Abstract
Autoimmune hemolytic anemia (AIHA) due to warm-acting IgA autoantibodies is rare. We explored the pathogenic mechanisms underlying destruction of red blood cells (RBCs) in a patient with severe AIHA mediated exclusively by polymeric immunoglobulin A (pIgA) anti-Band 3 autoantibodies. The follow-up period was 17 months. RBCs were not destroyed by complement activation as no deposition of complement was observed on the patient's RBCs. pIgA eluted from the patient's RBCs did not induce RBC destruction through phagocytosis by monocytes or antibody–dependent cell–mediated cytotoxicity by natural killer cells. Induction of eryptosis (ie, RBC apoptosis) due to direct alteration of the RBC membrane by pIgA autoantibodies was also excluded. By contrast, upon incubation with pIgA-opsonized RBCs, substantial RBC membrane transfers (ie, trogocytosis) to monocytes were observed that might contribute to RBC immune destruction. This effect was poorly inhibited by blockers of Fc receptors, excluding a major contribution of FcαRI to this process. Histologic analysis revealed a massive accumulation of agglutinated RBCs with little sign of erythrophagocytosis in the spleen. These results, together with the efficacy of splenectomy 17 months after AIHA onset, suggest that the trapping and subsequent sequestration of agglutinated RBCs in the spleen are the principal pathogenic mechanisms of pIgA-mediated AIHA.
Introduction
Autoimmune hemolytic anemia (AIHA) is characterized by hemolysis associated with the presence of immunoglobulin G (IgG), IgM, or IgA, and/or components of the complement system on red blood cells (RBCs). IgA class antibodies are present in approximately 14% of patients with warm-type autoimmune hemolytic anemia (wAIHA) and are almost always associated with IgG or IgM.1,2 In these cases, warm IgA autoantibodies (optimal reaction at 37°C) generally have a specificity spectrum similar to that of warm IgG autoantibodies.3 However, wAIHA associated exclusively with IgA antibodies, reacting optimally at 37°C, remains rare.1,4-8 Some of the features of these reported cases, including disease severity, high binding potency of IgA for RBCs with very little free antibody detectable in the serum, no strong evidence of blood group specificity, and in some cases, refractoriness to first-line treatment (administration of high doses of glucocorticosteroids), seem to be characteristic.1
IgA antibodies exist in several forms, monomeric IgA, dimeric IgA, and polymeric IgA, all of which interact to some extent with the human IgA Fc receptor (FcαRI/CD89).9 The distribution of polymeric and monomeric forms of IgA in the serum differs between species. Approximately 90% of the IgA present in the serum is monomeric in humans, but approximately the same proportion is polymeric in mice.10,11
The mechanisms of RBC destruction in IgA AIHA remain a matter of debate, but it is well established that IgG anti-RBC autoantibodies induce immune RBC destruction by activating complement and FcγR-bearing effector cells. In humans, IgA-mediated immune effector responses, such as phagocytosis, antibody–dependent cell–mediated cytotoxicity (ADCC), NADPH oxidase activation, and cytokine release, are induced primarily by the activation of FcαRI/CD89. Both monomeric IgA (mIgA) and dimeric IgA (dIgA) bind to FcαRI/CD89, and IgA immune complexes activate phagocytosis and other immune responses through the clustering of this receptor.12,13 One previous study investigated the mechanisms by which RBCs coated with IgA autoantibodies are destroyed in pure IgA-associated AIHA and demonstrated that IgA-coated RBCs failed to activate complement but triggered phagocytosis by monocytes in vitro.14 However, as the extent of phagocytosis was only moderate compared with that induced by IgG-coated RBCs, it has not been clear whether FcαRI–mediated erythrophagocytosis is indeed the major pathogenic mechanism responsible for the development of IgA-induced AIHA.
In this rarely documented severe AIHA, elucidation of the precise mechanism by which IgA autoantibodies provoke the destruction of RBCs is of particular importance to determine the most appropriate treatment. In this study, we explored the mechanism of destruction of RBCs sensitized with IgA autoantibodies isolated from a single case of severe wAIHA associated only with the IgA class during follow-up, in which splenectomy was the only successful treatment. Our results showed that eluted IgA anti-RBC autoantibodies were polymeric and triggered neither erythrophagocytosis nor complement activation, but that trapping and subsequent sequestration of agglutinated RBC in spleen is the pathogenic mechanism underlying IgA-mediated AIHA in this patient.
Methods
Case report: clinical aspects and routine exploration
A 41-year-old woman with no relevant medical history was referred to our department for unexplained acute–onset hemolytic anemia. The blood count revealed profound anemia with a hemoglobin (Hb) concentration of 4.2 g/dL, a high mean corpuscular volume (120 fL), a high reticulocyte count (600 × 109/L) and features of active hemolysis (lactate dehydrogenase 5× above the reference range and no haptoglobin detectable in the serum). No schistocytes or spherocytes were observed. The direct antiglobulin test (DAT) was strongly positive (4+) throughout the follow-up period of 17 months, from diagnosis to splenectomy, with an IgA pattern (negative for IgG and C3). Clinical examination showed mild splenomegaly. Both serologic and polymerase chain reaction tests for hepatitis C were positive. The patient (ABO type O) received 2 units of packed RBCs and treatment with prednisone was initiated at a daily dose of 1 mg/kg. Hb concentration increased to 8.5 g/dL within a week. However, the patient remained anemic and active hemolysis persisted despite continuous treatment with prednisone (1 mg/kg/d prednisone initially, with gradual tapering thereafter). She was therefore treated with rituximab (4 weekly infusions at 375 mg/m2) 4 months after AIHA onset, with no response. No response to mycophenolate mofetil was observed either. Splenectomy was therefore considered 17 months after first presentation. The spleen weighed 630 g and was deep red and firm. The patient's Hb concentration reached normal levels within 4 weeks after the procedure and remained normal (13.6 g/dL) 2 years after splenectomy. LDH levels remained slightly high, haptoglobin concentration was 0.07 g/L and prednisone could be tapered to 5 mg/d for the first time and then withdrawn. In routine tests several months after splenectomy, DAT remained positive for IgA (4+) and appeared slightly positive for IgG (1+). This study was approved by the Institutional Review Board of the Henri Mondor Hospital (CPP 08-008), Créteil, France. Informed consent was provided in accordance with the Declaration of Helsinki.
Characteristics of the autoantibody
Investigations included DAT, antibody screening tests, elution of antibodies from coated RBCs, searches for antibodies in serum and eluate, and characterization of the class and the specificity of the autoantibodies present in the eluate. Gel filtration, immunoblot, and Western blot analysis were also performed on the eluted autoantibody. All the samples were collected during the period between presentation and splenectomy. The eluates obtained were used immediately or stored at −80°C for further studies. (Details may be found in supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article.)
Erythrophagocytosis assay with monocytes
Adherent monocytes, isolated from normal peripheral blood mononuclear cells (PBMCs), were overlaid with normal unsensitized RBCs or with normal RBCs sensitized with e-IgA autoantibody. The slides were incubated for 2 hours, then fixed, and observed under a light microscope for assessment of RBC phagocytosis and adhesion; for details, see supple-mental Methods.
ADCC
The capacity of eluted-IgA (e-IgA) autoantibodies to trigger cytotoxic activity by normal PBMC subsets was evaluated in vitro on the basis of transient CD107a protein exposure at the cell surface.15 Data were collected by flow cytometry; for details, see supplemental Methods.
Eryptosis assay
The induction of eryptosis was assessed by AnnexinV-fluorescein isothiocyanate staining to detect phosphatidylserine (PS) exposure, and Fluo3/AM staining to detect increases in cytosolic Ca2+ concentrations. RBCs were tested after sensitization with e-IgA autoantibodies for 30 minutes at 37°C. Data were collected by flow cytometry; for details, see supple-mental Methods.
Trogocytosis assay
PBMCs and RBCs from donors were used for trogocytosis as previously described.16 RBCs were stained with the lipophilic green-emitting dye PKH67 (Sigma-Aldrich) according to the manufacturer's instructions. RBCs were then sensitized with the monoclonal HM16 IgG anti-human RhD (Diagast) or the human polyclonal IgG anti-RhD (Rhophylac; 100 μg/mL; LFB) for the positive controls, or with e-IgA autoantibodies. The RBCs, which served as targets, were incubated with PBMCs, at an RBC:PBMC ratio of 2:1, for 90 minutes. The cells were then washed with 0.5mM EDTA (ethylenediaminetetraacetic acid; Sigma-Aldrich) in phosphate-buffered saline. PBMCs subsets were stained with specific monoclonal antibodies (mAbs) for 30 minutes at 4°C, and flow cytometric analysis was then carried out. We investigated the involvement of the FcαRI in e-IgA–induced trogocytosis by treating PBMCs with a polyspecific FcR-blocking reagent (Miltenyi Biotec), which efficiently blocks the binding of microbeads or antibodies to the Fc receptor of FcR–expressing cells, including monocytes.17 Briefly, PBMCs in 90 μL of phosphate-buffered saline plus 0.2% bovine serum albumin were incubated twice for 10 minutes each with 10 μL of FcR-blocking Reagent (FcR-Blocker) at 4°C. Alternatively, PBMCs were treated with 50 μg/mL My43 IgM FcαRI blocking mAb for 30 minutes at 37°C.18 Treated PBMCs were washed, stained with the various mAbs (phycoerythrin-conjugated mAbs SK7 anti-CD3, SK3 anti-CD4, SK1 anti-CD8, MY31 anti-CD56; MΦP9 anti-CD14; BD Biosciences) and tested for trogocytosis. Data were collected and analyzed by flow cytometry. Synaptic transfer was assessed as the acquisition of PKH67 fluorescence from PKH67-labeled RBCs by the various leukocyte subset populations. This acquisition led to an increase in mean fluorescence intensity (mfi) for PKH67. For the detection of the spontaneous PKH67 fluorescence released from untreated RBCs and the transfer of this fluorescence to leukocytes, a T0 control sample (“instant contact”) was included in each experiment.
Histologic examinations
The patient's spleen was recovered after splenectomy, fixed in formalin, and routinely processed for histologic analysis. Formalin-fixed paraffin embedded tissue sections were stained with hematoxylin and eosin for histologic examination, and Perls to detect hemosiderin deposits.
Statistical analysis
Data are expressed as arithmetic mean ± SEM. We carried out 2-tailed Student t tests, Mann-Whitney U tests, or analysis of variance as appropriate with GraphPad Prism software, version 5.0. Values of P < .05 were considered statistically significant.
Results
An AIHA mediated exclusively by IgA autoantibodies in the absence of complement activation
On admission, DAT was strongly positive (score of 4+) with a monospecific anti-human IgA antiglobulin. Negative results were obtained with anti-IgG, anti-IgM, anti-C3d, and anti-C3b sera. No free circulating autoantibodies against RBCs were detected in the serum, as demonstrated by the negative result screening by indirect antiglobulin test with anti-IgG or anti-IgA. The DAT remained strongly positive (4+) for IgA throughout follow-up and the various treatments. Immunoglobulin levels were normal throughout follow-up, and no monoclonal gammapathy was detected. During sample collection, from admission to splenectomy, we observed only pure IgA DAT (score of 4+). No spontaneous agglutination of sensitized RBCs was observed.
Characterization of the e-IgA autoantibodies
As no autoantibodies were found in serum, IgA autoantibodies were studied exclusively with eluates obtained from the patient's RBCs. The eluted autoantibodies reacted at 37°C with a titer of 64 and at 22°C with a titer of 16, confirming that the AIHA was of the warm type. No common blood group specificity of the e-IgA autoantibodies was identified in the screening test. Further tests showed that e-IgA displayed little or no reactivity to RBCs treated with α-chymotrypsin and pronase-treated RBCs. (supplemental Table 1). Consistent with previous findings,19,20 these results suggested that the target antigen may be located on the third external loop of Band 3, on decay-accelerating factor (DAF; CD55), or on glycophorin B (GPB; CD235). Positive agglutination reactions against rare RBCs, including Inab (DAF-deficient), Mk/Mk (GPB-deficient), and Rhnull samples, ruled out specificity for DAF, GPB, and RhAG. Agglutination blocking tests showed that 2 mAbs specific for the third external loop of Band 3 inhibited the binding of e-IgA autoantibodies to RBCs, whereas mAbs directed against other proteins present on the surface of RBCs had no effect (supplemental Table 2). Thus, the target of the e-IgA autoantibody is located on the third loop of Band 3.
Gel fractionation analysis was carried out on the eluate to determine the elution profile of the proteins (supplemental Figure 1A). We identified 2 major protein peaks. The first peak (arrow 1; elution volume 61 mL) and the second peak (arrow 3; elution volume 97 mL) corresponded to the positions of the void volume and the albumin present in the buffer used for the elution technique, respectively. Immunoblot analysis of the individual fractions (supplemental Figure 1B) confirmed the presence of IgA in the first peak, indicating a polymeric form of IgA (pIgA), but also the absence of IgA at the theoretical position for a monomeric form of IgA (arrow 2; elution volume 80 mL). The demonstration by Western blotting of the presence of J-chains confirmed the presence of pIgA in the first peak (supplemental Figure 1C). These results clearly indicate that the IgA autoantibodies responsible for sensitization of the patient's RBCs were secreted in a polymeric form.
Lack of phagocytosis by monocytes and of ADCC by PBMC subsets against RBCs sensitized with e-IgA autoantibodies
The ability of normal monocytes to phagocytise common RBCs, sensitized with e-IgA autoantibodies, was assessed in vitro. Incubation with e-IgA–coated RBCs did not lead to erythrophagocytosis by monocytes (supplemental Figure 2A). The mean values obtained from several independent determinations were below the cutoff point for positivity (5%).21 We assessed the ADCC induced by various normal PBMC subsets against RBCs sensitized with e-IgA. Only minimal levels of CD107a staining were detected on CD56+ natural killer (NK) cells and CD8+ T cells (supplemental Figure 2B). Thus, pIgA autoantibodies only weakly trigger the cytotoxic process of immune effector cells.
Lack of activation of RBC eryptosis by e-IgA autoantibodies
Some IgG antibodies against blood group A can induce eryptosis (ie, RBC apoptosis) of A-positive RBCs.22 After incubation for 24 hours, e-IgA autoantibodies induced no significant increase in PS exposure and cytosolic Ca2+ concentrations over those obtained for nontreated RBCs (supplemental Figure 3). Thus, e-IgA autoantibodies were not considered to cause cytotoxic effects on RBCs through the activation of eryptosis.
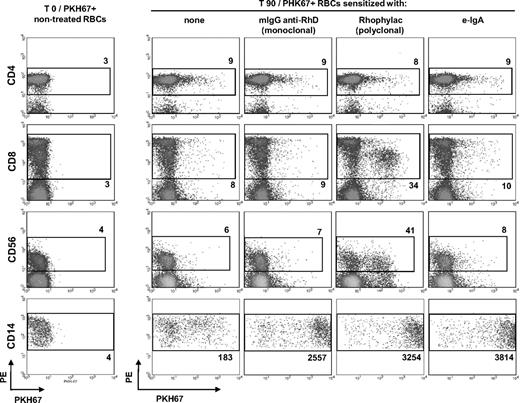
FcαRI–independent monocyte–mediated trogocytosis of RBCs sensitized with e-IgA autoantibodies
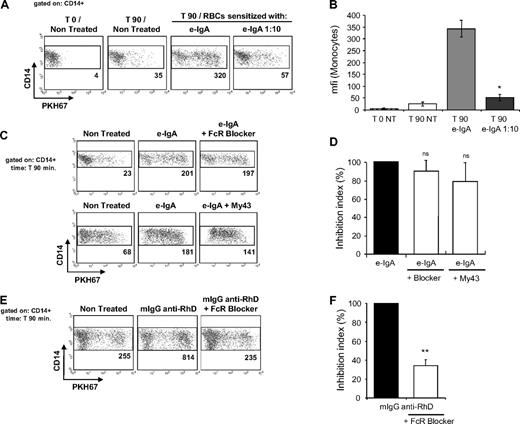
We evaluated trogocytosis, that is, membrane transfers occurring between sensitized RBCs and various PBMC populations (Figure 1). As previously reported,16 very low levels of membrane transfers occurred naturally between lymphocyte populations and nonsensitized RBCs incubated together for 90 minutes (lines I, II, and III; comparisons for lymphocytes before [column I] and 90 minutes [column II] after incubation with nontreated RBCs). Notably, the intensity of membrane exchanges between the various lymphocytes populations and RBCs was not significantly increased by sensitization with polymeric e-IgA autoantibodies (column V: e-IgA). However, in tests with CD14+ monocytes (line IV), we observed substantial membrane transfer from RBCs coated with e-IgA, mIgG anti-RhD, or Rhophylac (polyclonal). Trogocytosis between e-IgA/RBCs and monocytes was dose-dependent (Figure 2A-B), as it was markedly diminished when RBCs were sensitized with e-IgA autoantibodies diluted 1:10 (P < .05 vs e-IgA–treated RBCs; 2-tailed U test). We investigated the involvement of FcαRI in the higher levels of trogocytosis promoted by e-IgA autoantibodies by treating monocytes with either polyspecific FcR-Blocker or My43 FcαRI/CD89 blocking mAb before contact with e-IgA/RBCs (Figure 2C-D). However, this decreased the membrane transfer between e-IgA/RBCs and monocytes only very slightly. The 13.2% and 20.7% inhibitions obtained in the presence of FcR Blocker and My43 FcαRI/CD89 blocking mAb, respectively, was not statistically significant (P > .1 vs nontreated monocytes; analysis of variance). Thus, the observed trogocytosis was mostly independent of FcαRI on monocytes. By contrast, the treatment of monocytes with FcR-Blocker almost entirely abolished trogocytosis between monocytes and RBCs sensitized with a mIgG anti-RhD, as mfi values obtained after incubation of FcR-Blocker–pretreated monocytes with mIgG anti-RhD–coated RBCs were comparable with those obtained by nontreated monocytes coincubated with nonsensitized RBCs (Figure 2E-F; P < .01; t test).
Monocyte-mediated trogocytosis of RBCs sensitized with e-IgA autoantibodies. PKH-67–labeled RBCs were incubated with PBMCs for 90 minutes. Spontaneous membrane transfer between RBCs and various leukocyte populations (see the gates; column I), before (T0) and after coculture (T90) are visualized as an acquisition of PKH67 mfi by: CD4+ (line I), CD8+ (line II) T lymphocytes; CD56+ NK cells (line III) and CD14+ monocytes (line IV), respectively. RBCs were sensitized, or not (columns I and II, respectively), with a mAb directed against RhD (mIgG anti-RhD; column III), a polyclonal IgG against RhD (Rhophylac, column IV) or with eluted IgA autoantibodies (e-IgA; column V). Sensitization with mIgG anti-RhD and Rhophylac served as positive controls. Data were collected on a BD FACScan cytometer and analyzed with CellQuest Pro software Version 4.0. Dot plots show the results from one representative experiment. The numbers indicated represent the mfi of the gated populations. Similar results were obtained in 2 other independent experiments. We visualized and analyzed at least 3000 gated events for each cell population.
Monocyte-mediated trogocytosis of RBCs sensitized with e-IgA autoantibodies. PKH-67–labeled RBCs were incubated with PBMCs for 90 minutes. Spontaneous membrane transfer between RBCs and various leukocyte populations (see the gates; column I), before (T0) and after coculture (T90) are visualized as an acquisition of PKH67 mfi by: CD4+ (line I), CD8+ (line II) T lymphocytes; CD56+ NK cells (line III) and CD14+ monocytes (line IV), respectively. RBCs were sensitized, or not (columns I and II, respectively), with a mAb directed against RhD (mIgG anti-RhD; column III), a polyclonal IgG against RhD (Rhophylac, column IV) or with eluted IgA autoantibodies (e-IgA; column V). Sensitization with mIgG anti-RhD and Rhophylac served as positive controls. Data were collected on a BD FACScan cytometer and analyzed with CellQuest Pro software Version 4.0. Dot plots show the results from one representative experiment. The numbers indicated represent the mfi of the gated populations. Similar results were obtained in 2 other independent experiments. We visualized and analyzed at least 3000 gated events for each cell population.
Lack of involvement of FcαRI in monocyte–mediated trogocytosis of RBCs sensitized with e-IgA autoantibodies. (A-B) PBMCs were incubated with PKH67-labeled RBCs, left untreated or sensitized with e-IgA autoantibodies and the acquisition of PKH67 fluorescence by CD14+ monocytes was determined before (T0) and after 90 minutes (T90) of coculture. Eluted IgA autoantibodies were used pure or diluted 1:10 in Hanks buffered salt solution (n = 3). *P < .05 vs e-IgA–treated; U test. (C-D) Before incubation with RBCs, PBMCs were treated either with a polyspecific FcR blocking reagent (FcR blocker; n = 6) or with the My43–specific FcαRI/CD89 blocking antibody (50 μg/mL; n = 4); ns: not significantly different vs nontreated monocytes; t test. (E-F) PBMCs incubated with mAb against RhD-sensitized RBCs (mIgG anti-RhD; n = 3) were tested for trogocytosis after treatment with the FcR Blocker. **P < .01 vs untreated monocytes; 2-tailed paired t test. The events shown are gated on monocytes, based on forward scatter/side scatter parameters and CD14 staining. Data were collected on a BD FACScan cytometer and analyzed with CellQuest Pro software Version 4.0. Dot plots (left panels A,C,E) show the results from one representative experiment. The numbers indicate the mfi of the gated populations. Histograms (right panel B,D,F) show means and standard errors of the means from at least 3 independent determinations. The histograms in panel B show the mfi values. The histograms in panels D and F show the inhibition index for fluorescence transferred to CD14+; the value obtained for sensitized RBCs incubated with untreated monocytes represent 100%.
Lack of involvement of FcαRI in monocyte–mediated trogocytosis of RBCs sensitized with e-IgA autoantibodies. (A-B) PBMCs were incubated with PKH67-labeled RBCs, left untreated or sensitized with e-IgA autoantibodies and the acquisition of PKH67 fluorescence by CD14+ monocytes was determined before (T0) and after 90 minutes (T90) of coculture. Eluted IgA autoantibodies were used pure or diluted 1:10 in Hanks buffered salt solution (n = 3). *P < .05 vs e-IgA–treated; U test. (C-D) Before incubation with RBCs, PBMCs were treated either with a polyspecific FcR blocking reagent (FcR blocker; n = 6) or with the My43–specific FcαRI/CD89 blocking antibody (50 μg/mL; n = 4); ns: not significantly different vs nontreated monocytes; t test. (E-F) PBMCs incubated with mAb against RhD-sensitized RBCs (mIgG anti-RhD; n = 3) were tested for trogocytosis after treatment with the FcR Blocker. **P < .01 vs untreated monocytes; 2-tailed paired t test. The events shown are gated on monocytes, based on forward scatter/side scatter parameters and CD14 staining. Data were collected on a BD FACScan cytometer and analyzed with CellQuest Pro software Version 4.0. Dot plots (left panels A,C,E) show the results from one representative experiment. The numbers indicate the mfi of the gated populations. Histograms (right panel B,D,F) show means and standard errors of the means from at least 3 independent determinations. The histograms in panel B show the mfi values. The histograms in panels D and F show the inhibition index for fluorescence transferred to CD14+; the value obtained for sensitized RBCs incubated with untreated monocytes represent 100%.
Massive accumulation of agglutinated RBCs in the spleen
Histologic examination of splenic tissue after patient's splenectomy showed that the red pulp was highly congestive (Figure 3A), with splenic cords packed with agglutinated RBCs (Figure 3B arrow). Consequently, the white pulp was markedly atrophic and only very small periarteriolar lymphoid sheaths were recognizable (Figure 3A arrows). Very few erythrophagocytosis events were observed, as Perls staining showed only rare hemosiderin-containing macrophages (Figure 3C-D arrows). These data indicate that RBC sequestration due to massive hemagglutination in the spleen was the major pathogenic mechanism of AIHA associated exclusively with IgA autoantibodies in the patient.
Histologic examination of the patient's spleen. (A-B) Hematoxylin and eosin staining. Original magnifications ×25 (A) and ×400 (B). The red pulp is highly congestive and the white pulp is atrophic, with a marked reduction of the periarteriolar lymphoid sheaths (arrows in panel A). The splenic cords are packed with agglutinated RBCs (arrow in panel B). (C-D) Perls iron staining. Original magnification ×100 (C) and ×400 (D). Very few hemosiderin-containing macrophages are observed (arrows).
Histologic examination of the patient's spleen. (A-B) Hematoxylin and eosin staining. Original magnifications ×25 (A) and ×400 (B). The red pulp is highly congestive and the white pulp is atrophic, with a marked reduction of the periarteriolar lymphoid sheaths (arrows in panel A). The splenic cords are packed with agglutinated RBCs (arrow in panel B). (C-D) Perls iron staining. Original magnification ×100 (C) and ×400 (D). Very few hemosiderin-containing macrophages are observed (arrows).
Discussion
In this study, we took advantage of the opportunity provided by the identification of this serious case of wAIHA with IgA only to study the mechanisms by which RBCs sensitized with IgA were destroyed. Our analysis of the effector functions of e-IgA autoantibodies and histologic examination of the spleen demonstrated that anti-RBC IgA autoantibodies induced AIHA by a novel complement- and FcαRI-independent mechanism different from that of anti-RBC IgG autoantibodies. Our data indicate that, in this case, sequestration of the pIgA-opsonized RBCs resulting from hemagglutination in the spleen is the major pathogenic mechanism responsible for the development of AIHA.
Band 3 was identified as the target of the IgA on the patient's RBCs. Only one other case of wAIHA with IgA directed against Band 3 in a 3-year-old child has been reported.19 However, other cases associated with IgG or IgM autoantibodies with Band 3 specificity have been described.20,23,24 The clinical relevance of Band 3 as a target in AIHA may be related to the broad role played by this anion exchanger in RBC physiopathology and to its abundance, as it accounts for more than 30% of the proteins present on the RBC membrane. The absence of free IgA anti-Band 3 autoantibodies in the serum of the patient described here suggests that most autoantibodies are probably absorbed onto patient's RBCs. This may be partly due to the high density of Band 3 on RBCs and due to a high binding potency of the patient's IgA autoantibody to RBCs.
In this case of AIHA, there was no deposition of C3b on RBCs, as previously reported in a patient with AIHA associated only with the IgA class of autoantibodies,14 indicating a minimal role of complement activation in IgA-associated AIHA. These findings provide strong evidence against the notion that IgA is able to activate complement by the alternative and lectin pathways, as claimed in several in vitro studies.25,26 A few cases of IgA AIHA with complement activation have been described,1 but in most of these cases, the AIHA was not purely due to IgA AIHA, with IgG and/or IgM also sensitizing RBCs, leading to complement deposition. Our results are consistent with the recent demonstration of a lack of involvement of complement activation in the pathogenesis of IgA-induced AIHA in mice, as the development of AIHA associated with IgA anti-RBC monoclonal autoantibodies was not inhibited at all in C3-deficient mice.27
Erythrophagocytosis assays clearly showed that e-IgA/RBCs did not induce phagocytosis by monocytes in vitro, contrasting with the results obtained for anti-RhD IgG-sensitized RBCs. Data from erythrophagocytosis tests are generally strongly correlated with the severity of AIHA involving IgG.28 Our results differed somewhat from those of a previous study reporting moderate phagocytosis in a case of wAIHA with pure IgA.14 This discrepancy was not due to the use of monocytes from healthy donors in our study, as similar negative results were obtained during the first few days of the follow-up, in coculture experiments with monocytes and sensitized RBCs obtained from fresh blood samples of the patient (data not shown). Instead, the lack of erythrophagocytosis in this study may be due to the polymeric form of IgA autoantibodies, as documented by gel filtration analysis of e-IgA autoantibodies and the presence of J-chains. Furthermore, our results exclude a role for ADCC and antibody-induced eryptosis as a possible cause of the RBC destruction in our patient, although such mechanisms have recently been proposed for the development of anemia associated with different types of anti-RBC antibodies.22,29
The lack of in vitro erythrophagocytosis is consistent with the histologic finding of few signs of erythrophagocytosis in the spleen. Instead, we observed a RBC sequestration due to the marked accumulation of agglutinated RBCs in the spleen. In another reported case of wAIHA with pure IgA, the authors provided evidence of splenic sequestration of RBCs, documented through RBC survival studies with Cr51-labeled RBCs.14 Unfortunately, no histologic data for the spleen were presented for this other patient, but it is possible that anemia developed due to the accumulation of agglutinated RBCs in spleen, as observed in our case. Identical splenic lesions were seen in murine AIHA induced by pIgA and by IgM mAbs against RBCs.27
Trogocytosis experiments revealed a substantial level of membrane exchanges between e-IgA/RBCs and monocytes. These membrane exchanges may be facilitated by close contact occurring in the spleen due to a slow blood flow. The precise role of these exchanges in the development of AIHA remains unclear, but they may modify the RBC membrane, thereby promoting RBC agglutination and subsequent destruction in the spleen. Such a mechanism would account for the absence of spontaneous agglutination of both control RBCs coated in vitro with the e-IgA and sensitized RBCs obtained from the patient. It should also be stressed that the trogocytosis observed was mostly independent of FcαRI, suggesting the involvement of IgA receptors other than FcαRI, such as the receptors for poly Ig, asialoglycoprotein, Fcα/μ, or transferrin.13,18 Clearly, it would be of interest to identify the receptor involved in the in FcαRI–independent interaction between pIgA/RBCs and monocytes.
The in vitro results of this study, the efficacy of splenectomy treatment, and the histologic data for the spleen indicate that, in this case, IgA anti-RBC autoantibodies provoked anemia due to hemagglutination and subsequent sequestration of RBCs in spleen, independently of complement activation and FcαRI-bearing effector cells. No phagocytosis, by monocytes, of RBCs sensitized with pIgA was observed, but we cannot exclude the possibility that monomeric IgA autoantibodies efficiently trigger FcαRI–dependent erythrophagocytosis. Treatment protocols for wAIHA with pure IgA are generally identical to those used for other types of wAIHA, with corticosteroids used as the first–line treatment. Intravenous immunoglobulins, immunosuppressive drugs, and more recently rituximab have all been used in cases of treatment failure on corticosteroids. Splenectomy is generally considered a treatment of last resort. The goal of the various treatments is principally to stop both the production of autoantibodies and the destruction of sensitized RBCs. In very severe cases of AIHA, elucidation of the mechanism responsible for the immune destruction of RBCs would help to guide decisions concerning the choice of first-line treatment. Therefore, in clinical conditions, the autoantibody class, including IgA, and possibly the autoantibody specificity should be determined rapidly, to make it possible to offer appropriate treatment such as splenectomy in other similar cases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Hélène Jouault, Dr Mary Poupot, and Dr José A. Campillo for providing research and technical assistance.
This work was supported by the Etablissement Français du Sang, Ile-de-France, and partly by a grant from the Swiss National Foundation for Scientific Research.
Authorship
Contribution: P.C. designed and performed experiments, analyzed the data, and cowrote the manuscript; M.M. collected clinical data and helped to write the manuscript; D.J. and K.Y. characterized the autoantibody; C.C.-B. performed histologic analysis of the spleen; G.B. provided technical assistance; A.B. and J.-J.F. contributed to the design of the ADCC and trogocytosis experiments, respectively, and reviewed the manuscript; B.G. and P.B. contributed intellectually to the project and reviewed the manuscript; S.I. characterized the autoantibody, analyzed data, and cowrote the manuscript; and F.N.-P. designed and supervised the research, analyzed the data, and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: France Noizat-Pirenne, MD, PhD, Etablissement Français du Sang, Ile de France, Henri Mondor Hospital, 51 Ave du Maréchal de Lattre de Tassigny, 94000 Créteil, France; e-mail: france.noizat-pirenne@efs.sante.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal