Abstract
Adoptive transfer of genetically modified T cells is an attractive approach for generating antitumor immune responses. We treated a patient with advanced follicular lymphoma by administering a preparative chemotherapy regimen followed by autologous T cells genetically engineered to express a chimeric antigen receptor (CAR) that recognized the B-cell antigen CD19. The patient's lymphoma underwent a dramatic regression, and B-cell precursors were selectively eliminated from the patient's bone marrow after infusion of anti–CD19-CAR-transduced T cells. Blood B cells were absent for at least 39 weeks after anti–CD19-CAR-transduced T-cell infusion despite prompt recovery of other blood cell counts. Consistent with eradication of B-lineage cells, serum immunoglobulins decreased to very low levels after treatment. The prolonged and selective elimination of B-lineage cells could not be attributed to the chemotherapy that the patient received and indicated antigen-specific eradication of B-lineage cells. Adoptive transfer of anti–CD19-CAR-expressing T cells is a promising new approach for treating B-cell malignancies. This study is registered at www.clinicaltrials.gov as #NCT00924326.
Introduction
T cells can be genetically modified to express chimeric antigen receptors (CARs).1-5 CARs consist of an antigen-recognition moiety, such as antibody-derived, single-chain variable fragments, coupled to T-cell activation domains.1-4 T cells have been genetically engineered to express CARs that can recognize a variety of tumor-associated antigens, including the B-lineage antigen CD19, in a non-human leukocyte antigen-restricted manner.4-15 Expression of the cell-surface protein CD19 is restricted to normal mature B cells, malignant B cells, B-cell precursors, and plasma cells.16-19 We have designed a CAR that targets CD19 and initiated a clinical trial of autologous T cells expressing this CAR (www.clinicaltrials.gov; #NCT00924326).
Methods
This clinical trial was approved by the National Cancer Institute Institutional Review Board. Design and construction of the mouse stem cell virus-based splice-gag retroviral vector MSGV-FMC63-28Z encoding the anti-CD19 CAR used in our clinical trial have been described (GenBank HM852952).7 The anti-CD19 CAR contains an antigen-recognition moiety consisting of the variable regions of the FMC63 monoclonal antibody joined to part of the CD28 molecule and the signaling domains of the CD3ζ molecule.
Peripheral blood mononuclear cells were transduced with retroviruses encoding the anti-CD19 CAR and cultured in an almost identical manner as previously described.20 As measured by flow cytometry, the CAR was expressed on 64% of the infused cells, which were 98% CD3+ T cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The T cells were 66% CD8+ and 34% CD4+. The anti–CD19-CAR-transduced T cells specifically recognized CD19+ target cells (supplemental Table 1). Methods of T-cell preparation, flow cytometry, polymerase chain reaction, and immunohistochemistry are in the supplemental data. For the immunohistochemistry images in Figures 1 and 2, images were obtained via digital microscopy using an Olympus BX51 microscope (Olympus America) equipped with a UPlanFL 10×/0.3 numeric aperture and UPlanFL 40×/0.75 numeric aperture objectives. Images were captured using an Olympus DP70 digital camera system. Imaging software was Adobe Photoshop CS3 (Adobe Systems).
Results and discussion
The patient was diagnosed with grade 1, stage IVB follicular lymphoma in 2002. Before enrollment on our protocol, he had received the following treatments for his lymphoma: PACE (prednisone, doxorubicin, cyclophosphamide, and etoposide), an idiotype vaccine, the anti–CTLA-4 monoclonal antibody ipilimumab, and EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab). The last cycle of EPOCH-R was administered in January 2008. The EPOCH-R caused a partial remission; however, progressive disease was noted in July 2008. The patient received no further treatment before he was evaluated for enrollment on our trial of anti–CD19-CAR-transduced T cells.
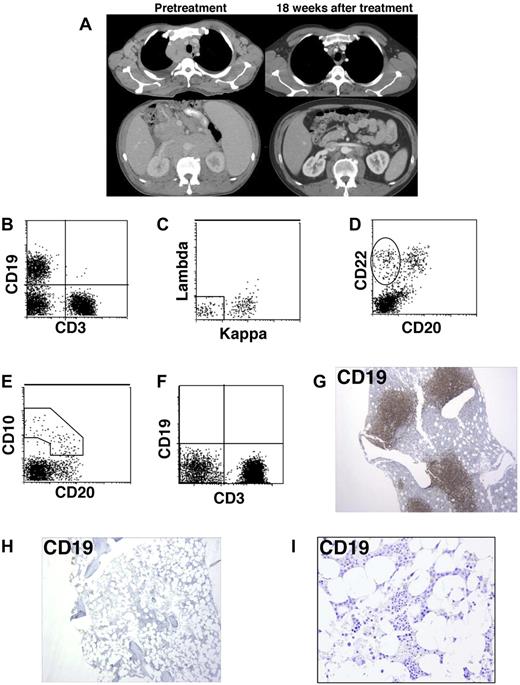
When we evaluated the patient in May 2009, he had progressive lymphoma that involved all major lymph node areas (Figure 1A). He had bilateral pleural effusions, night sweats, and a recent weight loss of 10 pounds. Flow cytometry of a fine needle aspirate from an enlarged cervical lymph node demonstrated a monoclonal B-cell process consistent with follicular lymphoma that uniformly expressed CD19, CD20, CD22, CD10, and IgM-kappa. Flow cytometry showed that 14.5% of the blood lymphoid cells had a phenotype that was consistent with the lymphoma and 0.7% of the blood lymphoid cells were normal polyclonal B cells (data not shown). Before treatment, 35% of bone marrow lymphoid cells expressed CD19 (Figure 1B). A total of 55% of these CD19+ cells were monoclonal κ-positive and λ-negative lymphoma cells; 45% of the bone marrow CD19+ cells were normal surface-immunoglobulin (Ig)–negative immature B-cell precursors (Figure 1C). The immature B-cell precursors demonstrated a pattern of antigen expression consistent with normal maturation, namely, CD22+ B cells with decreasing CD10 expression correlating with increasing CD20 expression (Figure 1D-E).21,22 Large numbers of bone marrow CD19+ cells and CD79a+ cells were detected by immunohistochemistry before treatment (Figures 1G, 2A).
B-lineage cells, including B-cell precursors, were eradicated from the bone marrow after treatment with anti–CD19-CAR-transduced T cells. (A) Representative pretreatment computed tomography scan images and images from 18 weeks after treatment demonstrate regression of lymphoma masses in the chest and abdomen after treatment with chemotherapy followed by anti–CD19-CAR-transduced T cells plus IL-2. (B) Flow cytometric evaluation of a pretreatment bone marrow aspirate was conducted with a forward versus side light scatter analysis gate of lymphoid cells. The left upper quadrant contains CD19+ B-lineage cells (35% of lymphoid cells), and the right lower quadrant contains CD3+ T cells. (C) Flow cytometric evaluation of a pretreatment bone marrow aspirate with a CD19+ analysis gate is shown. κ- and λ-negative, CD19+, mostly immature B-lineage cells that are not part of the malignant lymphoma clone are in the rectangle. The cells outside the rectangle are mostly lymphoma cells. (D) Flow cytometric evaluation of a pretreatment bone marrow aspirate with a forward versus side light scatter analysis gate of lymphoid cells. Immature B-cell precursors in the oval are CD22+ and CD20−. (E) Flow cytometric evaluation of a pretreatment bone marrow aspirate with a forward versus side light scatter analysis gate of lymphoid cells. Immature B-cell precursors in the polyhedral demonstrate decreasing CD10 correlating with increasing CD20 expression. (F) Flow cytometric evaluation of a bone marrow aspirate from 36 weeks after treatment with a forward versus side light scatter analysis gate of lymphoid cells. CD19+ B-lineage cells are absent. (G) Immunohistochemistry staining of a pretreatment bone marrow biopsy reveals a large population of CD19+ cells that includes lymphoma cells as well as nonmalignant B-lineage cells. (H) Immunohistochemistry staining of a bone marrow biopsy from 36 weeks after infusion of anti–CD19-CAR-transduced T cells demonstrates a complete absence of CD19+ cells. (I) High-power view of the same anti-CD19 staining shown in panel H.
B-lineage cells, including B-cell precursors, were eradicated from the bone marrow after treatment with anti–CD19-CAR-transduced T cells. (A) Representative pretreatment computed tomography scan images and images from 18 weeks after treatment demonstrate regression of lymphoma masses in the chest and abdomen after treatment with chemotherapy followed by anti–CD19-CAR-transduced T cells plus IL-2. (B) Flow cytometric evaluation of a pretreatment bone marrow aspirate was conducted with a forward versus side light scatter analysis gate of lymphoid cells. The left upper quadrant contains CD19+ B-lineage cells (35% of lymphoid cells), and the right lower quadrant contains CD3+ T cells. (C) Flow cytometric evaluation of a pretreatment bone marrow aspirate with a CD19+ analysis gate is shown. κ- and λ-negative, CD19+, mostly immature B-lineage cells that are not part of the malignant lymphoma clone are in the rectangle. The cells outside the rectangle are mostly lymphoma cells. (D) Flow cytometric evaluation of a pretreatment bone marrow aspirate with a forward versus side light scatter analysis gate of lymphoid cells. Immature B-cell precursors in the oval are CD22+ and CD20−. (E) Flow cytometric evaluation of a pretreatment bone marrow aspirate with a forward versus side light scatter analysis gate of lymphoid cells. Immature B-cell precursors in the polyhedral demonstrate decreasing CD10 correlating with increasing CD20 expression. (F) Flow cytometric evaluation of a bone marrow aspirate from 36 weeks after treatment with a forward versus side light scatter analysis gate of lymphoid cells. CD19+ B-lineage cells are absent. (G) Immunohistochemistry staining of a pretreatment bone marrow biopsy reveals a large population of CD19+ cells that includes lymphoma cells as well as nonmalignant B-lineage cells. (H) Immunohistochemistry staining of a bone marrow biopsy from 36 weeks after infusion of anti–CD19-CAR-transduced T cells demonstrates a complete absence of CD19+ cells. (I) High-power view of the same anti-CD19 staining shown in panel H.
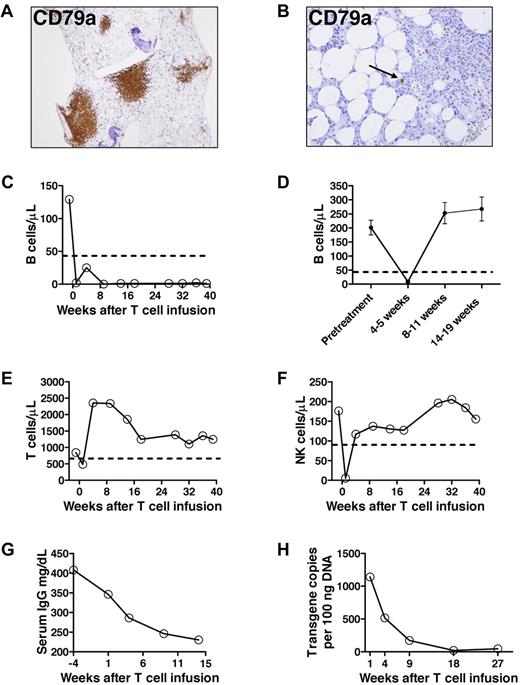
Prolonged B-cell depletion after anti–CD19-CAR-transduced T-cell infusion. (A) Immunohistochemistry staining of a pretreatment bone marrow biopsy shows a large population of CD79a+ cells. (B) Thirty-six weeks after anti–CD19-CAR-transduced T-cell infusion, rare CD79a+ cells were detected by immunohistochemisty staining of a bone marrow biopsy. The cells did not appear to be plasma cells morphologically. The number of CD79a+ cells was substantially below normal limits. The arrow indicates one of the rare CD79a+ cells. (C) The blood B-cell count of the patient treated with anti–CD19-CAR-transduced T cells is shown before treatment and at multiple time points after treatment. B cells were measured by flow cytometry for CD19. The dashed line indicates the lower limit of normal. Day 0 is the day of the second anti–CD19-CAR-transduced T-cell infusion. (D) The mean ± SEM blood B-cell count is shown for patients who received infusions of T cells targeted to either the NY-ESO antigen or the gp100 antigen. The patients all received the same chemotherapy and IL-2 regimen as the patient who received anti–CD19-CAR-transduced T cells. NY-ESO and gp100 are not expressed by B cells. Day 0 is the day of T-cell infusion. All available B-cell counts were included for each time point (pretreatment, n = 28; 4-5 weeks after T-cell infusion, n = 29; 8-11 weeks after T-cell infusion, n = 31; 14-19 weeks after T-cell infusion, n = 20). All patients with available samples had a B-cell count in the normal range by 14 to 19 weeks after T-cell infusion. (E) The blood CD3+ T-cell count of the patient treated with anti–CD19-CAR-transduced T cells is shown before treatment and at multiple time points after treatment. (F) The blood NK cell count of the patient treated with anti–CD19-CAR-transduced T cells is shown before treatment and at multiple time points after treatment. NK cells were measured by flow cytometry as CD3−, CD16+, CD56+ cells. (E-F) Day 0 is the day of the second anti–CD19-CAR-transduced T-cell infusion, and the dashed line indicates the lower limit of normal. (G) The serum IgG level of the patient treated with anti–CD19-CAR-transduced T cells is shown before treatment and at multiple time points after treatment. Day 0 is the day of the second anti–CD19-CAR-transduced T-cell infusion. (H) Real-time polymerase chain reaction was performed with a primer and probe set that was specific for the anti-CD19 CAR. Anti–CD19-CAR-transduced T cells were undetectable in pretreatment blood samples. The anti–CD19 CAR transgene was detected in the peripheral blood of the patient who received anti–CD19-CAR-transduced T cells from 1 to 27 weeks after anti–CD19-CAR-transduced T-cell infusion.
Prolonged B-cell depletion after anti–CD19-CAR-transduced T-cell infusion. (A) Immunohistochemistry staining of a pretreatment bone marrow biopsy shows a large population of CD79a+ cells. (B) Thirty-six weeks after anti–CD19-CAR-transduced T-cell infusion, rare CD79a+ cells were detected by immunohistochemisty staining of a bone marrow biopsy. The cells did not appear to be plasma cells morphologically. The number of CD79a+ cells was substantially below normal limits. The arrow indicates one of the rare CD79a+ cells. (C) The blood B-cell count of the patient treated with anti–CD19-CAR-transduced T cells is shown before treatment and at multiple time points after treatment. B cells were measured by flow cytometry for CD19. The dashed line indicates the lower limit of normal. Day 0 is the day of the second anti–CD19-CAR-transduced T-cell infusion. (D) The mean ± SEM blood B-cell count is shown for patients who received infusions of T cells targeted to either the NY-ESO antigen or the gp100 antigen. The patients all received the same chemotherapy and IL-2 regimen as the patient who received anti–CD19-CAR-transduced T cells. NY-ESO and gp100 are not expressed by B cells. Day 0 is the day of T-cell infusion. All available B-cell counts were included for each time point (pretreatment, n = 28; 4-5 weeks after T-cell infusion, n = 29; 8-11 weeks after T-cell infusion, n = 31; 14-19 weeks after T-cell infusion, n = 20). All patients with available samples had a B-cell count in the normal range by 14 to 19 weeks after T-cell infusion. (E) The blood CD3+ T-cell count of the patient treated with anti–CD19-CAR-transduced T cells is shown before treatment and at multiple time points after treatment. (F) The blood NK cell count of the patient treated with anti–CD19-CAR-transduced T cells is shown before treatment and at multiple time points after treatment. NK cells were measured by flow cytometry as CD3−, CD16+, CD56+ cells. (E-F) Day 0 is the day of the second anti–CD19-CAR-transduced T-cell infusion, and the dashed line indicates the lower limit of normal. (G) The serum IgG level of the patient treated with anti–CD19-CAR-transduced T cells is shown before treatment and at multiple time points after treatment. Day 0 is the day of the second anti–CD19-CAR-transduced T-cell infusion. (H) Real-time polymerase chain reaction was performed with a primer and probe set that was specific for the anti-CD19 CAR. Anti–CD19-CAR-transduced T cells were undetectable in pretreatment blood samples. The anti–CD19 CAR transgene was detected in the peripheral blood of the patient who received anti–CD19-CAR-transduced T cells from 1 to 27 weeks after anti–CD19-CAR-transduced T-cell infusion.
The patient underwent apheresis, and peripheral blood mononuclear cells were used to prepare anti–CD19-CAR-transduced T cells. The patient received a lymphocyte-depleting regimen consisting of 60 mg/kg cyclophosphamide daily for 2 days followed by 5 daily doses of 25 mg/m2 fludarabine. The day after the last fludarabine dose, the patient received 1 × 108 anti–CD19-CAR-transduced T cells intravenously. The next day, he received 3 × 108 anti–CD19-CAR-transduced T cells intravenously. After the second anti–CD19-CAR-transduced T-cell infusion, the patient received 720 000 IU/kg interleukin-2 (IL-2) intravenously every 8 hours. Eight doses of IL-2 were administered. The only acute toxicities that the patient experienced were cytopenias that were attributable to chemotherapy and a fever that lasted 2 days (maximum temperature, 38.5°C). The patient was discharged 11 days after his second anti–CD19-CAR-transduced T-cell infusion, and he resumed full-time employment.
After therapy, computed tomography scans revealed an impressive partial remission of the lymphoma that lasted 32 weeks (Figure 1A); 32 weeks after treatment, progressive CD19+ lymphoma was noted in right cervical and retroperitoneal lymph nodes.
Blood B cells were absent from 9 weeks after anti–CD19-CAR-transduced T-cell infusion until at least 39 weeks after anti–CD19-CAR-transduced T-cell infusion (Figure 2C; supplemental Figure 2). This prolonged B-cell depletion cannot be attributed to the chemotherapy that the patient received. Neither the New York esophageal squamous cell carcinoma antigen-1 (NY-ESO) nor the melanoma antigen gp100 is expressed by B cells.23,24 In prior clinical trials, patients treated with the same chemotherapy and IL-2 regimen as the patient described in this report along with T cells retrovirally transduced with receptors that recognized either NY-ESO or gp100 did not experience prolonged B-cell depletion (Figure 2D).
Except for B cells and a mild thrombocytopenia, all blood cell counts, including neutophils, erythrocytes, T cells, and NK cells, of the patient treated with anti–CD19-CAR-transduced T cells recovered to normal levels by 9 weeks after treatment (Figure 2E-F).
Thirty-six weeks after anti–CD19-CAR-transduced T cells were infused, CD19+ cells were absent from the bone marrow as measured by flow cytometry (Figure 1F) and immunohistochemistry (Figure 1H-I). CD79a+ cells were undetectable in the bone marrow by immunohistochemistry 14 weeks after treatment (data not shown). CD79a+ cells were detected at greatly below normal frequency 36 weeks after anti–CD19-CAR-transduced T-cell infusion (Figure 2B). CD79a is expressed earlier in B-cell development than CD19,25 so the presence of a small number of CD79a+ cells while CD19+ cells were absent suggests early recovery of B-lineage cells.
A decrease in serum IgG levels occurred after treatment (Figure 2G). Serum IgM was undetectable from 9 to at least 39 weeks after treatment. Serum IgA was 66.8 mg/dL before treatment. Serum IgA decreased to below the detectable limit of 10 mg/dL after treatment (supplemental Figure 3). Five months after treatment, the patient developed pneumonia of unknown etiology that required hospitalization. After a course of antibiotics, the patient recovered completely. The patient has subsequently received intravenous Ig replacement, and he has not had further infections.
The anti-CD19 CAR transgene was detected in peripheral blood mononuclear cells from one to 27 weeks after anti–CD19-CAR-transduced T-cell infusion with a quantitative real-time polymerase chain reaction assay (Figure 2H).
This is the first patient treated on our trial and the only patient with long enough follow-up to evaluate B-cell depletion. The prolonged elimination of CD19+ cells in this patient indicates in vivo antigen-specific activity of anti–CD19-CAR-expressing T cells. Our findings should encourage continued study of anti–CD19-CAR-transduced T cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Margaret Brown for flow cytometry, Manuel Van Deventer for Ig assays, Laura Devillier for T-cell preparation, and Hui Xu, Mary Black, and Zhili Zheng for technical assistance.
This work was supported by the Center for Cancer Research, National Cancer Institute, National Institutes of Health (intramural funding).
National Institutes of Health
Authorship
Contribution: J.N.K. designed the protocol, provided patient care, conducted experiments, analyzed data, and wrote the paper; W.H.W., J.E.J., D.-A.N.N., and B.J.L. provided patient care, assisted protocol design, and edited the paper; S.A.F. and R.A.M. provided reagents and interpreted data; M.E.D. conducted experiments and edited the paper; M.S.-S., I.M., and M.R. conducted experiments, interpreted data, and edited the paper; and S.A.R. designed the protocol, interpreted data, and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James N. Kochenderfer, National Institutes of Health, 10 Center Dr, CRC Rm 3-3888, Bethesda, MD 20892; e-mail: kochendj@mail.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal