143 (47.8) |
| Unfavorable | 74 (49.3) | 70 (47.0) | 144 (48.2) |
| Unknown/missing | 71 | 63 | 134 |
| Peripheral WBCs, ×103/μL | |||
| Median (IQR) | 3.9 (1.7-16.5) | 7.6 (3.2-26.3) | 5.5 (1.9-22.2) |
| Peripheral blast percentage | |||
| Median (IQR) | 10 (1-35) | 18 (3-46) | 14 (1-40) |
| Rh-123 efflux with or without zosuquidar | |||
| Median (IQR) | 2.0 (1.2-3.2) | 1.8 (1.2-3.1) | 2.0 (1.2-3.1) |
| Ratio > 1.3, no. (%) | 133 (70.4) | 123 (65.8) | 256 (68.1) |
| Unknown/missing | 32 | 25 | 57 |
| MRK 16 attaining | |||
| % positive, median (IQR) | 42 (18-87) | 32 (15-81) | 38 (15-84) |
| Unknown/missing | 48 | 32 | 80 |
| Characteristic . | Placebo . | Zosuquidar . | All patients . |
|---|---|---|---|
| Total | 221 | 212 | 433 |
| Disease, no. (%) | |||
| De novo AML | 137 (62) | 123 (59) | 260 (61%) |
| RAEB-t (WHO-AML) | 7 (3) | 15 (7) | 22 (5%) |
| HR-RAEB (IPSS) | 6 (3) | 8 (4) | 14 (3%) |
| Secondary AML or MDS | 70 (32) | 63 (30) | 133 (31%) |
| Unknown/missing | 1 | 3 | 4 |
| Diagnostic karyotype, no. (%) | |||
| Evaluable | 173 (78.3) | 164 (77.0) | 337 (77.6) |
| Not evaluable/not submitted | 48 (21.7) | 48 (23.0) | 96 (22.4) |
| Cytogenetic risk classification, no. (%) | |||
| Favorable | 8 (5.3) | 4 (2.7) | 12 (4.0) |
| Intermediate | 68 (45.3) | 75 (50.3) | 143 (47.8) |
| Unfavorable | 74 (49.3) | 70 (47.0) | 144 (48.2) |
| Unknown/missing | 71 | 63 | 134 |
| Peripheral WBCs, ×103/μL | |||
| Median (IQR) | 3.9 (1.7-16.5) | 7.6 (3.2-26.3) | 5.5 (1.9-22.2) |
| Peripheral blast percentage | |||
| Median (IQR) | 10 (1-35) | 18 (3-46) | 14 (1-40) |
| Rh-123 efflux with or without zosuquidar | |||
| Median (IQR) | 2.0 (1.2-3.2) | 1.8 (1.2-3.1) | 2.0 (1.2-3.1) |
| Ratio > 1.3, no. (%) | 133 (70.4) | 123 (65.8) | 256 (68.1) |
| Unknown/missing | 32 | 25 | 57 |
| MRK 16 attaining | |||
| % positive, median (IQR) | 42 (18-87) | 32 (15-81) | 38 (15-84) |
| Unknown/missing | 48 | 32 | 80 |
The only statistically significant differences in baseline disease characteristics between patients randomized to zosuquidar or placebo are for WBCs and peripheral blasts, with P values of .015 and .011, respectively.
Secondary AML indicates antecedent hematologic disease or chemotherapy or radiation therapy for a nonmyeloid malignancy; HR-RAEB, high-risk refractory anemia with excess blasts; RAEB-t, refractory anemia with excess blast in transformation; WHO, World Health Organization, WBC, white blood cell count; and Rh-123, rhodamine-123.
Using a cutoff of 10% cells staining positively, LRP was expressed by 90% (median 73% positive myeloblasts; IQR = 32%-91%), MRP1 by 78% (median 32% positive myeloblasts; IQR = 12%-57%), and BCRP by 84% of cases when using antibody bxp-21 (median 64%; IQR = 24%-90%) and by 55% of cases when using antibody bxp-34 (median 13%, IQR = 3%-32%). In 2.2% of samples, there was no detectable level of expression. This higher sensitivity of the bxp-21 antibody may reflect preferential binding of bxp-21 to a functionally inactive state of BCRP.23 Expression of BCRP (P = .001) and MRP1 (P = .007), but not LRP, was significantly associated with P-gp. LRP expression was highest in AML with monocytic component (P = .001). To evaluate whether non-Pgp efflux activity contributed to the rhodamine-123 efflux attributed to P-gp activity, we analyzed non-Pgp protein expression in relation to rhodamine-123 shift. Only BCRP expression correlated positively with rhodamine shift (P = .02) and the P-gp ratio (P < .001).
Overall survival
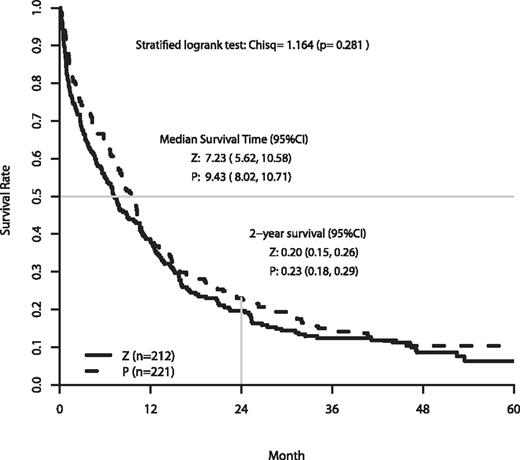
The analysis of OS is based on follow-up data as of June 2009. The median follow-up on patients still alive was 50.2 months. At the time of analysis, 388 patients had died. The efficacy outcomes are summarized in Table 3. A significant negative impact on OS was found with poorer performance status (PS), higher efflux ratio, and unfavorable cytogenetics. Figure 2 shows the OS for patients randomized to zosuquidar or placebo. There were no differences in the OS curves (P = .281, stratified log-rank test). The median and OS at 2 years were 7.2 months and 20% on zosuquidar and 9.4 months and 23% on placebo.
Efficacy of outcomes by assigned treatment
| Endpoint . | All patients . | Randomized . | P* . | |
|---|---|---|---|---|
| Placebo . | Zosuquidar . | |||
| No. of patients | 433 | 221 | 212 | — |
| Overall survival | ||||
| Median, mo | 8.3 | 9.4 | 7.2 | .281 |
| % at 2 y | 21 | 23 | 20 | |
| PFS | ||||
| Median, mo | 2.7 | 2.0 | 3.0 | .165 |
| Remissions (CR + CRi) | ||||
| No. (%) | 218 (50.3) | 108 (48.9) | 110 (51.9) | .583 |
| Treatment-related mortality | ||||
| % | 19.2 | 16.3 | 22.2 | .158 |
| Refractory disease | ||||
| % | 36.3 | 40.7 | 31.6 | .057 |
| Endpoint . | All patients . | Randomized . | P* . | |
|---|---|---|---|---|
| Placebo . | Zosuquidar . | |||
| No. of patients | 433 | 221 | 212 | — |
| Overall survival | ||||
| Median, mo | 8.3 | 9.4 | 7.2 | .281 |
| % at 2 y | 21 | 23 | 20 | |
| PFS | ||||
| Median, mo | 2.7 | 2.0 | 3.0 | .165 |
| Remissions (CR + CRi) | ||||
| No. (%) | 218 (50.3) | 108 (48.9) | 110 (51.9) | .583 |
| Treatment-related mortality | ||||
| % | 19.2 | 16.3 | 22.2 | .158 |
| Refractory disease | ||||
| % | 36.3 | 40.7 | 31.6 | .057 |
The total of CR, refractory disease, and treatment-related mortality exceeds 100% because 54 of the 157 patients with documented refractory disease died in the first 42 days.
— indicates not applicable; CRi, complete remission with incomplete platelet recovery; Treatment-related mortality, death from any cause within the first 42 days after enrollment.
Stratified log-rank test was used for OS and PFS comparisons, and Mantel-Haenszel test was used for other comparisons.
Remission rate, treatment-related mortality, and progression-free survival
Outcomes are summarized in Table 3. The remission rate (CR + CRp) was 51.9% (110 of 212) for patients treated with zosuquidar and 48.9% (108 of 221) for those treated with placebo (P = .583). There was not a significant difference in the CR rates alone (46.2%, 98 of 212 on zosuquidar vs 43.4%, 96 of 221 on placebo) P = .617). Slightly more patients who were randomized to receive placebo and achieved a CR required a second induction cycle than patients who received zosuquidar (12 of 98, 12.2% on zosuquidar vs 21 of 96, 21.9% on placebo; P = .087). The number of patients with refractory AML was similar between the 2 arms. All-cause mortality in the first 42 days of induction was not different between cohorts (zosuquidar: 22.2% vs placebo: 16.3%; P = .158). A total of 413 subjects were evaluable for PFS (202 on zosuquidar and 212 on placebo). There were no differences in median PFS (3.0 months on zosuquidar and 2.0 months on placebo, P = .160).
Multivariable analysis of prognostic factors on remission rate and OS
Although randomization was performed appropriately in this trial, there were nominally significant imbalances in sex (P = .042) and PS (P < .0001; Tables 1, 2). More women were randomly assigned to receive zosuquidar than placebo (48.6% vs 38.5%), fewer patients with a performance status of 0 were on the zosuquidar than placebo arm (22.9% vs 37.1%), and more patients with a performance status of 2 or 3 received zosuquidar than placebo (28.1% vs 13.2%).
To determine whether the imbalance in gender and ECOG PS affected OS, we performed a stratified Cox regression analysis. The results are summarized in Table 4. The stratification factors used for randomization (age < 70 vs ≥ 70] years) and disease type (de novo AML or RAEB-t vs secondary AML) were included as strata in both unadjusted and adjusted models in Table 4. The hazard ratios (HRs) were expressed as zosuquidar/placebo. Thus, a ratio less than 1 would indicate an improved outcome with zosuquidar. The unadjusted HR for treatment effect was 1.12 (95% CI, 0.92-1.37, P = .260). In the adjusted model, the following factors were associated with a significantly worse outcome: poorer ECOG PS (PS = 2 vs PS = 0, HR = 1.85; PS = 3 vs PS = 0, HR = 2.20), higher efflux ratio (> 1.3 vs ≤ 1.3, HR = 1.43), and cytogenetics (unfavorable vs intermediate, HR = 2.04). However, the HR estimate for treatment effect was not different from the unadjusted one (Table 4).
Unadjusted and adjusted hazard ratios for treatment effect: effect of baseline characteristics on comparison of OS between patients treated with zosuquidar or placebo
| Factor . | Unadjusted model . | Adjusted model . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Treatment (zosuquidar vs placebo) | 1.12 (0.91, 1.37) | .282 | 1.20 (0.89, 1.40) | .338 |
| Sex (male vs female) | — | — | 1.12 (0.90, 1.40) | .320 |
| ECOG PS (1 vs 0) | — | — | 1.11 (0.85, 1.44) | .454 |
| ECOG PS (2 vs 0) | — | — | 1.85 (1.29, 2.65) | .001 |
| ECOG PS (3 vs 0) | — | — | 2.20 (1.28, 3.79) | .004 |
| P-gp ratio (> 1.3 vs ≤ 1.3) | — | — | 1.43 (1.10, 1.85) | .007 |
| Cytogenetics (intermediate risk vs unfavorable) | — | — | 2.04 (1.54, 2.69) | < .001 |
| Cytogenetics (intermediate risk vs unknown) | — | — | 1.13 (0.85, 1.51) | .399 |
| Cytogenetics (intermediate risk vs favorable) | — | — | 0.61 (0.26, 1.40) | .240 |
| Factor . | Unadjusted model . | Adjusted model . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Treatment (zosuquidar vs placebo) | 1.12 (0.91, 1.37) | .282 | 1.20 (0.89, 1.40) | .338 |
| Sex (male vs female) | — | — | 1.12 (0.90, 1.40) | .320 |
| ECOG PS (1 vs 0) | — | — | 1.11 (0.85, 1.44) | .454 |
| ECOG PS (2 vs 0) | — | — | 1.85 (1.29, 2.65) | .001 |
| ECOG PS (3 vs 0) | — | — | 2.20 (1.28, 3.79) | .004 |
| P-gp ratio (> 1.3 vs ≤ 1.3) | — | — | 1.43 (1.10, 1.85) | .007 |
| Cytogenetics (intermediate risk vs unfavorable) | — | — | 2.04 (1.54, 2.69) | < .001 |
| Cytogenetics (intermediate risk vs unknown) | — | — | 1.13 (0.85, 1.51) | .399 |
| Cytogenetics (intermediate risk vs favorable) | — | — | 0.61 (0.26, 1.40) | .240 |
Stratified Cox regression models were used for both adjusted and unadjusted models. The stratification factors used for randomization (age < 70 vs ≥ 70 years, disease type [de novo AML or RAEB-t, RAEB, secondary AML]) were included as strata in the models. The HRs were expressed as zosuquidar/placebo. A ratio < 1 would indicate an improved outcome with zosuquidar.
— indicates not applicable.
A secondary objective of ECOG 3999 was to assess for correlations between OS and P-gp efflux activity or modulation by zosuquidar (P-gp ratio) and between OS and expression of P-gp or non-P-gp proteins. Patients with rhodamine shift greater than the optimal cutoff of 48% had a median OS of 6.8 months compared with a OS of 10.6 months for patients with less shift (P = .001). The optimal cutoff level for the P-gp ratio was less than or equal to 1.9 compared with the ratio less than or equal to 1.3 that we had calculated based on the median MFI shift. For both thresholds, higher ratios were significantly associated with shorter OS (P < .0001). There was no correlation between P-gp, LRP, MRP1 expression, and OS. BCRP as measured by bxp-34 expression was weakly correlated with 2-year survival (24% vs 14%; P = .038).
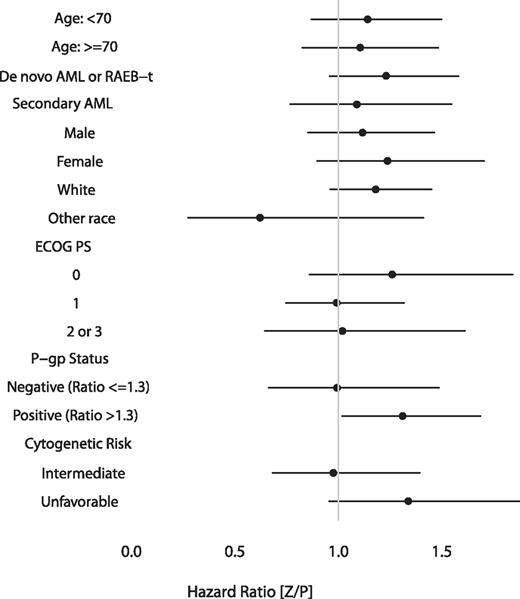
We also performed subgroup analyses to determine whether zosuquidar improved the OS for any clinically meaningful subgroup. The Forest plot (Figure 3) of the HRs demonstrates no evidence of improvement with zosuquidar within analyzed subgroups. The apparent improvement in patients who were nonwhite is difficult to interpret. The sample size (n = 26) was too small to adjust for potential confounding factors. Otherwise, there were no subgroups for which the difference in OS was in favor of treatment with zosuquidar.
Forest plot representation of subgroup analysis of OS. A ratio less than 1 indicates a benefit to zosuquidar.
Forest plot representation of subgroup analysis of OS. A ratio less than 1 indicates a benefit to zosuquidar.
Given the correlation between P-gp status and cytogenetic risk and their independent prognostic importance (Table 4), we analyzed for interactions. For patients with favorable or intermediate-risk cytogenetics, a lower P-gp ratio correlated with a longer OS (13.1 vs 10.2 months, P = .004). We were unable to identify a subgroup based on the combination of P-gp status and cytogenetic risk that benefitted from zosuquidar.
Adverse events
All enrolled patients who received at least one dose of zosuquidar or placebo during induction were monitored for the occurrence of adverse events (439 patients, 219 on zosuquidar and 210 on placebo). The most common adverse events were related to the period of prolonged and significant myelosuppression as is anticipated with induction chemotherapy. As summarized in Table 5, more than 90% of patients experienced clinically significant fever and neutropenia or infection with neutropenia and thrombocytopenia. Severe hemorrhagic complications were uncommon, with epistaxis being the most common at 5%. The median time of neutrophil recovery to greater than 1000 in all patients was 22 days (range, 1-84 days). The median time to recovery of platelets to greater than 100 000 was estimated for all patients to be 36 days (range, 1-65 days).
Adverse events (grade ≥ 3) observed in 5% or more of patients treated with either zosuquidar or placebo
| . | Zosuquidar (n = 219), % . | Placebo (n = 220), % . |
|---|---|---|
| Hematologic | ||
| Hemoglobin | 73 | 69 |
| Leukocytes | 93 | 96 |
| Neutrophils | 91 | 92 |
| Platelets | 96 | 96 |
| Transfusion: platelets | 9 | 11 |
| Transfusion: PRBCs | 8 | 8 |
| Nonhematologic | ||
| Supraventricular arrhythmias | 7 | 4 |
| Hypotension | 7 | 4 |
| Fatigue | 17 | 10 |
| Anorexia | 13 | 9 |
| Nausea | 8 | 6 |
| Stomatitis | 9 | 5 |
| Diarrhea without prior colostomy | 7 | 6 |
| Epistaxis | 6 | 6 |
| Petechiae | 3 | 6 |
| Bilirubin | 16 | 14 |
| Febrile neutropenia | 42 | 38 |
| Infection with grade 3 or 4 neutropenia | 54 | 55 |
| Ataxia | 8 | 1 |
| Confusion | 8 | 1 |
| Hallucinations | 11 | 5 |
| Dyspnea | 10 | 10 |
| Hypoxia | 7 | 6 |
| Pneumonitis/pulmonary infiltrates | 6 | 5 |
| . | Zosuquidar (n = 219), % . | Placebo (n = 220), % . |
|---|---|---|
| Hematologic | ||
| Hemoglobin | 73 | 69 |
| Leukocytes | 93 | 96 |
| Neutrophils | 91 | 92 |
| Platelets | 96 | 96 |
| Transfusion: platelets | 9 | 11 |
| Transfusion: PRBCs | 8 | 8 |
| Nonhematologic | ||
| Supraventricular arrhythmias | 7 | 4 |
| Hypotension | 7 | 4 |
| Fatigue | 17 | 10 |
| Anorexia | 13 | 9 |
| Nausea | 8 | 6 |
| Stomatitis | 9 | 5 |
| Diarrhea without prior colostomy | 7 | 6 |
| Epistaxis | 6 | 6 |
| Petechiae | 3 | 6 |
| Bilirubin | 16 | 14 |
| Febrile neutropenia | 42 | 38 |
| Infection with grade 3 or 4 neutropenia | 54 | 55 |
| Ataxia | 8 | 1 |
| Confusion | 8 | 1 |
| Hallucinations | 11 | 5 |
| Dyspnea | 10 | 10 |
| Hypoxia | 7 | 6 |
| Pneumonitis/pulmonary infiltrates | 6 | 5 |
PRBCs indicates packed red blood cells.
Nonhematologic toxicities that occurred more commonly in patients treated with zosuquidar were gastrointestinal and neurologic, specifically ataxia, confusion, or hallucinations. However, only when all gastrointestinal events were combined, an increased frequency with zosuquidar was demonstrated.
Serial measurements of cardiac ejection fraction demonstrated no evidence of increased cardiac toxicity with zosuquidar. There were no differences in left ventricular ejection fraction between baseline and after recovery from induction or consolidation II in either cohort (63%, 59.8%, and 59.3% for zosuquidar; 63.7%, 60.6%, and 62.3% for placebo).
Pharmacokinetic analysis
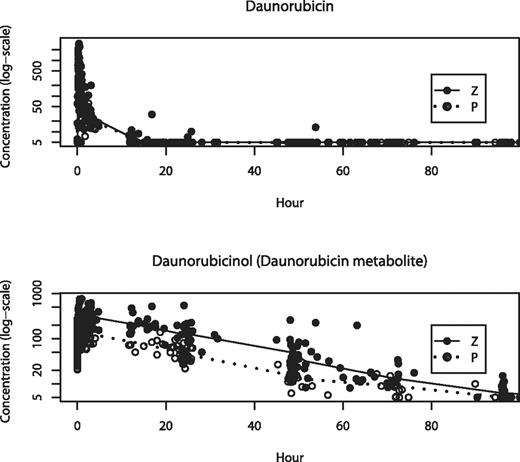
The primary pharmacokinetic objective was to compare the systemic exposure of daunorubicin and daunorubicinol in the presence or absence of zosuquidar. As can be appreciated in the time concentration plots (Figure 4), there was no significant difference in the concentrations of daunorubicin measured after the third dose through day 7 between patients treated with zosuquidar or placebo. However, the concentrations of daunorubicinol were in general greater for the patients who received zosuquidar compared with placebo.
Measured concentrations of daunorubicin and daunorubicinol over time. Patients treated with zosuquidar (●) or placebo (○). Values are median concentrations and expressed logarithmically. Time 0 is immediately before the third dose of daunorubicin.
Measured concentrations of daunorubicin and daunorubicinol over time. Patients treated with zosuquidar (●) or placebo (○). Values are median concentrations and expressed logarithmically. Time 0 is immediately before the third dose of daunorubicin.
Discussion
In this large randomized, double-blind, placebo-controlled trial of the P-gp modulator zosuquidar in adults older than 60 years with newly diagnosed AML or high-risk MDS, there was no demonstrable improvement in OS, PFS, or CR rates with zosuquidar. Functional measurements of P-gp efflux activity (rhodamine shift) and modulation by zosuquidar (P-gp ratio) correlated strongly with OS in ECOG 3999. However, there was no improvement in OS for all patients or for patients with a P-pg ratio more than 1.3.
Few trials to date have shown benefit to P-gp modulation. SWOG 9126, a trial of CSA in relapsed or refractory AML, is the notable exception.6 However, in the SWOG trial, the improvement was restricted to event-free survival but not in the primary endpoint of CR rate or OS. It was impossible, furthermore, to exclude the possibility that the prolonged infusion of daunorubicin or the altered pharmacokinetics of daunorubicin were the explanations for the improved outcome with CSA. Furthermore, the benefit was restricted to the P-gp+ subgroup. The latter observation is noteworthy in that the frequency of zosuquidar modulation of rhodamine-123 efflux was 67.5% in ECOG 3999, which was consistent with the prevalence of P-gp positivity in the 3 trials of PSC-833 in older adults with AML.7,8,11 So the negative results of 4 trials in elderly AML are not attributable to a low prevalence of the therapeutic target. Furthermore, subgroup analyses did not demonstrate a positive treatment effect in the P-gp+ group compared with the P-gp− group in any of the 3 trials of PSC-833 or in ECOG 3999.
The therapeutic value of P-gp modulation may be limited by the existence of multiple other efflux pumps or efflux-independent mechanisms of chemotherapy resistance in older adults.3,27,28 The expression of efflux proteins other than P-gp on AML blasts has been correlated with an inferior prognosis.23,29–34 However, in ECOG 3999, despite coexpression of multiple efflux proteins by myeloblasts in the majority of patients, we were unable to demonstrate a direct correlation between OS and percentage of positive myeloblasts or intensity of staining for MRP, LRP, or BCRP. The lack of prognostic significance of non-P-gp protein expression may be the result of our patient cohorts (all older patients with newly diagnosed AML and almost half with poor-risk cytogenetics), the increased statistical power of our large sample size, or the fact that the primary endpoint of ECOG 3999 was OS and the correlation between expression and outcomes in several prior studies was with CR rate or event-free survival. The current finding that undifferentiated AML, defined as CD65 negativity, was associated with high expression of P-gp, BCRP, and MRP as well as elevated Pgp ratio supports the validity of our antibody staining methodology.23,32,33 In agreement with ECOG 3999, Schaich et al reported that the prognostic effect of MRP1 demonstrable in younger patients was not evident in patients older than 60 years.33
BCRP may have contributed to the non–Pgp-mediated drug efflux activity observed in ECOG 3999. The correlation of BCRP and efflux is relevant as BCRP is not inhibited by zosuquidar.18 In support of this, Legrand proposed that in patients with unfavorable cytogenetics modulation of both P-gp and MRP1 may be necessary to improve treatment results.35 Furthermore, data on mitoxantrone accumulation suggest that, although P-gp was the most efficient efflux pump, BCRP effectively transported mitoxantrone when P-gp and MRP1 were inhibited in vitro.23 Our differential protein expression data with bxp-21 and bxp-34 suggest that functionally active BCRP, recognized with antibody bxp-34, was expressed by only a small fraction of myeloblasts (median, 13%) compared with total BCRP (active plus inactive state) recognized by bxp-21 (median, 64%) in the majority of patients. It is conceivable that the inhibition of P-gp by zosuquidar in vivo caused the conversion of BCRP into a functionally active state. The clinical significance of BCRP-mediated resistance is suggested by the marginal correlation with 2-year OS.
Resistance resulting from nonefflux mechanisms may also explain the failure of zosuquidar to improve outcome in patients with clear evidence of zosuquidar modulation of rhodamine-123 efflux in vitro. In multivariable analysis, cytogenetic risk stratification and P-gp status were independent prognostic factors. This result is consistent with Leith et al who demonstrated that a combination of P-gp expression, secondary AML, and poor-risk cytogenetics identified the group with the worse outcome.3 In ECOG 3999, the P-gp ratio did not add further significant prognostic impact to the dismal outcome observed in patients with poor-risk cytogenetics. One potential explanation is that P-gp–mediated efflux is only one of several mechanisms of resistance. The inability to detect any subgroup for which zosuquidar modulation was of benefit suggests that other mechanisms of resistance overwhelm any potential benefit of zosuquidar modulation.
Finally, a recent phase 1 trial raises the concern that the dose of 550 mg initiated 1 hour before the injection of daunorubicin and continued for 5 additional hours was not sufficient to modulate P-gp. Lancet et al reported the administration of zosuquidar (700 or 800 mg) over 72 hours commencing 4 hours before the first daunorubicin dose.36 The rationale was based on the in vitro effect of the duration of zosuquidar exposure on the IC50 for daunorubicin in a P-gp–expressing cell line, which demonstrated at least 12 hours of coexposure was required to reverse daunorubicin resistance. The dose and schedule of zosuquidar in ECOG 3999 were selected to provide maximal inhibition during the 2 distribution phases of daunorubicin and to minimize the potential for increased toxicity resulting from prolonged clearance of daunorubicin and its metabolites. Prior studies of rhodamine-123 efflux by CD56+ natural killer cells or CD33+ leukemia blasts demonstrated a direct, rapid, and reversible concentration-effect relationship.16,18,37 The increased concentration of daunorubicinol observed in patients treated with zosuquidar suggests that a meaningful pharmacodynamic effect was achieved in ECOG 3999. Only a randomized trial of alternative schedules of zosuquidar could resolve the issue of whether the infusion time of zosuquidar was inadequate in ECOG 3999.
However, further trials of zosuquidar or other P-gp modulators will confront the possibility of an increased frequency of severe organ toxicity (eg, mucositis or prolonged myelosuppression) because of the pharmacokinetic interactions with chemotherapy. The mortality rate in the placebo arm of ECOG 3999 compared favorably to other studies in older adults. However, there was a trend toward an increased number of deaths in the first 42 days for patients treated with zosuquidar (Table 3). This trend was observed in all subgroups. Prolonged exposure to zosuquidar has also been linked to unacceptable incidence of ataxia, confusion, and hallucinations.
In conclusion, the addition of zosuquidar, a potent and selective modulator of P-gp–mediated drug efflux in vitro, to standard induction chemotherapy failed to improve the outcome of older patients with newly diagnosed AML. The coexpression of non-Pgp proteins and nonefflux mechanisms of resistance appears to limit the therapeutic benefit of P-gp modulation. Thus, future trials of P-gp modulators are unlikely to demonstrate benefit and alternative strategies should be sought.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Rick J. Scheper, Vrije Universiteit, Amsterdam, The Netherlands for providing antibodies (LRP, MRP, and BCRP).
This work was supported in part by the US Public Health Service (grants CA23318, CA66636, CA21115, CA49883, CA13650, CA14958, CA11083, CA15488, and CA17145) and the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. This study was conducted by the Eastern Cooperative Oncology Group (Dr Robert L. Comis, Chair).
Contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
National Institutes of Health
Authorship
Contribution: L.D.C., M.R.L., J.M.R., and M.S.T. designed the study; L.D.C., S.L., M.S.T., M.R.L., E.M.P., and R.P.K. obtained and analyzed data; H.U., R.P.K., and E.P. performed statistical analyses; L.D.C., H.U., E.M.P., and H.M.L. wrote the paper; E.M.P. analyzed flow cytometric and efflux studies; J.M.B. reviewed the diagnostic marrow aspirates and biopsies; and all authors read, gave comments, and approved of the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Larry D. Cripe, Indiana University Simon Cancer Center, 535 Barnhill Dr, Indianapolis, IN 46202; e-mail: lcripe@iupui.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal