Abstract
Focal adhesion kinase (FAK) is a non-receptor tyrosine kinase that has been extensively studied in fibroblasts; however its function in hematopoiesis remains an enigma. FAK is thought to be expressed in myeloid and erythroid progenitors, and its expression is enhanced in response to cytokines such as granu-locyte macrophage colony-stimulating factor. Furthermore, bone marrow cells cultured in granulocyte macrophage colony-stimulating factor show active migration and chemoattractant-induced polarization, which correlates with FAK induction. While loss of FAK in mice results in embryonic lethality, we have deleted FAK in the adult bone marrow. We show an essential role for FAK in regulating hemolytic, myelotoxic, as well as acute inflammatory stress responses in vivo. In vitro, loss of FAK in erythroid and myeloid progenitor's results in impaired cytokine induced growth and survival, as well as defects in the activation and expression of antiapoptotic proteins caspase 3 and Bcl-xL. Additionally, reduced migration and adhesion of myeloid cells on extracellular matrix proteins, as well as impaired activation of Rac GTPase is also observed in the absence of FAK. Our studies reveal an essential role for FAK in integrating growth/survival and adhesion based functions in myeloid and erythroid cells predominantly under conditions of stress.
Introduction
Focal adhesion kinase (FAK) is a ubiquitously expressed nonreceptor protein tyrosine kinase.1,2 Its function in mammalian system has been largely explored in fibroblasts, where it plays an essential role in regulating focal adhesions. Attachment of cells to extracellular matrix proteins (ECM) such as fibronectin (FN) results in rapid activation of FAK. While integrins are thought to be the primary activators of FAK; growth factor receptors and cytokines such as thrombopoietin (TPO), epidermal growth factor receptor, as well as platelet-derived growth factor receptor also use FAK as a signaling module.3-6
In response to receptor activation, FAK undergoes rapid phosphorylation on tyrosine residue 397. Phosphorylation of this site on FAK allows for the binding of additional Src homology-2 (SH2) containing proteins such as members of the Src family kinases (SFK).7,8 SFKs further enhance the phosphorylation of FAK by phosphorylating additional tyrosine residues on FAK.9 These additional phosphorylated tyrosine residues allow for the recruitment of additional SH3 and SH2 domain consisting proteins including adaptor molecules, which results in an overall amplification of FAK-induced signals in these cells.10,11 While several downstream signaling molecules have been shown to be activated by FAK, some of the most common substrates include phosphoinositol-3Kinase, phospholipase C-γ, and various members of the Rho family GTPases including Rac and Rho.12-14
Although the role of FAK has been well characterized in fibroblasts, its physiologic function in primary hematopoietic erythroid and myeloid progenitor cells remains enigmatic. Using conditional knockout mice of FAK, in which FAK was deleted only in megakaryocytes, Hitchcock et al showed that loss of FAK in the megakaryocytic lineage results is enhanced megakaryopoiesis, which is associated with significant increase in megakaryocytic progenitors (CFU-MK), mature megakaryocytes, megakaryocytic ploidy, and moderate increase in resting platelet number and platelet recovery following a thrombocytopenic stress.6 In addition to the expression and function of FAK in megakaryopoiesis, FAK is also expressed in granulocyte macrophage progenitor cells, mast cells, as well as erythroid progenitors.15-20 Primary wild-type (WT) bone marrow (BM)–derived cells cultured in the presence of granulocyte macrophage colony-stimulating factor (GM-CSF) show a significant increase in the expression of FAK.15 In contrast, stimulation of these cells with interleukin-3 (IL-3) does not induce FAK expression.15 Furthermore, BM cells cultured in the presence of GM-CSF show active migration and chemoattractant-induced polarization, which is associated with FAK induction.15 FAK is also highly expressed in BM cells derived from patients with acute myeloid leukemia (AML).21 FAK-positive AML cells demonstrate significantly greater ability to migrate and resistance to daunorubicin, compared with FAK-negative cells.21 In AML patients, FAK expression is also associated with high blast cell count, early death, and shorter survival rate.21
While studies suggest that FAK is expressed in BM-derived myeloid and erythroid progenitors; its physiologic role in these cells is unknown. This is partly because Fak deletion in mice results in embryonic lethality around day 8.5, thus precluding the analysis of definitive hematopoiesis in these mice. Therefore, to determine the physiologic role of FAK in BM-derived myeloid and erythroid cells in vivo and in vitro, we have deleted FAK in the adult BM compartment by performing a genetic cross between Fak-floxed mice and Mx-Cre mice. Our studies reveal novel function(s) of FAK in BM-derived progenitor cells under steady-state conditions as well as under conditions of stress. In addition, we provide biochemical mechanism(s) by which FAK contributes to various defects in these cells.
Methods
Mice
Generation of mice containing a conditional loxP-flanked allele of FAK, Fakflox/flox, is described in Beggs et al.22 Mx-Cre mice were purchased from Jackson Laboratories. All mice were maintained in a pathogen-free facility at Indiana University School of Medicine, Indianapolis. All studies were approved by the Indiana University Laboratory Animal Resource Center. Conditional Fakflox/flox mice were genotyped using tail DNA as described previously.22 Briefly, polymerase chain reaction (PCR) was performed using following primers: P2, 5′-GAATGCTACAGGAACCAAATAAC-3′ and P3, 5′-GAGAATCCAGCTTTGGCTGTTG-3′. The resultant 290-bp band for WT FAK allele and 400-bp band corresponding to the Fakflox/flox allele were separated on a 1.2% agarose gel. In order to generate experimental Fakflox/flox; Mx-Cre mice, mice containing the Fakflox/flox allele were crossed to mice containing the type I interferon-inducible Mx-Cre transgene. To induce expression of the Mx-Cre transgene, mice were given a 300-μg intraperitoneal injection of synthetic double-stranded RNA, polyinosinic-polycytidylic acid [poly (I):(C); GE Healthcare Life Sciences] on 3 separate days at 1-day intervals. To determine Cre-mediated recombination of FAK in BM and spleen after a month of poly (I):(C) injection, the following primers were used: P1, 5′-GACCTTCAACTTCTCATTTCTCC-3′ and P2, 5′-GAATGCTACAGGAACCAAATAAC-3′. Cre-mediated deletion of FAK was detected as a 327-bp fragment, whereas WT FAK was observed as a 1.6-kb fragment.
Single-cell suspension from BM and spleen
BM was obtained from tibia, iliac crest, and femurs. Briefly, a cell suspension was collected by flushing the BM with a syringe filled with Iscove modified Dulbecco medium (IMDM; Invitrogen). Red blood cells (RBCs) in the BM were lysed in a RBC lysis buffer containing 155mM ammonium chloride, 10mM potassium bicarbonate, and 0.1mM EDTA (ethylenediaminetetraacetic acid) for 5 minutes at room temperature. The resultant cell pellet was suspended in phosphate-buffered saline (PBS) containing 0.2% bovine serum albumin (BSA) after washing with IMDM and stained with flow antibodies. Spleen was placed in a Petri dish and flushed with IMDM into single-cell suspension and passaged through a 200-μm nylon mesh.
PB analysis
Peripheral blood (PB; 20 μL/mouse) was collected from the tail vein with an EDTA-coated capillary tube before and after 5-flurouracil (5-FU) and phenylhydrazine (PHZ) injection. White blood cells, red blood cells (RBCs), neutrophils, lymphocytes, basophils, eosinophils, and platelets in each sample were counted using an automatic hemavet (Drew Scientific).
5-FU- and PHZ-induced stress hematopoiesis
Age- and sex-matched mice were injected intraperitoneally with 5-FU (150 mg/kg in sterile saline; Abraxis Pharmaceutical Products). Control animals received sterile PBS. 5-FU–injected mice were euthanized at different time points (3, 9, and 14 days). PB was collected from tail vein in EDTA-treated tubes. Total BM was obtained from 2 femurs, 2 tibias, and 2 iliac crest bones and suspended in IMDM. RBCs were removed by lysis in RBC lysis buffer for 5 minutes on ice. PHZ was administered subcutaneously (100 mg/kg). Hematocrits were assayed via microcapillary centrifugation.
Antibodies for flow cytometry
For the analysis of monocytes, granulocytes, erythrocytes, and B and T lymphocytes, the following monoclonal antibodies conjugated with either phycoerythrin (PE) or fluorescein isothiocyanate (FITC) were used. Rat anti–mouse monoclonal antibodies specific for the mature cell lineage antigens CD45R (B220, Clone RA3-6B2), Gr-1 (Ly-6G, Clone RB6-8C5), CD4 (L3T4, Clone RM4-5), CD8a (Ly-2, Clone 53-6.7), TER119 (TER119), and Mac-1 (CD11b, Clone M1/70), CD3 (clone 17A2), CD43 (clone S7), CD19 (1D3), immunoglobulin D (IgD), IgM (clone R6-60.2), Sca-1(LY-6A/E, clone D7), CD71 (transferin receptor, clone C2), CD49d, and CD49e (all purchased from BD Biosciences) were used. Isotype control staining experiments were performed using PE-conjugated IgG2a and FITC-conjugated IgG2b antibodies.
Methylcellulose assay
Low-density BM (LDBM) cells (1.5 × 104 cells) from WT and Fak−/− mice were cultured in 1 mL methylcellulose complete medium containing 1% methylcellulose (StemCell Technologies), 30% fetal bovine serum (FBS), 2% penicillin-streptomycin, 1% BSA, and 10−4M β-mercaptoethanol in the presence of 4 U/mL erythropoietin (EPO; Amgen), 100 ng/mL M-CSF, 100 ng/mL stem cell factor (SCF), 50 ng/mL FLT3, 100 ng/mL TPO, 100 ng/mL G-CSF, 10 ng/mL IL-6, and 10 ng/mL IL-3 (PeproTech). The cultures were incubated at 37°C in a humidified atmosphere of 5% CO2. Colonies were counted under the inverted microscope after 7 to 10 days of culture.
Myeloid cell migration in vivo
WT and Fak−/− mice were challenged with 4% thioglycollate (Sigma-Aldrich) by intraperitoneal injection (2 mL) as described previously.23,24 Mice were killed by cervical dislocation after 4 hours or 4 days, and peritoneal cells were harvested by injecting 6 mL Dulbecco-PBS through the peritoneal wall. Peritoneal lavage was collected, and RBCs present in the lavage fluid were removed by RBC lysis buffer. Cells were centrifuged and resuspended in 1 mL RPMI medium containing 10% FBS. The number of cells in lavage was determined using a hemocytometer. Cells were stained with FITC-conjugated anti–Gr-1, PE-conjugated anti–Mac-1, and PE-conjugated anti-F4/80 antibody and analyzed by flow cytometric analysis (BD Pharmingen).
Generation of BM-derived myeloid and erythroid progenitors
BM-derived myeloid progenitors were generated from 8- to 12-week-old WT and Fak−/− mice femurs, tibias, and iliac crest. Briefly, BM cells were flushed into a 50-mL Falcon tube using syringe-needle and IMDM. Cells were collected by centrifugation at 800g for 5 minutes (Beckman Coulter) at room temperature, and RBCs were lysed with RBC lysis buffer for 5 minutes at room temperature. The cells were centrifuged and resuspended in 5 mL IMDM. LDBM cells were isolated by density gradient centrifugation using Histopaque 1083 (Sigma-Aldrich). LDBM cells were cultured in complete media consisting of IMDM, 20% FBS supplemented with 1% penicillin/streptomycin and in the presence of 100 ng/mL of M-CSF (PeproTech) for generating macrophages. For neutrophils, LDBM cells were cultured in complete media containing IMDM, 10% FBS supplemented with 1% penicillin/streptomycin, and in the presence of 1 ng/mL G-CSF and 5 ng/mL IL-3 (PeproTech). Erythroid progenitors were generated by isolating c-KIT positive cells from BM using stem cell technologies c-KIT selection kit. c-KIT–positive cells were cultured in StemPro-34 medium supplemented with 1 × Stempro-34 nutrient, 2.5 U/mL EPO, 100 ng/mL SCF, 1μM dexamethasone, 1μM β-estradiol, 40 ng/mL insulin-like growth factor-1, 75 μg/mL transferring, 0.1mM 2-mercaptoethanol, and 0.5% BSA.
Proliferation assay
Proliferation was assessed by incorporation of radioactive thymidine in WT and Fak−/− progenitors. Briefly, cells were starved in starvation medium containing 0.2% BSA in IMDM without any growth factors. Macrophage, neutrophil, or erythroid progenitors (5 × 104) were plated in a 96-well plate in 200 μL complete medium either in the absence or in the presence of indicated concentration of growth factors. Cells were cultured for 48 hours and subsequently pulsed with 1.0 μCi (0.037 MBq) [3H] thymidine for6 hours. Cells were harvested using an automated 96-well cell harvester (Brandel), and thymidine incorporation was determined as counts per minute (cpm).
Assessment of apoptosis in myeloid and erythroid cells
Apoptosis was assessed by examining the percentage of cells able to bind Annexin V and 7-AAD (Apoptosis Detection kit from BD Biosciences). Apoptosis assays were performed as outlined in the manufacturer's instructions and analyzed by flow cytometric analysis using a FACScan (Becton Dickinson).
Myeloid cell migration assay in vitro on extracelluar matrix proteins
Haptotactic cell migration assay was performed as previously described.25 Briefly, the bottom of transwell filters (8-μM pore filter; Costar) were coated with 20 μg/mL FN peptide CH296 (Takara), collagen, and laminin (Sigma-Aldrich) for 2 hours at 37°C and rinsed twice with PBS containing 2% BSA. The ECM-coated filters were placed in the lower chamber containing 500 μL complete medium with or without M-CSF (100 ng/mL). Macrophages (2.5 × 105) were resuspended in 100 μL IMDM and allowed to migrate toward the bottom of the top chamber. After 20 hours of incubation at 37°C, nonmigrated cells in the upper chamber were removed with a cotton swab. The migrated cells that attached to the bottom surface of the membrane were stained with 0.1% crystal violet dissolved in 0.1M borate, pH 9.0, and 2% ethanol for 5 minutes at room temperature. The number of migrated cells per membrane was counted in 10 random fields with an inverted microscope using 20× objective lens.
Wound-healing assay
Wound-healing assay was performed as described previously.25 Briefly, WT and Fak−/− BM–derived macrophages (BMMs; 2 × 106) were plated in a 6-well tissue-culture plate overnight in order to form a confluent monolayer. An artificial wound was created by scraping the confluent monolayer with a yellow pipet tip, and the cells migrating into wounded area were monitored microscopically at different time intervals. Images were taken immediately and at indicated time intervals. In addition, cells were counted at indicated time points following wound initiation.
Adhesion assay on extracellular matrix proteins
Adhesion assay was performed as described earlier.26 Briefly, flat-bottom 96-well polystyrene plates (BD Biosciences) were coated with 20 μg/mL FN fragment CH-296, collagen, and laminin in PBS for 1 hour at 37°C. Wells were washed once with PBS, incubated with heat-inactivated BSA for 1 hour at 37°C to block nonspecific sites, and again washed twice with PBS. To examine cell adhesion on the coated surface, 1 × 105 cells were added to each well and incubated at 37°C for different time intervals (15 minutes, 30 minutes, 45 minutes, and 1 hour). At the end of the incubation, unbound cells were removed carefully by aspiration, and wells were washed twice with cold PBS. Adherent cells were fixed with 3.5% formaldehyde and stained with 0.1% crystal violet. The stain was eluted with 10% acetic acid, and absorbance was determined at 600 nm using a microplate reader (Spectramax 250; Molecular Device).
Western blot analysis
WT and Fak−/− progenitors were starved in the absence of serum and growth factors. Cells were stimulated with G-CSF, FN, or SCF and EPO. The reaction was stopped by adding cold PBS, and cells were lysed in lysis buffer containing phosphatase inhibitors (Cell Signaling). All samples were subjected to protein estimation using BCA protein quantification method (Pierce). Equal amount of protein was loaded onto sodium dodecyl sulfate polyacrylamide gel electrophoresis. Separated proteins were transferred to a nitrocellulose membrane. The membrane was blocked with 5% nonfat dry milk in Tris-buffered saline Tween 20 (TBS-T). Western blot analysis was performed using an anti-FAK monoclonal antibody raised against amino acids 903-1052 (H1) of FAK of murine origin (Santa Cruz Biotechnology), anti–phospho AKT, anti–total AKT, anti–caspase 3, anti-p53 (1CI2), anti–phospho Lyn, anti–phospho ERK1/2 (Cell Signaling), and anti–Bcl-xL antibodies (Santa Cruz Biotechnology). Super-signal West Dura extended duration detection system (Pierce) was used according to the manufacturer's instructions to visualize protein signal.
Rac activity assay
Rac activation assay was performed according to the manufacturer's instructions using a nonradioactive Rac activity assay kit (Millipore). Briefly, WT and Fak−/− cells were starved overnight in the absence of serum and growth factors. Cells were replated on 20 μg/mL FN fragment CH-296–coated plates for indicated times. The reaction was stopped by adding cold PBS. Cells were lysed in a buffer containing 50mM Tris-HCl, pH 7.0, 0.5% NP-40, 500mM NaCl, 5mM MgCl2, 5% glycerol, 10 μg/mL leupeptin, 1mM orthovanadate, and 1mM phenylmethylsulfonyl fluoride. Cell lysates were immunoprecipitated with a glutathione S-transferase fusion-protein corresponding to the p21-binding domain bound to glutathione-agarose. Immunoprecipitated complex was subjected to 16% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and activated Rac was detected by immunoblotting using an anti-Rac1 antibody (Cell Signaling).
Statistical analysis
Statistical significance was determined by t test with comparison to control group, and the differences were considered significant if P < .05.
Results
Generation of mice deficient in the expression of FAK in hematopoietic progenitor cells
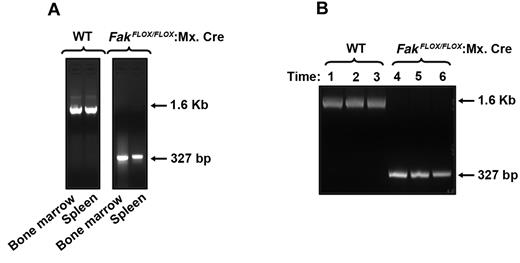
To determine the role of FAK in hematopoiesis, we generated FAK-deficient mice by using cre/loxP method. Since disruption of the Fak gene in mice by gene targeting methodology causes early embryonic lethality, we chose to use mice containing a conditional loxP-flanked allele of Fak termed Fakflox/flox. The Fakflox/flox allele contains 2 loxP sites flanking the second kinase domain of FAK. Recombination between 2 loxP sites, catalyzed by Cre recombinase, causes deletion of the kinase domain and abrogates expression of the FAK protein.22 To allow inducible deletion of the FAK gene, we crossed Fakflox/flox mice with type I interferon-inducible Mx.Cre transgene containing mice. Injection of poly (I):(C) causes the release of type I interferon in Fakflox/flox:Mx.Cre mice, thereby efficiently deleting the FAK gene in the BM and spleen of these mice (Figure 1). These mice were used to study normal and stress-induced hematopoiesis. Poly (I):(C)-treated FAKflox/flox mice lacking the Mx.Cre transgene were designated as WT mice, and Fakflox/flox mice bearing the Mx.Cre transgene were designated as Fak−/−. For experiments in this study, comparisons were made between Fakflox/flox mice with or without the Mx.Cre transgene but treated equivalently with poly (I):(C). As seen in Figure 1A, no change in the presence of Fak sequences in the BM and spleen after poly (I):(C) treatment of Fakflox/flox (WT) mice were observed, while robust deletion of WT FAK sequences was observed in Fakflox/flox:Mx.Cre mice. To further confirm the deletion of Fak in BM of Fakflox/flo:Mx.Cre mice, we performed a time course study in response to poly (I):(C) treatment. DNA was extracted from BM after 1, 2, and 3 months following poly (I):(C) induction and subjected to PCR analysis. As seen in Figure 1B, WT Fak gene was completely deleted in FAKflox/flox:Mx.Cre BM (lanes 4-6), whereas WT Fak sequences were present in WT BM cells at all time points examined (lanes 1-3).
Cre-mediated deletion of Fak in BM and spleen. (A) Cre-mediated deletion of FAK was induced by 3 intraperitoneal injections of 300 μg poly (I):(C) at 1-day intervals. One month after the final injection, DNA was extracted from BM and spleen and analyzed by PCR. Cre-mediated deletion of Fak was detected as a 327-bp fragment and that of WT Fak was observed as a 1.6-kb fragment. (B) Cre-mediated deletion of Fak after various times after poly (I):(C) treatment. One, 2, and 3 months following final injection of poly (I):(C), DNA was extracted from BM and analyzed by PCR. Cre-mediated deletion of Fak was detected as a 327-bp fragment and that of WT Fak was observed as a 1.6-kb fragment. Lanes 1, 2, and 3 represent WT FAK bands 1, 2, and 3 months after induction, respectively. Lanes 4, 5, and 6 represent Fak deletion in BM 1, 2, and 3 months after induction, respectively.
Cre-mediated deletion of Fak in BM and spleen. (A) Cre-mediated deletion of FAK was induced by 3 intraperitoneal injections of 300 μg poly (I):(C) at 1-day intervals. One month after the final injection, DNA was extracted from BM and spleen and analyzed by PCR. Cre-mediated deletion of Fak was detected as a 327-bp fragment and that of WT Fak was observed as a 1.6-kb fragment. (B) Cre-mediated deletion of Fak after various times after poly (I):(C) treatment. One, 2, and 3 months following final injection of poly (I):(C), DNA was extracted from BM and analyzed by PCR. Cre-mediated deletion of Fak was detected as a 327-bp fragment and that of WT Fak was observed as a 1.6-kb fragment. Lanes 1, 2, and 3 represent WT FAK bands 1, 2, and 3 months after induction, respectively. Lanes 4, 5, and 6 represent Fak deletion in BM 1, 2, and 3 months after induction, respectively.
Altered steady-state myelopoiesis- and cytokine-induced erythropoieisis in FAK-deficient mice
To determine if deletion of FAK affects the development of hematopoietic cells, a thorough analysis of the hematopoietic compartment of Fak−/− mice was performed. Total and differential cell counts from PB of Fak−/− mice showed no profound changes, although a modest but significant increase in platelet count as well as a decrease in basophil count was observed in these mice relative to WT controls (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). All other leukocyte populations were present at normal numbers and frequencies (data not shown). Since previous studies have shown that myeloid progenitors in the BM express FAK, we next examined the percentage of Gr-1/Mac-1 double-positive myeloid progenitors in the BM of WT and Fak−/− mice. As seen in Figure 2A-B, a modest but significant decrease in the percentage of Gr-1/Mac-1 double-positive cells in the BM of Fak−/− mice was observed relative to WT controls. Furthermore, the reduction in Gr-1/Mac-1 double-positive Fak−/− BM cells was associated with a significant increase in apoptosis as determined by an increase in the percentage of Annexin V–positive cells in Fak−/− BM relative to controls (Figure 2C-D). Consistent with the reduction in the percentage of Gr-1/Mac-1 double-positive myeloid progenitors in the BM of Fak−/− mice; colony-forming ability of BM cells from these mice was also significantly reduced compared with controls in response to multiple cytokine combinations (Figure 2E and supplemental Figure 2). Since FAK has been implicated in regulating actin-based functions in other cell types, we examined the ability of Fak−/− BM progenitors to migrate in response to SCF and stromal cell–derived factor-1 (SDF-1) on ECM protein FN. As seen in Figure 2F and G, a significant reduction in the migration of Fak−/− progenitors was observed under all conditions examined relative to WT controls.
Impaired myelopoiesis in Fak−/− mice. (A) Whole BM cells from WT and Fak−/− mice were analyzed for the expression of myeloid cell marker Gr-1 and Mac-1 by flow cytometry. The percentage of Gr-1/Mac-1 high double-positive cells (R2) and Gr-1/Mac-1 low positive cells (R3) in the BM are indicated in the square of each dot blot. Shown are results from a representative experiment. (B) Bar graph represents the mean values of Gr-1/Mac-1 high double-positive cells in the BM of WT and Fak−/− mice (n = 8; *P < .05). (C) Whole BM cells from WT and Fak−/− mice were stained with Annexin V and 7-AAD and analyzed by flow cytometric analysis. Numbers in each quadrant represent the percentage of cells in various stages of apoptosis. (D) Quantitative analysis of the percentage of BM cells undergoing early apoptosis (Annexin V) in WT and Fak−/− mice. Bars represent the mean of 3 independent experiments consisting of 3 pairs of mice of each genotype for each experiment (n = 9; *P < .05). (E) Reduced CFU-Cs in Fak−/− BM. LDBM cells (1.5 × 104) from WT and Fak−/− mice were plated in a methylcellulose colony-forming assay in the presence of the indicated cytokines. Colonies were enumerated 7 days later. Bar graph shows the mean number of colonies from 3 independent mice of each genotype plated in triplicates. *P < .05, Fak−/− versus WT. Similar results were obtained in 3 additional pairs of mice of each genotype. (F) WT and Fak−/− progenitors were subjected to an in vitro migration assay in response to SDF-1 or SCF on FN. Briefly, LDBM cells isolated from WT and Fak−/− mice were cultured in the presence of SCF, TPO, IL-6, and FLT-3 for 2 days. Cells (0.5 × 106) were subjected to transwell migration assay for 5 hours at 37°C. After 5 hours of incubation at 37°C, nonmigrated cells in the upper chamber were removed with a cotton swab. The migrated cells attached to the bottom surface of the membrane were stained with 0.1% crystal violet, dissolved in 0.1M borate, pH 9.0, and 2% ethanol for 5 minutes at room temperature. Photomicrographs shown are progenitor cells migrated on FN in response to SDF-1 or SCF. (G) The number of migrated cells per membrane was counted in 10 random fields with an inverted microscope using 20× objective lens; cells migrated into the lower chamber in response to SDF-1 or SCF are counted using hemocytometer from replicates of 3 from 1 experiment using 3 mice of each genotype. *P < .05, Fak−/− vs WT, mean ± SEM. (H) Primary erythroid progenitor proliferation was assessed by incorporation of radioactive thymidine in WT and Fak−/− cells. Briefly, erythroid progenitor cells grown for 2 days were starved in Stem Pro 34 medium without any growth factors or supplements for 4 hours. Erythroid progenitor cells (5 × 104) were placed in a 96-well plate in 200 μL complete medium either in the absence or in the presence of indicated concentration of EPO, SCF alone, or in combination. Cells were cultured for 48 hours and subsequently pulsed with 1.0 μCi (0.037 MBq) [3H] thymidine for 6 hours. Cells were harvested using an automated 96-well cell harvester, and thymidine incorporation was determined as cpm. Bar graph shows pooled data from 2 independent experiments performed in replicates of 4 using 4 mice per genotype per experiment. *P < .05, Fak−/− vs WT, mean ± SEM. (I) Quantitative analysis of the percentage of primary erythroid cells undergoing early apoptosis (Annexin V) in WT and Fak−/− cells. Cells were harvested after 4 days of culture and starved in absence of growth factors or supplements for 6 hours. Bar graph represents the mean of Annexin V–positive cells for 1 independent experiment performed in triplicates. *P < .05, Fak−/− vs WT, mean ± SD. (J) Erythroid progenitor cells were cultured for 4 days and starved in absence of growth factors or supplements for 4 hours and stimulated with SCF and EPO at 37°C. Cell lysates (35 μg) were subjected to Western blot analysis using indicated antibodies.
Impaired myelopoiesis in Fak−/− mice. (A) Whole BM cells from WT and Fak−/− mice were analyzed for the expression of myeloid cell marker Gr-1 and Mac-1 by flow cytometry. The percentage of Gr-1/Mac-1 high double-positive cells (R2) and Gr-1/Mac-1 low positive cells (R3) in the BM are indicated in the square of each dot blot. Shown are results from a representative experiment. (B) Bar graph represents the mean values of Gr-1/Mac-1 high double-positive cells in the BM of WT and Fak−/− mice (n = 8; *P < .05). (C) Whole BM cells from WT and Fak−/− mice were stained with Annexin V and 7-AAD and analyzed by flow cytometric analysis. Numbers in each quadrant represent the percentage of cells in various stages of apoptosis. (D) Quantitative analysis of the percentage of BM cells undergoing early apoptosis (Annexin V) in WT and Fak−/− mice. Bars represent the mean of 3 independent experiments consisting of 3 pairs of mice of each genotype for each experiment (n = 9; *P < .05). (E) Reduced CFU-Cs in Fak−/− BM. LDBM cells (1.5 × 104) from WT and Fak−/− mice were plated in a methylcellulose colony-forming assay in the presence of the indicated cytokines. Colonies were enumerated 7 days later. Bar graph shows the mean number of colonies from 3 independent mice of each genotype plated in triplicates. *P < .05, Fak−/− versus WT. Similar results were obtained in 3 additional pairs of mice of each genotype. (F) WT and Fak−/− progenitors were subjected to an in vitro migration assay in response to SDF-1 or SCF on FN. Briefly, LDBM cells isolated from WT and Fak−/− mice were cultured in the presence of SCF, TPO, IL-6, and FLT-3 for 2 days. Cells (0.5 × 106) were subjected to transwell migration assay for 5 hours at 37°C. After 5 hours of incubation at 37°C, nonmigrated cells in the upper chamber were removed with a cotton swab. The migrated cells attached to the bottom surface of the membrane were stained with 0.1% crystal violet, dissolved in 0.1M borate, pH 9.0, and 2% ethanol for 5 minutes at room temperature. Photomicrographs shown are progenitor cells migrated on FN in response to SDF-1 or SCF. (G) The number of migrated cells per membrane was counted in 10 random fields with an inverted microscope using 20× objective lens; cells migrated into the lower chamber in response to SDF-1 or SCF are counted using hemocytometer from replicates of 3 from 1 experiment using 3 mice of each genotype. *P < .05, Fak−/− vs WT, mean ± SEM. (H) Primary erythroid progenitor proliferation was assessed by incorporation of radioactive thymidine in WT and Fak−/− cells. Briefly, erythroid progenitor cells grown for 2 days were starved in Stem Pro 34 medium without any growth factors or supplements for 4 hours. Erythroid progenitor cells (5 × 104) were placed in a 96-well plate in 200 μL complete medium either in the absence or in the presence of indicated concentration of EPO, SCF alone, or in combination. Cells were cultured for 48 hours and subsequently pulsed with 1.0 μCi (0.037 MBq) [3H] thymidine for 6 hours. Cells were harvested using an automated 96-well cell harvester, and thymidine incorporation was determined as cpm. Bar graph shows pooled data from 2 independent experiments performed in replicates of 4 using 4 mice per genotype per experiment. *P < .05, Fak−/− vs WT, mean ± SEM. (I) Quantitative analysis of the percentage of primary erythroid cells undergoing early apoptosis (Annexin V) in WT and Fak−/− cells. Cells were harvested after 4 days of culture and starved in absence of growth factors or supplements for 6 hours. Bar graph represents the mean of Annexin V–positive cells for 1 independent experiment performed in triplicates. *P < .05, Fak−/− vs WT, mean ± SD. (J) Erythroid progenitor cells were cultured for 4 days and starved in absence of growth factors or supplements for 4 hours and stimulated with SCF and EPO at 37°C. Cell lysates (35 μg) were subjected to Western blot analysis using indicated antibodies.
Since FAK has also been shown to be expressed in erythroid cells and reduced numbers of progenitors were observed in response to SCF and EPO in Fak−/− BM (Figure 2E), we next analyzed the effect of FAK deletion on erythroid progenitor cell proliferation and survival in vitro. B-derived erythroid progenitors were starved and stimulated with SCF and/or EPO and thymidine incorporation was assessed. As seen in Figure 2H, a significant reduction in the growth of Fak−/− erythroid progenitors was observed relative to controls in response to SCF but not EPO. Further, a significant increase in apoptosis of erythroid progenitors was also observed upon cytokine withdrawal in cells lacking FAK (Figure 2I). Since cytokines can result in autophosphorylation of FAK, which can create binding sites for SH2 domains of SFKs, leading to growth and proliferation, we examined the activation of Lyn SFK in Fak−/− erythroid progenitors. As seen in Figure 2J, while no profound changes in the activation of AKT and ERK MAP kinase were observed, activation of Lyn SFK was signi-ficantly impaired in Fak−/− progenitors (supplemental Figure 3). Additionally, upon cytokine withdrawal, a significant increase in the expression of p53 as well as cleaved form of caspase 3 was also observed (Figure 2J and supplemental Figure 3). Taken together, these results suggest that FAK plays an essential role in regulating cytokine-induced growth and survival in BM-derived myeloid and erythroid progenitors.
Impaired erythropoiesis and myelopoiesis in FAK−/− mice in response PHZ and 5-FU treatment
To assess the ability of Fak−/− hematopoietic system to cope with erythropoietic stress, PHZ was administered to Fak−/− mice to induce hemolytic anemia. Specifically, Fak−/− mice (and controls) were treated with PHZ (100 mg/kg) at day 0, and hematocrits were monitored over a 12-day period. Among Fak−/− mice, delayed recovery and deficient hematocrits were observed. In particular, in both mutant and WT mice, hematocrits initially fell at day 4 to approximately 25%. In WT mice, hematocrits thereafter increased to approximately 40%, while in Fak−/− mice, hematocrits remained below approximately 20% until day 8 (Figure 3A). In addition, Fak−/− mice did not support efficient erythrosplenomegaly (Figure 3D) and exhibited decreased splenic cellularity and weight on days 4 and 8 (Figure 3B-C). All mice survived, however, and by day 12, hematocrits recovered to baseline levels. Flow cytometric analysis as performed on days 4 and 8 revealed deficient erythroid progenitor pools in Fak−/− spleens, including approximately 50% reduction in Ter119/CD71+ erythroblasts (Figure 3E-F) and approximately 70% reduction in absolute erythroid burst-forming units and erythroid colony-forming units (Figure 3G). Furthermore, cellularity in Fak−/− BM in this hemolytic model was not altered, but frequency of BM CD71hi/Ter119+ erythroid progenitors was impaired (Figure 3H-I).
Defective erythropoiesis in Fak−/− mice during PHZ-induced anemia. WT and Fak−/− mice were treated with PHZ (100 mg/kg at day 0). At the indicated time points, hematocrits (A), spleen weight (B), splenic cellularity (C), photomicrographs of spleen (D), flow cytometry based analysis of the frequency (percentage) of Ter119/CD71 positive erythroblasts (R3, R4, and R5) in the spleen (E), absolute number of Ter119/CD71-positive erythroblasts in the spleen (F), absolute number of erythroid burst-forming units and erythroid colony-forming units in the spleen (G), frequency (percentage) of Ter119/CD71-positive erythroblasts (R3) in the BM (H), and percent of Ter119/CD71-positive erythroblasts in the BM were assessed (I). For each analysis, mean ± SEM are illustrated (n = 3 mice per group, 2 independent experiments were performed; *P < .05). (J) Cre-mediated deletion of Fak was detected in BM and spleen after 8 days of PHZ treatment as a 327-bp fragment. WT allele of Fak was detected as a 1.6-kb fragment. Lanes 1, 2, and 3 represent WT Fak bands after 8 days of PHZ treatment in BM and spleen, respectively. Lanes 4, 5, and 6 show Fak deletion in BM and spleen after 8 days of PHZ treatment, respectively.
Defective erythropoiesis in Fak−/− mice during PHZ-induced anemia. WT and Fak−/− mice were treated with PHZ (100 mg/kg at day 0). At the indicated time points, hematocrits (A), spleen weight (B), splenic cellularity (C), photomicrographs of spleen (D), flow cytometry based analysis of the frequency (percentage) of Ter119/CD71 positive erythroblasts (R3, R4, and R5) in the spleen (E), absolute number of Ter119/CD71-positive erythroblasts in the spleen (F), absolute number of erythroid burst-forming units and erythroid colony-forming units in the spleen (G), frequency (percentage) of Ter119/CD71-positive erythroblasts (R3) in the BM (H), and percent of Ter119/CD71-positive erythroblasts in the BM were assessed (I). For each analysis, mean ± SEM are illustrated (n = 3 mice per group, 2 independent experiments were performed; *P < .05). (J) Cre-mediated deletion of Fak was detected in BM and spleen after 8 days of PHZ treatment as a 327-bp fragment. WT allele of Fak was detected as a 1.6-kb fragment. Lanes 1, 2, and 3 represent WT Fak bands after 8 days of PHZ treatment in BM and spleen, respectively. Lanes 4, 5, and 6 show Fak deletion in BM and spleen after 8 days of PHZ treatment, respectively.
We next examined the effects of 5-FU induced myelotoxicity on the myeloid compartment of Fak−/− mice. 5-FU was administered at a standard dose of 150 mg/kg. No major alterations in the PB counts of Fak−/− mice were observed relative to controls (data not shown). In contrast, the recovery of relatively primitive pheno-typically defined BM progenitors as defined by the expression of Kit, Sca-1 and negative for lineage markers (KSL cells) was significantly reduced (Figure 4A-B). Consistently, the recovery of Gr-1/Mac-1 double-positive myeloid progenitors in the BM of Fak−/− mice was also significantly delayed and reduced relative to WT controls at all time points examined including on days 3, 9, and 14 after 5-FU treatment (Figure 4C-D and supplemental Figure 4).
Delayed and reduced recovery of Fak−/− myeloid progenitors in response to 5-FU. (A) Whole BM cells from WT and Fak−/− mice were harvested at 0, 3, 9, and 14 days after 5-FU injection and stained with FITC-conjugated anti–Mac-1, anti-B220, anti-CD3, anti–Gr-1, anti-F4/80, and anti-Ter119 antibodies. Subsequently, cells were stained with PE-conjugated antibody to Sca-1 and APC-conjugated–c-Kit antibody. c-Kit and Sca-1 expression were determined on lineage negative cells (LSK cells). Upper right quadrant in each dot blot indicates the percentage of LSK cells in WT and Fak−/− mice at 0, 3, 9, and 14 days after 5-FU treatment. (B) Line chart represents the mean value of percent LSK cells in WT and Fak−/− BM at the indicated time points after 5-FU treatment (n = 3-6 mice; *P < .05). (C) The percentage of Gr-1/Mac-1 double-positive cells in the BM of representative WT and Fak−/− mouse is indicated in the upper right quadrant of each dot blot in response to 5-FU at indicated days after 5-FU treatment. (D) Line chart represents the percentage of Gr-1/Mac-1 double-positive cells in WT and Fak−/− mice at 0, 3, 9, and 14 days after 5-FU treatment from 2 independent experiments (n = 3 mice per group, mean ± SEM; *P < .05).
Delayed and reduced recovery of Fak−/− myeloid progenitors in response to 5-FU. (A) Whole BM cells from WT and Fak−/− mice were harvested at 0, 3, 9, and 14 days after 5-FU injection and stained with FITC-conjugated anti–Mac-1, anti-B220, anti-CD3, anti–Gr-1, anti-F4/80, and anti-Ter119 antibodies. Subsequently, cells were stained with PE-conjugated antibody to Sca-1 and APC-conjugated–c-Kit antibody. c-Kit and Sca-1 expression were determined on lineage negative cells (LSK cells). Upper right quadrant in each dot blot indicates the percentage of LSK cells in WT and Fak−/− mice at 0, 3, 9, and 14 days after 5-FU treatment. (B) Line chart represents the mean value of percent LSK cells in WT and Fak−/− BM at the indicated time points after 5-FU treatment (n = 3-6 mice; *P < .05). (C) The percentage of Gr-1/Mac-1 double-positive cells in the BM of representative WT and Fak−/− mouse is indicated in the upper right quadrant of each dot blot in response to 5-FU at indicated days after 5-FU treatment. (D) Line chart represents the percentage of Gr-1/Mac-1 double-positive cells in WT and Fak−/− mice at 0, 3, 9, and 14 days after 5-FU treatment from 2 independent experiments (n = 3 mice per group, mean ± SEM; *P < .05).
Impaired recruitment of myeloid cells to sites of inflammation in a model of acute peritonitis
To extend the studies related to the role of FAK in stress hematopoiesis, we next subjected WT and Fak−/− mice to inflammatory stress by injecting mice with thioglycollate. Thioglycollate induces acute inflammation in mice and results in the recruitment/accumulation of myeloid cells in the peritoneal cavity. Thioglycollate (4%) was injected intraperitoneally into WT and Fak−/− mice, and the recruitment of myeloid cells in the peritoneal cavity after 4 hours or 4 days was analyzed by flow cytometry. Four hours after the thioglycollate injection, WT and Fak−/− mice were killed, and cells were harvested from the peritoneal cavity followed by staining with anti–Gr-1 and –Mac-1 antibodies. Flow analysis revealed a significant reduction in the accumulation/recruitment of FAK-deficient Gr-1/Mac-1–positive cells compared with WT controls (Figure 5A-D). Likewise, 4 days after thioglycollate injection, a significant reduction in the number of F4/80-positive cells was also observed in FAK-deficient mice compared with controls (Figure 5E-H).
Impaired recruitment of myeloid cells to sites of inflammation in a model of acute peritonitis. (A) Impaired accumulation of Gr-1/Mac-1 double-positive cells in the inflamed peritoneum of Fak−/− mice. WT and FAK−/− mice were given intraperitoneal injections of 4% thioglycollate. Peritoneal lavage was collected 4 hours after injection, and Gr-1 and Mac-1 expression was examined by flow cytometry. Dot blots represent Gr-1/Mac-1–positive cells in the peritoneal cavity of WT and Fak−/− mice, analyzed following PBS or thioglycollate injection. (B) Quantitative analysis of the percentage of Gr-1/Mac-1 double-positive cells in the peritoneal cavity of WT and Fak−/− mice after PBS or thioglycollate injections (n = 3; *P < .05). (C) Quantitative analysis of the total number of Gr-1/Mac-1–positive cells recruited into peritoneal cavity of WT and Fak−/− mice after PBS or thioglycollate injections (n = 3; *P < .05). (D) DNA was extracted from peritoneal cavity-derived cells and analyzed by PCR. Cre-mediated deletion of Fak was detected as a 327-bp fragment, and WT Fak was observed as a1.6-kb fragment. Lanes 1, 2, and 3 represent WT Fak bands after 4 hours of thioglycollate treatment in peritoneal cavity-derived cells. Lanes 4, 5, and 6 shows Fak deletion in peritoneal cavity-derived cells after 4 hours of thioglycollate treatment. (E) WT and FAK−/− mice were given intraperitoneal injections of 4% thioglycollate. Peritoneal lavage was collected 4 days after injection, and F4/80-positive cells were detected by flow cytometry. Upper left quadrant of each dot blot represents the percentage of phycoerythrin-conjugated F4/80-positive cells in WT and FAK−/− peritoneum after PBS or thioglycollate injections. (F) Quantitative analysis of the percentage of F4/80 positive cells in the peritoneal cavity of WT and Fak−/− mice after PBS or thioglycollate injections (n = 3; *P < .05, 4 pairs of WT and Fak−/− mice). (G) Quantitative analysis of the total number of F4/80 cells recruited to the peritoneal cavity of WT and Fak−/− mice after PBS or thioglycollate injection (n = 3; *P < .05, 4 pairs of WT and Fak−/−). (H) DNA was extracted from peritoneal cavity-derived cells and analyzed by PCR. Cre-mediated deletion of FAK was detected as a 327-bp fragment, and the WT Fak allele was observed as a 1.6-kb fragment. Lanes 1, 2, and 3 represent WT Fak bands in the peritoneal cavity-derived cells after 4 days of thioglycollate treatment. Lanes 4, 5, and 6 represent FAK deletion in cells recruited into the peritoneal cavity after 4 days of thioglycollate treatment.
Impaired recruitment of myeloid cells to sites of inflammation in a model of acute peritonitis. (A) Impaired accumulation of Gr-1/Mac-1 double-positive cells in the inflamed peritoneum of Fak−/− mice. WT and FAK−/− mice were given intraperitoneal injections of 4% thioglycollate. Peritoneal lavage was collected 4 hours after injection, and Gr-1 and Mac-1 expression was examined by flow cytometry. Dot blots represent Gr-1/Mac-1–positive cells in the peritoneal cavity of WT and Fak−/− mice, analyzed following PBS or thioglycollate injection. (B) Quantitative analysis of the percentage of Gr-1/Mac-1 double-positive cells in the peritoneal cavity of WT and Fak−/− mice after PBS or thioglycollate injections (n = 3; *P < .05). (C) Quantitative analysis of the total number of Gr-1/Mac-1–positive cells recruited into peritoneal cavity of WT and Fak−/− mice after PBS or thioglycollate injections (n = 3; *P < .05). (D) DNA was extracted from peritoneal cavity-derived cells and analyzed by PCR. Cre-mediated deletion of Fak was detected as a 327-bp fragment, and WT Fak was observed as a1.6-kb fragment. Lanes 1, 2, and 3 represent WT Fak bands after 4 hours of thioglycollate treatment in peritoneal cavity-derived cells. Lanes 4, 5, and 6 shows Fak deletion in peritoneal cavity-derived cells after 4 hours of thioglycollate treatment. (E) WT and FAK−/− mice were given intraperitoneal injections of 4% thioglycollate. Peritoneal lavage was collected 4 days after injection, and F4/80-positive cells were detected by flow cytometry. Upper left quadrant of each dot blot represents the percentage of phycoerythrin-conjugated F4/80-positive cells in WT and FAK−/− peritoneum after PBS or thioglycollate injections. (F) Quantitative analysis of the percentage of F4/80 positive cells in the peritoneal cavity of WT and Fak−/− mice after PBS or thioglycollate injections (n = 3; *P < .05, 4 pairs of WT and Fak−/− mice). (G) Quantitative analysis of the total number of F4/80 cells recruited to the peritoneal cavity of WT and Fak−/− mice after PBS or thioglycollate injection (n = 3; *P < .05, 4 pairs of WT and Fak−/−). (H) DNA was extracted from peritoneal cavity-derived cells and analyzed by PCR. Cre-mediated deletion of FAK was detected as a 327-bp fragment, and the WT Fak allele was observed as a 1.6-kb fragment. Lanes 1, 2, and 3 represent WT Fak bands in the peritoneal cavity-derived cells after 4 days of thioglycollate treatment. Lanes 4, 5, and 6 represent FAK deletion in cells recruited into the peritoneal cavity after 4 days of thioglycollate treatment.
Effect of FAK deletion on myeloid cell growth and survival
To further explore the mechanism behind reduced accumulation of F4/80-positive cells in the peritoneal cavity of Fak−/− mice in response to thioglycollate, we generated BMM progenitors in vitro from WT and Fak−/− mice by culturing BM cells for 3 days in the presence of M-CSF. Figures 6A-B demonstrates loss of FAK protein in Fak−/− progenitors. Figure 6C shows that cells grown in the presence of M-CSF from FAK-deficient mice do not incorporate thymidine to same extent as WT cells. To determine whether this defect was due to changes in the maturation of FAK-deficient cells grown in M-CSF, we performed flow cytometric analysis on these cells using an anti-F4/80 antibody and observed no significant differences in the rate of maturation/differentiation (Figure 6D). F4/80 is a cell surface marker whose expression is restricted to the macrophage lineage.
Deletion of FAK in BMM and neutrophil cells results in reduced proliferation, survival, and activation of antiapoptotic proteins. (A) Cre-mediated Fak deletion in BMMs: after 1 month of poly (I):(C) injection, BM cells from WT and Fak−/− mice were cultured for 7 days, and DNA was extracted. Lanes 1 and 2 represent WT Fak bands, and lanes 3 and 4 represent FAK deleted bands. (B) Western blot analysis demonstrating the expression of Fak in WT and Fak−/− cells. Equal amount of cell lysates were subjected to Western blot analysis. Blot was probed with an antibody specific to FAK (raised against amino acids 903-1052). The same blot was reprobed for β-actin to show equal loading. (C) Cells were subjected to proliferation assay in the absence of growth factors as well as in the presence of indicated concentrations of M-CSF. After 48 hours of culture, cells were pulsed with [3H] thymidine for 6 hours. Concentrations of M-CSF are shown on the x-axis, and mean thymidine incorporation (in cpm) are shown on the y-axis. Shown are results from 1 of 3 independent experiments performed in quadruplicate; *P < .05. (D) Expression of F4/80 on WT and Fak−/− cells. Cells were stained with PE-conjugated anti-F4/80 antibody and subjected to flow cytometric analysis. Solid histograms indicate the level of F4/80 expression on the surface of WT and Fak−/− cells, while open histograms indicate the level of expression using an isotype control antibody. (E) BM cells grown in G-CSF were subjected to proliferation assay in the absence of growth factors and in the presence of indicated concentrations of various cytokines. After 48 hours of culture, cells were pulsed with [3H] thymidine for 6 hours. Concentrations of various cytokines are shown on the x-axis, and mean thymidine incorporation (in cpm) is shown on the y-axis. Bar graph shows data from 1 experiment performed in replicates of 4 (*P < .05). Similar findings were observed in 3 additional independent experiments. (F) BM cells grown in G-CSF derived from WT and FAK-deficient mice were starved of growth factors for indicated times and subjected to flow cytometric analysis after staining with Annexin V and 7-AAD. The percentages of early and late apoptotic cells at each time point are indicated. Shown is a representative dot blot from 1 of 3 independent experiments. (G) WT and Fak-deficient cells grown in the presence of IL-3 and G-CSF were starved of growth factors and stimulated for 5 minutes with G-CSF. Upper panel shows the activation of AKT as assessed by Western blot analysis using an anti–phospho AKT antibody. Bottom panel shows total AKT levels in each lane. (H) Actively growing neutrophils derived from WT and Fak−/− mice were subjected to Western blot analysis using an anti–caspase 3 (upper panel), anti–Bcl-xL (middle panel), and anti–β-actin (bottom panel) antibody. Similar results were observed in 2 additional independent experiments.
Deletion of FAK in BMM and neutrophil cells results in reduced proliferation, survival, and activation of antiapoptotic proteins. (A) Cre-mediated Fak deletion in BMMs: after 1 month of poly (I):(C) injection, BM cells from WT and Fak−/− mice were cultured for 7 days, and DNA was extracted. Lanes 1 and 2 represent WT Fak bands, and lanes 3 and 4 represent FAK deleted bands. (B) Western blot analysis demonstrating the expression of Fak in WT and Fak−/− cells. Equal amount of cell lysates were subjected to Western blot analysis. Blot was probed with an antibody specific to FAK (raised against amino acids 903-1052). The same blot was reprobed for β-actin to show equal loading. (C) Cells were subjected to proliferation assay in the absence of growth factors as well as in the presence of indicated concentrations of M-CSF. After 48 hours of culture, cells were pulsed with [3H] thymidine for 6 hours. Concentrations of M-CSF are shown on the x-axis, and mean thymidine incorporation (in cpm) are shown on the y-axis. Shown are results from 1 of 3 independent experiments performed in quadruplicate; *P < .05. (D) Expression of F4/80 on WT and Fak−/− cells. Cells were stained with PE-conjugated anti-F4/80 antibody and subjected to flow cytometric analysis. Solid histograms indicate the level of F4/80 expression on the surface of WT and Fak−/− cells, while open histograms indicate the level of expression using an isotype control antibody. (E) BM cells grown in G-CSF were subjected to proliferation assay in the absence of growth factors and in the presence of indicated concentrations of various cytokines. After 48 hours of culture, cells were pulsed with [3H] thymidine for 6 hours. Concentrations of various cytokines are shown on the x-axis, and mean thymidine incorporation (in cpm) is shown on the y-axis. Bar graph shows data from 1 experiment performed in replicates of 4 (*P < .05). Similar findings were observed in 3 additional independent experiments. (F) BM cells grown in G-CSF derived from WT and FAK-deficient mice were starved of growth factors for indicated times and subjected to flow cytometric analysis after staining with Annexin V and 7-AAD. The percentages of early and late apoptotic cells at each time point are indicated. Shown is a representative dot blot from 1 of 3 independent experiments. (G) WT and Fak-deficient cells grown in the presence of IL-3 and G-CSF were starved of growth factors and stimulated for 5 minutes with G-CSF. Upper panel shows the activation of AKT as assessed by Western blot analysis using an anti–phospho AKT antibody. Bottom panel shows total AKT levels in each lane. (H) Actively growing neutrophils derived from WT and Fak−/− mice were subjected to Western blot analysis using an anti–caspase 3 (upper panel), anti–Bcl-xL (middle panel), and anti–β-actin (bottom panel) antibody. Similar results were observed in 2 additional independent experiments.
Since we observed enhanced apoptosis and reduced colony-forming ability of FAK-deficient BM cells in response to cytokines such as G-CSF and IL-3 (Figure 2E), we explored the growth, differentiation and survival of these cells in liquid culture in the presence of these cytokines in vitro in more detail. BM cells from WT and Fak−/− mice were cultured in the presence of G-CSF and IL-3 for 3 days and subjected to thymidine incorporation assay. As seen in Figure 6E, Fak−/− cells grown under these conditions were significantly impaired in their ability to proliferate in response to G-CSF and IL-3 compared with WT controls. To assess whether Fak−/− neutrophil progenitors could be more sensitive to apoptosis upon cytokine withdrawal, we subjected in vitro cultured WT and Fak−/− cells to cytokine deprivation (ie, removed G-CSF and IL-3) for the indicated times and assessed apoptosis by staining the cells with Annexin V and 7-AAD. As seen in Figure 6F, loss of FAK in these cells rendered them more susceptible to cytokine withdrawal-induced apoptosis at every time point examined compared with WT controls.
Previous studies have shown that cytokines such as GM-CSF and G-CSF play an essential role in regulating the survival of myeloid cells by regulating the expression of caspase 3 and Bcl-xL.27,28 To determine whether the enhanced apoptosis due to FAK deficiency in response to G-CSF was related to impaired expression of caspase 3 and Bcl-xL, we performed Western blot analysis on cells derived from WT and FAK−/− mice and looked at the expression of caspase 3 and Bcl-xL. As seen in Figure 6G-H, loss of FAK in these cells not only resulted in enhanced expression of cleaved caspase 3 and reduced expression of Bcl-xL, but also resulted in reduced activation of Akt. Taken together, these results suggest that FAK plays an essential role in regulating cytokine-induced growth and survival by regulating the activation of the Akt, caspase 3, and Bcl-xL in myeloid progenitors.
Defects in actin-based functions due to FAK deficiency in BMMs
Since FAK also regulates actin-based functions in fibroblasts, we next explored the possibility that BMMs from FAK-deficient mice might be impaired in their ability to mediate cytoskeleton based functions, which may contribute to the impaired recruitment in the peritoneal cavity upon thioglycollate challenge. To test this possi-bility, we performed transwell migration assays using cells from WT and Fak−/− mice and found significant reduction in the migratory ability of Fak−/− BMMs on wells coated with the ECM proteins FN, laminin, and collagen (Figure 7A-B). Consistently, FAK-deficient BMMs demonstrated impaired wound repair ability compared with WT controls (Figure 7C-D). Impaired migration and wound-healing ability of FAK-deficient cells was, in part, associated with reduced ability of these cells to adhere to ECM proteins including FN, collagen, and laminin (Figure 7E-G). These defects were not due to changes in the expression of integrin molecules on Fak−/− BMMs (Figure 7H).
Deficiency of FAK in BMMs alters actin-based functions. (A) WT and Fak−/− cells (2.5 × 105) were subjected to an in vitro migration assay on FN, laminin, and collagen. Images were acquired through a Zeiss Axioskop 2 Plus microscope equipped with a Plan-Neofluar 20×/0.5 objective lens, and were captured with an Axiocam MRC-5 camera and Axiovision 4 software (all from Zeiss). (B) A quantitative assessment of the number of WT and Fak−/− cells migrated through wells coated with FN, laminin, and collagen. Bars represent mean number of cells migrated ± standard deviation on various extracellular matrix proteins. Ten fields were scored in each experiment. Similar results were observed in 3 additional experiments (n = 3; *P < .05, WT vs Fak−/−). (C) WT and Fak−/− cells were cultured for 8 days in 24-well plates in the presence of 100 ng/mL M-CSF. An artificial wound was created in the monolayer using a pipet tip. Images were taken immediately and again at indicated time periods after creating the wound. Photomicrograph is from an independent experiment. (D) Bar graph shows quantitative analysis of the number of migrated cells in the wounded area. Data are from 1 representative experiment (n = 3; *P < .05 WT vs Fak−/−). WT and Fak−/− cells (5 × 105) were subjected to an in vitro adhesion assay on FN, laminin, and collagen. Adhesion was assessed by measuring absorbance at indicated times on extracellular matrix proteins FN (E), laminin (F), and collagen (G). Bar graph represents the optical density of adherent cells at 600 nm. Data shown are from 1 representative experiment; *P < .05, WT vs Fak−/−. Similar findings were observed in 3 independent mice. (H) Expression of integrins on WT and Fak−/− cells. Cells were stained with PE-conjugated anti-α4β1 and PE-conjugated anti-α5β1 antibody and subjected to flow cytometric analysis. Top panel solid histograms indicate the level of α4β1 and α5β1 expression on the surface of WT cells and bottom panel solid histograms indicate the level of α4β1 and α5β1 expression on the surface of Fak−/− cells. Open histograms demonstrate the level of expression using an isotype control antibody in both panels. Similar results were observed in 3 independent experiments. (I) WT and FAK-deficient cells grown in the presence of M-CSF for 3 days were starved of growth factors and stimulated on FN for indicated times. Lysates were subjected to Rac activity assay. Quantification of the level of active Rac is shown in bar graphs. Bottom panels show the level of active Rac (Rac GTP) and total Rac protein from 1 of 2 independent experiments performed.
Deficiency of FAK in BMMs alters actin-based functions. (A) WT and Fak−/− cells (2.5 × 105) were subjected to an in vitro migration assay on FN, laminin, and collagen. Images were acquired through a Zeiss Axioskop 2 Plus microscope equipped with a Plan-Neofluar 20×/0.5 objective lens, and were captured with an Axiocam MRC-5 camera and Axiovision 4 software (all from Zeiss). (B) A quantitative assessment of the number of WT and Fak−/− cells migrated through wells coated with FN, laminin, and collagen. Bars represent mean number of cells migrated ± standard deviation on various extracellular matrix proteins. Ten fields were scored in each experiment. Similar results were observed in 3 additional experiments (n = 3; *P < .05, WT vs Fak−/−). (C) WT and Fak−/− cells were cultured for 8 days in 24-well plates in the presence of 100 ng/mL M-CSF. An artificial wound was created in the monolayer using a pipet tip. Images were taken immediately and again at indicated time periods after creating the wound. Photomicrograph is from an independent experiment. (D) Bar graph shows quantitative analysis of the number of migrated cells in the wounded area. Data are from 1 representative experiment (n = 3; *P < .05 WT vs Fak−/−). WT and Fak−/− cells (5 × 105) were subjected to an in vitro adhesion assay on FN, laminin, and collagen. Adhesion was assessed by measuring absorbance at indicated times on extracellular matrix proteins FN (E), laminin (F), and collagen (G). Bar graph represents the optical density of adherent cells at 600 nm. Data shown are from 1 representative experiment; *P < .05, WT vs Fak−/−. Similar findings were observed in 3 independent mice. (H) Expression of integrins on WT and Fak−/− cells. Cells were stained with PE-conjugated anti-α4β1 and PE-conjugated anti-α5β1 antibody and subjected to flow cytometric analysis. Top panel solid histograms indicate the level of α4β1 and α5β1 expression on the surface of WT cells and bottom panel solid histograms indicate the level of α4β1 and α5β1 expression on the surface of Fak−/− cells. Open histograms demonstrate the level of expression using an isotype control antibody in both panels. Similar results were observed in 3 independent experiments. (I) WT and FAK-deficient cells grown in the presence of M-CSF for 3 days were starved of growth factors and stimulated on FN for indicated times. Lysates were subjected to Rac activity assay. Quantification of the level of active Rac is shown in bar graphs. Bottom panels show the level of active Rac (Rac GTP) and total Rac protein from 1 of 2 independent experiments performed.
In fibroblasts, Rac GTPases have been shown to be regulated by FAK. Since FAK-deficient cells are significantly impaired in regulating actin based functions, which are regulated to large extent by Rac GTPase, we next examined Rac-GTP levels in WT and Fak−/− BMMs by stimulating these cells on FN-coated plates for various time points. As seen in Figure 7I, loss of FAK in BMMs impairs the activation of Rac when stimulated via an ECM protein such as FN. Taken together, these results suggest that FAK not only regulates survival and growth of myeloid cells under steady-state conditions, but it does so also under conditions of stress. Furthermore, FAK also regulates cytoskeletal functions, and therefore functions as an integrator of both growth/survival and actin-based functions in myeloid cells.
Discussion
Previous studies have linked FAK signaling to disassembly of integrin-based adhesion sites, in regulating lamellipodia formation as well as adhesion turnover in migrating cells. FAK-deficient fibroblasts show excess focal contact formation and defects in cell movement.29 In the hematopoietic system, loss of FAK in mature macrophages using lineage restricted deletion (using lysozyme Cre mice) results in enhanced protrusive activity, reduced adhesion turnover, and impaired lamellipodia formation.30 Lineage-restricted deletion of FAK in platelets points to a negative role for FAK in megakaryopoiesis and platelet function.6 In contrast, loss of FAK specifically in mature neutrophils (using lysozyme Cre mice) does not alter adhesion/trafficking of these cells in vitro or in vivo, but results in impaired pathogen-killing, including a significant reduction in NADPH oxidase–mediated superoxide production and complement-mediated phagocytosis.31 Thus, while the role of FAK in mature hematopoietic cells has been described using mice in which the FAK gene has been deleted in a lineage-restricted manner in mature/committed cells; how the loss of FAK impacts cellular functions including growth, survival, differentiation, and migration of immature BM-derived myeloid and erythroid progenitors is not known. In the present study, by performing a BM progenitor-specific (MX.Cre; deletes FAK in very primitive hematopoietic cells) conditional deletion of FAK, we show that FAK, which is mostly known for its function in nonhematopoietic cells or in regulating cell adhesion and migration in fibroblasts, plays an essential role in regulating cytokine- and stress-induced responses in primary BM-derived erythroid and myeloid progenitors. These results depict a previously undescribed role for FAK in BM-derived erythroid and myeloid progenitors.
Our results demonstrating that loss of FAK in BM-derived myeloid progenitor's results in enhanced apoptosis under steady-state conditions while erythropoiesis remains unperturbed prompted us to examine the role of FAK in stress erythropoiesis. To this end, PHZ treatment of Fak−/− mice was associated with impaired erythrosplenomegaly, pronounced reduction in erythroblasts in the spleen and BM, lower hematocrits, and delayed recovery. While stress erythropoiesis can be contributed by several factors, KIT and EpoR signaling have been shown to play a prominent role in this context. With respect to KIT, mutants of KIT lacking Src family kinase binding sites (Y567) result in decreased red cell production and delayed recovery in response to PHZ-induced hemolytic anemia.32 Since FAK has been shown to activate Src kinases, it is conceivable that the impaired stress erythropoiesis in FAK−/− mice is partly due to altered KIT-induced activation of SFKs in vivo. Alternatively, recent studies have shown that EpoR lacking tyrosine residues show persistent anemia upon PHZ treatment, which can be restored by reconstituting Stat5 binding site.33 Thus, it is likely that impaired erythropoiesis during PHZ-induced anemia in FAK−/− mice might reflect involvement of both KIT- and EpoR-directed signals. Consistent with this notion, our studies performed in vitro demonstrate enhanced apoptosis and reduced SCF- and EPO-induced growth due to FAK deficiency in erythroid progenitors, which is associated with reduced Lyn SFK phosphorylation and enhanced p53 levels as well as caspase 3 cleavage products. FAK has been shown to regulate p53 levels and apoptosis. Furthermore, high p53 expression has been shown to induce apoptosis in fetal liver-derived erythroblasts as a result of loss of Mdm2 (a known negative regulator of p53)34 . Importantly, deficiency of Lyn in erythroblasts also results in enhanced SCF-induced apoptosis and proliferation.35 Our observations as a result of FAK deficiency in erythroid cells are somewhat in contrast to those recently reported in megakaryocytes lacking FAK.6 Interestingly, in these cells, just like in erythroid progenitors, loss of FAK results in reduced activation of Lyn. However, in megakaryocytes Lyn functions as a negative regulator of megakaryopoiesis.36 Thus, while activation of Lyn SFK is modulated in both megakaryocytes and erythroid cells due to FAK deficiency, the functional outcomes appear to be distinct.
In myeloid cells, our results suggest an essential role for FAK in regulating M-CSF– and G-CSF–induced growth and survival. The defects in growth and survival due to lack of FAK in these cells are related in large part to reduced Akt activation, enhanced cleaved caspase 3 expression, and reduced expression of antiapoptotic protein Bcl-xL. Previous studies have shown that G-CSF plays an essential role in inhibiting the activation of caspase 3 as well as in inducing the expression of antiapoptotic protein Bcl-xL.27,28 In addition to these prosurvival pathways, studies conducted in endothelial cells as well as fibroblasts show that defects in Fak−/− cell proliferation could be restored by p53 inactivation. While FAK activation of Akt can inhibit p53 activation in some cases,37,38 recent studies suggest that FAK-mediated p53 inactivation can be mediated by FAK nuclear translocation, binding to p53, and Mdm2-dependent p53 ubiquitination.39 It would be interesting to determine whether these additional pathways also contribute to growth factor–induced proliferation and survival in Fak−/− myeloid progenitors.
In addition to regulating cytokine-dependent growth and survival in myeloid progenitors, our results also suggest an essential role for FAK in protecting myeloid cells from cytotoxic effects of 5-FU. Removal of FAK signaling (Fak−/− mice) delays and impairs the recovery of myeloid cells in response to antineoplastic agents such as 5-FU. These results are consistent with previous studies demonstrating that down-regulation of FAK in melanoma cell lines results in increased sensitivity of these cells to 5-FU.40 While Akt may be clearly involved in sensitizing BM-derived myeloid cells to 5-FU–induced apoptosis and reduced recovery in the absence of FAK; previous studies have also implicated p53 in this process. In human colon cancer cell lines, 5-FU–induced apoptosis appears to be p53-dependent, as disruption of p53 in these cells results in marked resistance to the effects of 5-FU.41 Thus, if 5-FU–induced apoptosis depends on a p53-mediated pathway, FAK expression may provide resistance to 5-FU by suppressing this pathway. We are currently investigating this possibility.
In addition to playing an essential role in hemolytic and myelotoxic stress, our results suggest that FAK also contributes to inflammatory stress. We show that loss of FAK in BM-derived myeloid progenitors impairs the recruitment of Gr-1/Mac-1, as well as F4/80-positive cells in the peritoneal cavity as early as 4 hours after thioglycollate treatment. While in vitro, these defects were associated with reduced adhesion and migration on ECM proteins; in vivo this is likely to be due to cumulative defects in growth, survival, and actin-based functions in Fak−/− myeloid progenitors. Since defects in adhesion and migration were observed in response to all 3 stimuli including collagen, FN, and laminin; deficiency of FAK likely results in a general impairment in actin-based functions in Fak−/− cells as opposed to specific defects to certain stimuli. While Fak−/− cell migration, adhesion, and wound-healing were significantly altered compared with controls, defects were only partial, suggesting FAK-independent mechanisms may also contribute to some of these processes. To this end, we have recently shown that deficiency of p85α subunit of class IA phosphoinositol-3Kinase also results in similar defects as the ones observed in Fak−/− cells.42 In both FAK-deficient, and p85α-deficient BMMs, reduced activation of Rac GTPases was observed, although the reduction was more profound in cells lacking p85α.42 Interestingly, deficiency of Rac GTPases by themselves also impairs actin-based functions in Rac1- and Rac2-deficient myeloid progenitors.43 Thus, some of the actin-based defects in Fak−/− cells are likely due to impaired activation of Rho family GTPases.
The mechanism(s) by which FAK regulates growth/survival and migration via cytokine receptors and integrin molecules in myeloid and erythroid progenitors is likely to be complex. It has been well-documented that FAK contains in its amino terminal end a FERM domain,44 which can directly interact with growth factor receptors.45 Interestingly, JAK2 has been shown to interact with the Box1 region of cytokine receptors via its FERM domain.46 Thus, it is possible that FAK interacts with cytokine receptors in a similar manner. FAK has also been shown to associate with the activated platelet-derived growth factor and epidermal growth factor receptors via the FAK amino terminus domain. Previous studies in fibroblasts have shown that physical interactions between the amino terminus end of FAK with activated epidermal growth factor and platelet-derived growth factor receptors and that of the carboxy terminus domain of FAK with sites of integrin receptor clustering is essential for efficient migration.5 Whether similar interactions occur in myeloid and erythroid progenitors remains to be determined.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We would like to thank Marilyn Wales for her administrative support.
This work is supported by National Institutes of Health grant nos. R01 HL077177, R01 HL075816, and R01 HL08111.
National Institutes of Health
Authorship
Contribution: S.V. designed and performed the research and wrote the manuscript; B.R. designed and performed the research; P.H. performed the research; J.M. performed the research. H.E.B. provided the reagent; and R.K. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sasidhar Vemula, Herman B Wells Center for Pediatric Research, Indiana University School of Medicine, Cancer Research Institute, 1044 W. Walnut St, Rm 169, Indianapolis, IN 46202; e-mail: svemula@iupui.edu; or Reuben Kapur, Herman B Wells Center for Pediatric Research, Indiana University School of Medicine, Cancer Research Institute, 1044 W. Walnut St, Rm 168, Indianapolis, IN 46202; e-mail: rkapur@iupui.edu.

![Figure 2. Impaired myelopoiesis in Fak−/− mice. (A) Whole BM cells from WT and Fak−/− mice were analyzed for the expression of myeloid cell marker Gr-1 and Mac-1 by flow cytometry. The percentage of Gr-1/Mac-1 high double-positive cells (R2) and Gr-1/Mac-1 low positive cells (R3) in the BM are indicated in the square of each dot blot. Shown are results from a representative experiment. (B) Bar graph represents the mean values of Gr-1/Mac-1 high double-positive cells in the BM of WT and Fak−/− mice (n = 8; *P < .05). (C) Whole BM cells from WT and Fak−/− mice were stained with Annexin V and 7-AAD and analyzed by flow cytometric analysis. Numbers in each quadrant represent the percentage of cells in various stages of apoptosis. (D) Quantitative analysis of the percentage of BM cells undergoing early apoptosis (Annexin V) in WT and Fak−/− mice. Bars represent the mean of 3 independent experiments consisting of 3 pairs of mice of each genotype for each experiment (n = 9; *P < .05). (E) Reduced CFU-Cs in Fak−/− BM. LDBM cells (1.5 × 104) from WT and Fak−/− mice were plated in a methylcellulose colony-forming assay in the presence of the indicated cytokines. Colonies were enumerated 7 days later. Bar graph shows the mean number of colonies from 3 independent mice of each genotype plated in triplicates. *P < .05, Fak−/− versus WT. Similar results were obtained in 3 additional pairs of mice of each genotype. (F) WT and Fak−/− progenitors were subjected to an in vitro migration assay in response to SDF-1 or SCF on FN. Briefly, LDBM cells isolated from WT and Fak−/− mice were cultured in the presence of SCF, TPO, IL-6, and FLT-3 for 2 days. Cells (0.5 × 106) were subjected to transwell migration assay for 5 hours at 37°C. After 5 hours of incubation at 37°C, nonmigrated cells in the upper chamber were removed with a cotton swab. The migrated cells attached to the bottom surface of the membrane were stained with 0.1% crystal violet, dissolved in 0.1M borate, pH 9.0, and 2% ethanol for 5 minutes at room temperature. Photomicrographs shown are progenitor cells migrated on FN in response to SDF-1 or SCF. (G) The number of migrated cells per membrane was counted in 10 random fields with an inverted microscope using 20× objective lens; cells migrated into the lower chamber in response to SDF-1 or SCF are counted using hemocytometer from replicates of 3 from 1 experiment using 3 mice of each genotype. *P < .05, Fak−/− vs WT, mean ± SEM. (H) Primary erythroid progenitor proliferation was assessed by incorporation of radioactive thymidine in WT and Fak−/− cells. Briefly, erythroid progenitor cells grown for 2 days were starved in Stem Pro 34 medium without any growth factors or supplements for 4 hours. Erythroid progenitor cells (5 × 104) were placed in a 96-well plate in 200 μL complete medium either in the absence or in the presence of indicated concentration of EPO, SCF alone, or in combination. Cells were cultured for 48 hours and subsequently pulsed with 1.0 μCi (0.037 MBq) [3H] thymidine for 6 hours. Cells were harvested using an automated 96-well cell harvester, and thymidine incorporation was determined as cpm. Bar graph shows pooled data from 2 independent experiments performed in replicates of 4 using 4 mice per genotype per experiment. *P < .05, Fak−/− vs WT, mean ± SEM. (I) Quantitative analysis of the percentage of primary erythroid cells undergoing early apoptosis (Annexin V) in WT and Fak−/− cells. Cells were harvested after 4 days of culture and starved in absence of growth factors or supplements for 6 hours. Bar graph represents the mean of Annexin V–positive cells for 1 independent experiment performed in triplicates. *P < .05, Fak−/− vs WT, mean ± SD. (J) Erythroid progenitor cells were cultured for 4 days and starved in absence of growth factors or supplements for 4 hours and stimulated with SCF and EPO at 37°C. Cell lysates (35 μg) were subjected to Western blot analysis using indicated antibodies.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/20/10.1182_blood-2010-01-262790/7/m_zh89991059770002.jpeg?Expires=1769170253&Signature=eS4Xp0NNVsdI1059zCAK8GhrqxiPT3yiOGDy-2d4B0viLct~Ukxey3h4IFlCFcnkDjjNhAYdEbapIKt9nQoZLEUViZ~hFesr24zs6lqyv2Wdq70JIM06E9aBINv0dlycMsvLWyhGwj0-Wah2EhnXkgsltwH9P6pLaPjaeNSATO~PE1c0uhVPItAfjLyZZIZdVMQc0nl1lkdLAWCKZXtteqZfwKTze~e40sxGnqoVTenaywUnnhFTXOE9JbhSCMG6DqGmbNu~ZOej-aRhRozD3cBjm08m8ktDCKxT-Id2M1beG0sG9Ba7wtKRnL4w-tjR~dYyOM-ZZVkZO0CMUySIEQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
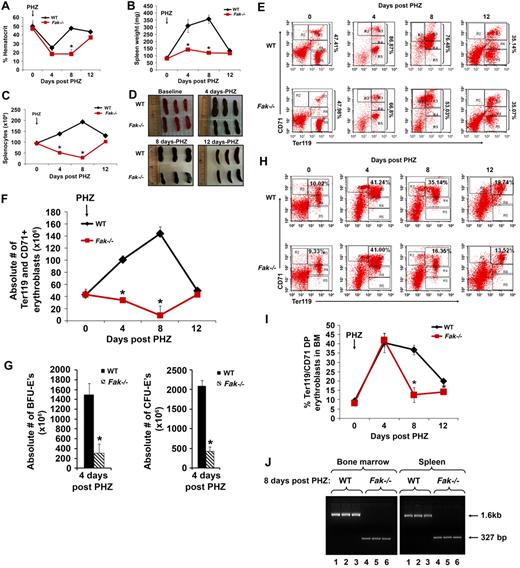
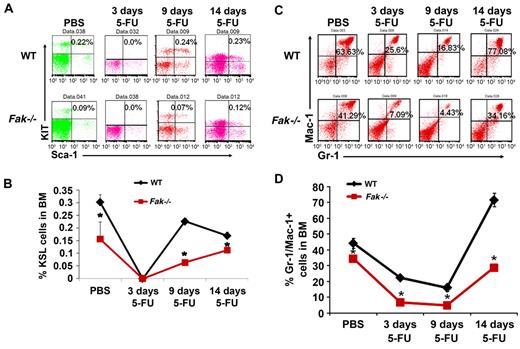
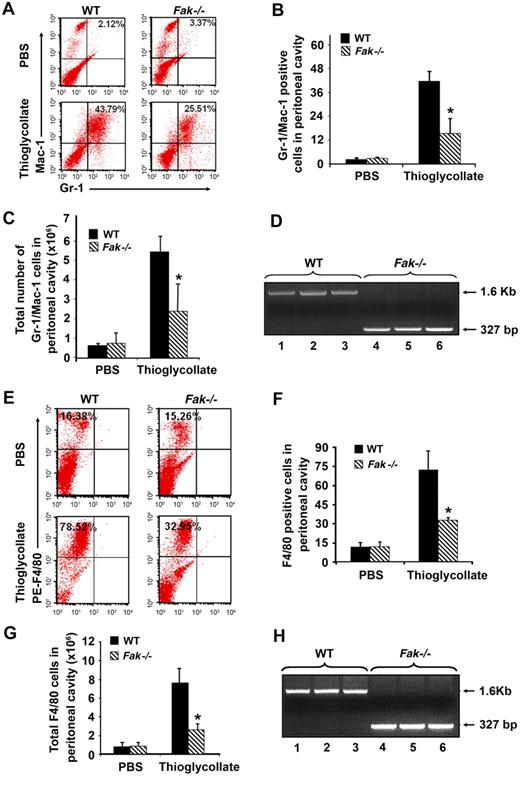
![Figure 6. Deletion of FAK in BMM and neutrophil cells results in reduced proliferation, survival, and activation of antiapoptotic proteins. (A) Cre-mediated Fak deletion in BMMs: after 1 month of poly (I):(C) injection, BM cells from WT and Fak−/− mice were cultured for 7 days, and DNA was extracted. Lanes 1 and 2 represent WT Fak bands, and lanes 3 and 4 represent FAK deleted bands. (B) Western blot analysis demonstrating the expression of Fak in WT and Fak−/− cells. Equal amount of cell lysates were subjected to Western blot analysis. Blot was probed with an antibody specific to FAK (raised against amino acids 903-1052). The same blot was reprobed for β-actin to show equal loading. (C) Cells were subjected to proliferation assay in the absence of growth factors as well as in the presence of indicated concentrations of M-CSF. After 48 hours of culture, cells were pulsed with [3H] thymidine for 6 hours. Concentrations of M-CSF are shown on the x-axis, and mean thymidine incorporation (in cpm) are shown on the y-axis. Shown are results from 1 of 3 independent experiments performed in quadruplicate; *P < .05. (D) Expression of F4/80 on WT and Fak−/− cells. Cells were stained with PE-conjugated anti-F4/80 antibody and subjected to flow cytometric analysis. Solid histograms indicate the level of F4/80 expression on the surface of WT and Fak−/− cells, while open histograms indicate the level of expression using an isotype control antibody. (E) BM cells grown in G-CSF were subjected to proliferation assay in the absence of growth factors and in the presence of indicated concentrations of various cytokines. After 48 hours of culture, cells were pulsed with [3H] thymidine for 6 hours. Concentrations of various cytokines are shown on the x-axis, and mean thymidine incorporation (in cpm) is shown on the y-axis. Bar graph shows data from 1 experiment performed in replicates of 4 (*P < .05). Similar findings were observed in 3 additional independent experiments. (F) BM cells grown in G-CSF derived from WT and FAK-deficient mice were starved of growth factors for indicated times and subjected to flow cytometric analysis after staining with Annexin V and 7-AAD. The percentages of early and late apoptotic cells at each time point are indicated. Shown is a representative dot blot from 1 of 3 independent experiments. (G) WT and Fak-deficient cells grown in the presence of IL-3 and G-CSF were starved of growth factors and stimulated for 5 minutes with G-CSF. Upper panel shows the activation of AKT as assessed by Western blot analysis using an anti–phospho AKT antibody. Bottom panel shows total AKT levels in each lane. (H) Actively growing neutrophils derived from WT and Fak−/− mice were subjected to Western blot analysis using an anti–caspase 3 (upper panel), anti–Bcl-xL (middle panel), and anti–β-actin (bottom panel) antibody. Similar results were observed in 2 additional independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/20/10.1182_blood-2010-01-262790/7/m_zh89991059770006.jpeg?Expires=1769170253&Signature=FMeCDPmsQI9OpBryVN823r1q8cLWlBkkOLuqG9bzhwGtagqlfF8LpNhfsL29EnmvTpb74hdP3glQO9R-bOgI7Mn~kGRkh3uqJCsxbVJxkbA5XgwdUqQj8VDYykn56f~tu4Qmj1LDCHOLTTrQEF9q0Hm~y2sBzg0xyqpg4Xq8tCJRh0AUX8Xqqw5YlGAM0NVpih0Xxicq6hbgmyhlWjCt2B4h83DbVyc~niLd18u1~B4olMDQtN28FR6-1FlgRGlA48LH86CP8OQnHDAL1MJy9~MPlQGntcWEW0wKBNQ9pK8CIs8C~gKjOysXU0MGfEfpNhsaXfyRlPOmAFeoNiKI2Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
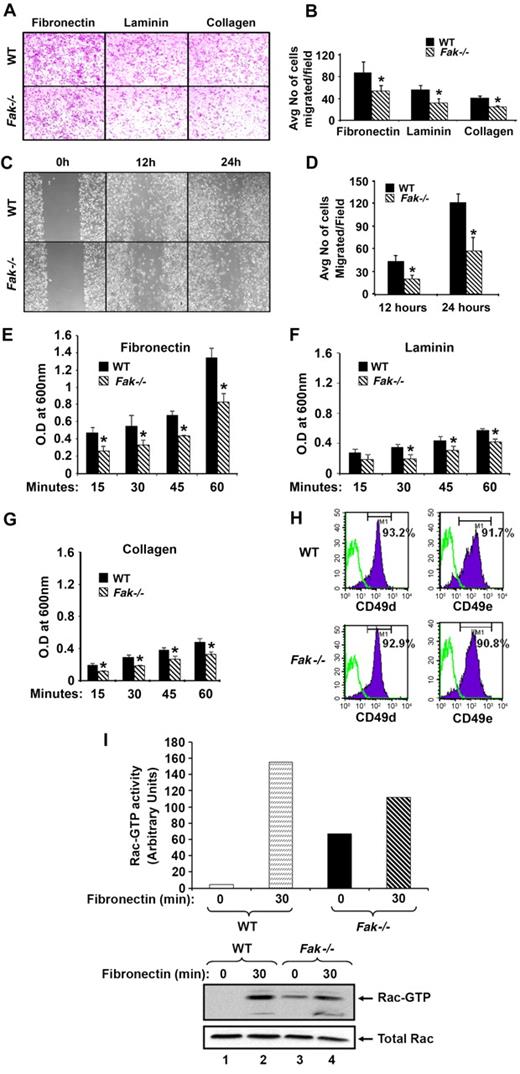
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal