Abstract
CD40 ligand (CD40L), identified as a costimulatory molecule expressed on T cells, is also expressed and functional on platelets. We investigated the thrombotic and inflammatory contributions of platelet CD40L in atherosclerosis. Although CD40L-deficient (Cd40l−/−) platelets exhibited impaired platelet aggregation and thrombus stability, the effects of platelet CD40L on inflammatory processes in atherosclerosis were more remarkable. Repeated injections of activated Cd40l−/− platelets into Apoe−/− mice strongly decreased both platelet and leukocyte adhesion to the endothelium and decreased plasma CCL2 levels compared with wild-type platelets. Moreover, Cd40l−/− platelets failed to form proinflammatory platelet-leukocyte aggregates. Expression of CD40L on platelets was required for platelet-induced atherosclerosis as injection of Cd40l−/− platelets in contrast to Cd40l+/+ platelets did not promote lesion formation. Remarkably, injection of Cd40l+/+, but not Cd40l−/−, platelets transiently decreased the amount of regulatory T cells (Tregs) in blood and spleen. Depletion of Tregs in mice injected with activated Cd40l−/− platelets abrogated the athero-protective effect, indicating that CD40L on platelets mediates the reduction of Tregs leading to accelerated atherosclerosis. We conclude that platelet CD40L plays a pivotal role in atherosclerosis, not only by affecting platelet-platelet interactions but especially by activating leukocytes, thereby increasing platelet-leukocyte and leukocyte-endothelium interactions.
Introduction
CD40 ligand (CD40L; CD154) and its receptor CD40, costimulatory molecules of the tumor necrosis factor (TNF) and TNF receptor family, have important roles in modulating immune responses and inflammation.1,2 In atherosclerosis, a chronic inflammatory disease of the large arteries involving multiple immune cell subsets,3,4 the cross-cellular interaction of CD40L with CD40 plays a major role. Deficiency or inhibition of CD40L or CD40 in hyperlipidemic (apolipoprotein E [Apoe−/−] or low density lipoprotein receptor [Ldlr−/−]) mice not only reduced the atherosclerotic lesion formation but also resulted in a clinically favorable plaque phenotype featuring extensive fibrosis and only a few inflammatory cells.5-10 Recently, we demonstrated that these phenotypic changes depend on the CD40-TRAF6, but not CD40-TRAF2/3/5 axis in leukocytes.9,10
CD40L is expressed on a plethora of cell types present in or around atherosclerotic plaques, such as T cells, macrophages, smooth muscle cells, and endothelial cells.1 In addition, it is found on platelets, which are on activation the most important source of circulating, soluble CD40L.11 However, the contribution of platelet CD40L to atherosclerosis has remained unclear to date.
As early as 1998, Henn et al reported that activated platelets express CD40L, which induces endothelial cells to secrete chemokines and express adhesion molecules, thereby initiating an inflammatory response of the vessel wall.11 However, later it was reported that platelet CD40L acts in an autocrine way by binding to integrin-αIIbβ3, contributing to the stabilization of thrombi,12,13 suggesting a role for platelet CD40L in inflammation and thrombosis, both imperative in atherosclerosis.
Although the role of platelets in hemostasis and thrombosis is well established, their function as potent immune cells, capable of initiating and mediating inflammation in the vasculature, is just emerging.14-17 For instance, adhesion of activated platelets via P-selectin, glycoprotein (GP)Ibα, and αIIbβ3 to the endothelium induces expression of adhesion molecules, such as vascular cellular adhesion molecule-1 (VCAM-1), intracellular adhesion molecule-1, E-selectin, chemokines (eg, CCL2, CXCL4, and CCL5), and matrix metalloproteinases (MMP-1, MMP-2, and MMP-9). These factors facilitate leukocyte recruitment into the lesion, thus accelerating atherosclerotic plaque formation.18-24
Moreover, activated platelets can indirectly support leukocyte recruitment via formation of platelet-leukocyte aggregates (PLAs).25 Through P-selectin, platelets bind to the P-selectin glycoprotein ligand on leukocytes, and the multicellular conjugates produce chemokines, such as CCL2 and CCL5, and cytokines, such as interleukin-1β (IL-1β), to further activate leukocytes.23-28 In vitro observations have indicated that these conjugates tether and roll on endothelial cells with a higher avidity than nonconjugated leukocytes, thereby enhancing endothelial activation.26-28 In addition, PLAs have been observed in prethrombotic or prothrombotic clinical conditions, and may provide a suitable predictor of acute myocardial infarction.29,30
It was demonstrated that repeated injection of thrombin-activated platelets into Apoe−/− mice resulted in acceleration of atherosclerosis, which was caused by platelet-mediated activation of the endothelium and P-selectin-dependent formation of PLAs.23
Considering the role of platelet CD40L in both thrombosis and inflammation, we investigated the atherogenic contribution of platelet CD40L. We demonstrate that platelet CD40L promotes both leukocyte and platelet adhesion to the endothelium and mediates the formation of PLAs. Repeated intravenous injection of activated Cd40l−/− platelets into Apoe−/− mice prevented the profound increase of atherosclerosis and the disruption of T-cell homeostasis (T-effector cell/Treg balance) that was observed after injection of activated Cd40l+/+ platelets.
Methods
Mice
Cd40l−/−Apoe−/− and Cd40−/−Apoe−/− mice were generated by interbreeding Cd40l−/− (kind gift of R. Flavell) and Cd40−/− mice (kind gift of R. Noelle), respectively, with Apoe−/− mice (The Jackson Laboratory), all on a C57Bl/6 background. All mice were housed and bred according to institutional guidelines. Experiments were approved by the Maastricht University animal experimental and care committees.
Platelet function assays
Blood was obtained from the retro-orbital plexus and collected into citrate containing tubes. For clot retraction, platelet-rich plasma (PRP) was prepared by centrifugation, and the platelet concentration was adjusted to 2 × 108 platelets/mL with Tyrode-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) containing CaCl2 (2mM). PRP was placed in a glass tube and incubated at 37°C for 5 minutes. Thrombin (11nM, Sigma-Aldrich) was added, and clot retraction was recorded at different time points by photographic images. Expression levels of P-selectin and GPIbα were measured by flow cytometry (FACSCanto II, BD Biosciences). Bleeding time was assessed in 6- to 8-week-old mice. Tails were cut 2 mm from the end and placed in 37°C 0.9% saline solution. The times to cessation of bleeding and any rebleeding were recorded.31
Measurement of thrombus formation under flow
Platelet adhesion experiments under flow conditions were performed with mouse blood collected into D-phenylalanyl-L-prolyl-L-arginine chloromethylketone (PPACK) and heparin.32 Blood was perfused over coverslips coated with collagen or fibrinogen mounted on a transparent, parallel-plate flow perfusion chamber.33 Alternatively, coverslips were coated with mouse von Willebrand factor (VWF), using a rabbit antibody against human VWF (1:500; Dako).34 Flow chambers were perfused at a shear rate of 1000 or 1700 seconds for 4 minutes. To assess platelet adhesion, microscopic phase-contrast images were recorded using a Visitech digital imaging system equipped with 2 intensified, charge-coupled device cameras.35 Images were captured with a 40×/1.3 numeric aperture (NA) UV-transparent objective and 1.5× optical magnification. Morphometric analysis of thrombus size was performed using Metamorph .5.0.0 software, using predefined values of platelet numbers per feature.36 Thrombus stability was assessed from recorded image sequences by off-line counting of embolizing platelets.
Platelet-leukocyte interactions
Laminar flow assays were performed as described.37 Washed Cd40l−/−Apoe−/− or Cd40l+/+Apoe−/− platelets were immobilized at the lower wall of a flow chamber on the stage of an Olympus IX 50 inverted phase-contrast microscope (Olympus Optical). Adherent platelets were activated with thrombin (1.1nM) at 37°C. Leukocytes were then perfused through the chamber for 10 minutes at 1000 seconds. The number of firmly adherent cells was quantified in multiple microscopic fields by analysis of images recorded with a JVC3 charge-coupled device video camera and recorder.
Platelet-leukocyte aggregate formation was also studied by flow cytometry. Washed platelets were prepared from PRP, resuspended in HEPES buffer, and activated with thrombin (1.1nM) at 37°C. Leukocytes were isolated from the sediments obtained after centrifugation and removal of PRP. After lysis of erythrocytes (Pharmlyse kit, BD Biosciences), leukocytes were washed twice with ice-cold Hanks HEPES buffer, added to the activated platelets, and incubated for 20 minutes at 37°C to generate platelet-leukocyte aggregates. Samples were stained with antibodies against CD11b, CD41, or isotype control antibodies and analyzed by flow cytometry (FACSCanto II, BD Biosciences).
Platelet-endothelium and leukocyte-endothelium interactions
Intravital microscopy was performed to monitor leukocyte-endothelium interactions along the atherosclerotic carotid artery. Apoe−/− mice were anesthetized by an intraperitoneal injection of 0.15 to 0.20 mL of a mixture of ketamine and xylazine. The left carotid artery was exposed as described previously.10 Rhodamine 6G (Invitrogen) was administered intravenously in mice, which had been injected with activated Cd40l+/+Apoe−/− or Cd40l−/−Apoe−/− platelets once every 5 days for 12 weeks. The carotid arteries were exposed, and arrest of labeled leukocytes was analyzed by epifluorescence microscopy (Zeiss Axiotech, 20×0.5 water immersion). Leukocytes were considered adherent when they remained stationary for more than 30 seconds.38
In a separate set of experiments, thrombin-activated, calcein (Invitrogen)-labeled platelets (3 × 107 in 100 μL of Tyrode-HEPES) from Cd40l+/+Apoe−/− or Cd40l−/−Apoe−/− mice were adoptively transferred into 17-week-old Apoe−/− mice. Platelet adhesion to the external carotid artery was recorded. Subsequently, rhodamine 6G was administered and leukocyte adhesion was visualized. The right jugular vein was cannulated with polyethylene tubing (PE10) for intravenous administration of platelets and rhodamine 6G. Intravital microscopy was performed using an Olympus BX51 microscope equipped with a Hamamatsu 9100-02 EM charge-coupled device camera and a 10×/0.3 saline-immersion objective. For image acquisition and analysis Olympus cellR software was used.
Atherosclerosis induction and measurement
Preparation of donor platelets.
Blood from Cd40l+/+Apoe−/− and Cd40l−/−Apoe−/− donor mice was obtained from the retro-orbital plexus and collected in acid-citrate-dextrose-containing tubes. PRP was prepared and platelets were washed extensively. Washed platelets were activated with 0.5nM thrombin for 15 minutes, followed by neutralization with an equimolar dose of hirudin. Activated Cd40l−/−/Apoe−/− or Cd40l+/+Apoe−/− platelets (3 × 107/20 g body weight) were administered every 5 days to Apoe−/− acceptor mice via tail vein injections.23
Plaque initiation.
To study the effects of platelet CD40L on plaque development, injections of activated Cd40l+/+Apoe−/− platelets, Cd40l−/−Apoe−/− platelets, or vehicle started at the age of 5 weeks (n = 11/group), when no signs of atherosclerosis were present in the aortic arch, and continued until 17 weeks of age, when the first fatty streak lesions appeared. The animals were fed a normal chow diet.
Advanced plaque development.
Advanced atherosclerotic plaques were induced by placing slightly constrictive silastic collars around both carotid arteries in 14-week-old Apoe−/− mice,39 which were primed with a 0.21% cholesterol diet for 3 weeks. Injections of activated Cd40l+/+Apoe−/− platelets, activated Cd40l−/−Apoe−/− platelets, or vehicle (every fifth day) started 5 days after collar placement (n = 10/group), and lasted for 6 weeks during which time the mice continued to consume the atherogenic diet.
Established plaques.
The effect of platelet injections on established plaques was determined using 17-week-old Apoe−/− mice, which already displayed atherosclerotic plaques. The mice were injected with activated Cd40l−/−Apoe−/− or Cd40l+/+Apoe−/− platelets or vehicle (n = 8/group), every fifth day for 12 additional weeks. Atherosclerosis was quantified at week 29 and compared with lesion size of 17-week-old Apoe−/− mice, which did not receive platelet injections. For all studies, plasma cholesterol levels were determined in duplicate using a colorimetric assay (CHOD-PAP, Roche Diagnostics).
Tissue processing, histology, and morphometry
At the end of the experimental period, mice were killed after 4 hours of fasting. Blood was obtained from the retro-orbital plexus. The arterial tree was perfused for 5 minutes with phosphate-buffered saline (PBS) containing sodium nitroprusside (Sigma-Aldrich), followed by 1% paraformaldehyde. The aortic arch including its main branch points was removed, fixed overnight in 1% paraformaldehyde, longitudinally embedded in paraffin, and sectioned. Twenty consecutive sections (4 μm) representing the central area of the aortic arch with an intact morphology of the arch and branch points were selected.6 For histologic analysis of atherosclerosis, 4 sections (20 μm apart) were stained with hematoxylin and eosin. Aortic root lesions were analyzed using serial sections of 4 μm with 40-μm intervals, beginning from the onset of the aortic valves until the valves had disappeared. In Apoe−/− mice subjected to collar placement, the right carotid artery, proximal from the collar, was analyzed. To determine plaque volume, plaque area was measured on cross sections, 100 μm apart, thereby covering the entire plaque. Atherosclerotic lesions were analyzed and classified as either initial or advanced lesions, based on histologic criteria defined by Virmani et al.40 The number of atherosclerotic plaques and the presence or absence of lipid cores were determined. Plaque area was calculated using a Leica DM3000 light microscope and a 10/×0.3 NA on 20/×0.5 objective (Leica Microsystems) coupled to a computerized morphometry system (Leica Qwin, Version 3.5.1). Images were captured using a Leica DFC 425c camera.
(Immuno)histochemistry
Aortic sections were immunolabeled with anti-Mac3 monoclonal antibody (mAb; 1:30; BD Biosciences PharMingen) to detect macrophages, anti-CD3 (1:200; Dako) to detect T lymphocytes, anti-CD45 mAb (1:5000; BD Biosciences) to detect leukocytes, anticleaved caspase-3 mAb (1:100; Cell Signaling Technology) to detect apoptosis, anti–α-smooth muscle actin mAb (1:500; Dako) as a marker for vascular smooth muscle cells, and anti-CCL2 (1:200, eBioscience) for the detection and localization of the chemokine CCL2. Antibody staining was visualized by diaminobenzidine or Vector red or blue. For CCL2, double immunohistochemistry was performed using the cell type-specific markers Mac3 (1:30; BD Biosciences) and FVIII (1:250; Dako). For visualization, 3-amino-9-ethylcarbazole and vector blue were used. Perl staining was used to detect iron deposition and Sirius red staining for analysis of collagen content.
Immune phenotyping of Apoe−/− mice
To determine potential effects of platelet injections on the immune system, cells from blood, spleen, and lymph nodes were harvested on death and analyzed for the relative distribution of monocyte, T-cell, and B-cell subsets. Cells were stained with antibodies against CD3, CD4, CD8, CD25, Foxp3, CD11b, Ly6C, Ly6G, CD11c, and B220 (all from BD Biosciences or eBioscience) and analyzed by flow cytometry (FACSCanto II, BD Biosciences). Isotype IgG was used as a control.
Analysis of regulatory T-cell function
CD4+CD25+ and CD4+CD25− T cells were isolated from spleens of activated Cd40l−/−Apoe−/− or Cd40l+/+Apoe−/− platelet-treated (for 5 weeks) mice using the Dynabeads FlowComp Mouse CD4+CD25+ Treg kit (Invitrogen) according to the instructions of the manufacturer. CD4+CD25− T cells from both strains were pooled and labeled using carboxyfluorescein succinimidyl ester (CFSE; Sigma-Aldrich). These responder cells were cocultured with differing dilutions of CD4+CD25+ Tregs from either Cd40l−/−Apoe−/− or Cd40l+/+Apoe−/− platelet-treated mice. Cells were stimulated with agonistic antibodies to CD3 and CD28 (eBioscience), and effector cell proliferation [CFSE] dilution) was assessed by flow cytometry.
For the analysis of Treg cell function in vivo in the presence of activated platelets, Apoe−/− mice (n = 30) were injected intravenously every fifth day for 5 weeks with thrombin-activated Cd40l−/−Apoe−/− or Cd40l+/+Apoe−/− platelets and weekly with an anti-CD25 antibody (200 μg intraperitoneally, clone PC61) or phosphate-buffered saline. After the treatment period, mice were killed and atherosclerosis was analyzed as described in “Tissue processing, histology, and morphometry.”
Analysis of chemokine and cytokine profiles
Concentrations of cytokines IL-6, IL-10, CCL2, interferon-γ, TNF-α and IL-12p70 were measured in plasma and in the supernatant of platelet-stimulated bone marrow-derived macrophages with a Cytometric Bead Array (mouse inflammation kit; BD Biosciences) or by Luminex technology (Mouse Cytokine 20-Plex Panel; Invitrogen). Enzyme-linked immunosorbent assays (ELISAs) were used to quantify CCL5, IL-1β, and sVCAM levels in plasma (BD Biosciences).
Macrophage phagocytosis assay
To investigate whether platelet CD40L affects macrophage phagocytosis, bone marrow-derived macrophages were cultured as described previously.10,41 Subsequently, bone marrow–derived macrophages were cocultured for 20 hours with or without platelets from Cd40l+/+Apoe−/− or Cd40l−/−Apoe−/− mice in 24-well plates (1:20 or 3.5 × 105 macrophages with 7 × 106 platelets per well). The platelets were washed away with phosphate-buffered saline, and the bone marrow–derived macrophages were incubated for 3 hours in Optimem-1 (Invitrogen) with 7 × 106 fluorescently labeled beads (1:20, Invitrogen). After residual beads were washed away, the uptake was assessed by flow cytometry.
Statistical analysis
Results are given as mean plus or minus SEM. Data from Cd40l−/−Apoe−/− mice were compared with Cd40l+/+Apoe−/− mice, Cd40−/−Apoe−/− mice, and vehicle-treated mice by a nonparametric Mann-Whitney U test or a Fisher exact test. A value of P less than .05 was considered statistically significant.
Results
Platelet CD40L promotes thrombus formation
Cd40l+/+Apoe−/− and Cd40l−/−Apoe−/− mice exhibited normal platelet counts and clotting parameters, as well as similar expression of platelet surface/activation markers (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
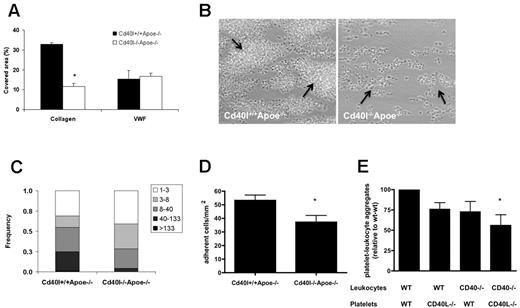
Blood from these mice was used to determine the role of CD40L in flow-dependent thrombus formation on various thrombogenic surfaces. Perfusion of Cd40l+/+Apoe−/− or Cd40l−/−Apoe−/− blood over immobilized VWF (Figure 1A), but not fibrinogen (not shown), at high shear rate resulted in similar platelet adhesion. In marked contrast, blood perfusion over type I collagen provoked the formation of multilayered platelet aggregates, which was markedly impaired when Cd40l−/−Apoe−/− blood was used. Analysis of platelet deposition showed that 32.8% ± 0.9% of the surface was covered with Cd40l+/+Apoe−/− platelets, in contrast to a surface coverage of 11.7% ± 4.3% with Cd40l−/−Apoe−/− platelets (Figure 1A-B). Both the height and size of Cd40l−/−Apoe−/− platelet aggregates were reduced (Figure 1B-C). The effect of CD40L deficiency was not the result of thrombus instability because no shedding of emboli was observed in real-time video images, but it was rather explained by reduced platelet activation (50% lower surface P-selectin). This suggested that CD40L-dependent thrombus formation is most crucial in the more advanced stages of atherosclerosis, when collagen is directly exposed to the blood.
CD40L enhances collagen-dependent thrombus formation in Apoe-deficient mice under flow. (A) Heparin/D-phenylalanyl-L-prolyl-L-arginine chloromethylketone-anticoagulated blood from Cd40l+/+Apoe−/− or Cd40l−/−Apoe−/− mice was passed over VWF or collagen at shear rates of 1700 or 1000 seconds, respectively. (B) Representative phase-contrast images for platelets perfused over collagen are shown after 4 minutes of flow. Arrows indicate platelet aggregates (n = 4-6). *P < .05. (C) Size distribution of multiplatelet thrombi on collagen, established from morphometric image analysis reported as numbers of platelets per agglomerate. *P < .001. (D) Role of CD40L and CD40 in the formation of platelet leukocyte-aggregate mice. Numbers of leukocytes (cells/mm2) on immobilized Cd40l+/+Apoe−/− or Cd40l−/−Apoe−/− platelets (n = 6). *P < .05. (E) After in vitro coincubation, flow cytometric analysis of frequencies of CD41+CD11b+ cell aggregates, demonstrating the contribution of both platelet CD40L and leukocyte CD40 (n = 6). *P < .05 vs WT/WT.
CD40L enhances collagen-dependent thrombus formation in Apoe-deficient mice under flow. (A) Heparin/D-phenylalanyl-L-prolyl-L-arginine chloromethylketone-anticoagulated blood from Cd40l+/+Apoe−/− or Cd40l−/−Apoe−/− mice was passed over VWF or collagen at shear rates of 1700 or 1000 seconds, respectively. (B) Representative phase-contrast images for platelets perfused over collagen are shown after 4 minutes of flow. Arrows indicate platelet aggregates (n = 4-6). *P < .05. (C) Size distribution of multiplatelet thrombi on collagen, established from morphometric image analysis reported as numbers of platelets per agglomerate. *P < .001. (D) Role of CD40L and CD40 in the formation of platelet leukocyte-aggregate mice. Numbers of leukocytes (cells/mm2) on immobilized Cd40l+/+Apoe−/− or Cd40l−/−Apoe−/− platelets (n = 6). *P < .05. (E) After in vitro coincubation, flow cytometric analysis of frequencies of CD41+CD11b+ cell aggregates, demonstrating the contribution of both platelet CD40L and leukocyte CD40 (n = 6). *P < .05 vs WT/WT.
CD40L facilitates platelet-leukocyte interactions
To further investigate the interaction of platelet CD40L with leukocytes, we analyzed the adhesion of Apoe−/− leukocytes to immobilized thrombin-activated Cd40l−/−Apoe−/− or Cd40l+/+Apoe−/− platelets. In contrast to the abundant leukocyte adhesion elicited by Cd40l+/+Apoe−/− platelets, deficiency of platelet CD40L resulted in a 30% decrease of adhered leukocytes (Figure 1D).
Activated platelets in the circulation are known to bind leukocytes and form proinflammatory PLAs.23,42 To investigate PLA formation in vitro, we used platelets and leukocytes isolated from Cd40l−/−Apoe−/−, Cd40−/−Apoe−/−, or Apoe−/− mice. Flow cytometric analysis demonstrated that the formation of CD11b+CD41+ PLAs was impaired when CD40L was absent from platelets or when CD40 was absent from leukocytes. Lowest numbers of PLAs were detected with both platelets lacking CD40L and leukocytes lacking CD40, indicating that the interaction between platelet CD40L and leukocyte CD40 facilitates PLA formation (Figure 1E).
Platelet CD40L mediates leukocyte recruitment via CCL2
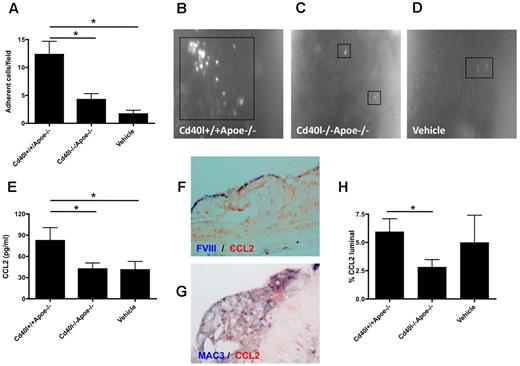
Leukocyte adhesion of to the endothelium, followed by transmigration into the diseased arterial wall, is a pivotal process contributing to the acceleration of atherosclerosis. We studied whether repeated injection of thrombin-activated platelets from Cd40l−/−Apoe−/− or Cd40l+/+Apoe−/− mice (injection every 5 days for 10 weeks) affected leukocyte recruitment in recipient Apoe−/− mice. Intravital microscopy of the left common carotid artery revealed that Cd40l+/+Apoe−/− platelets, but not Cd40l−/−Apoe−/− platelets, caused a substantial adhesion of leukocytes compared with vehicle-treated mice (Figure 2A-D). This experiment demonstrated that long-term injection with activated platelets led to increased leukocyte sequestration to the endothelium, an effect that was fully prevented in the absence of platelet CD40L. Interestingly, this effect on leukocyte adhesion was already detected directly after one injection of thrombin-activated platelets (Figure 3B,D). Using cytometric bead assays, we analyzed cytokine levels in plasma from the animals injected with activated platelets. Injection of activated Cd40l+/+Apoe−/− platelets caused a significant increase in the expression of monocyte CCL2, which was absent when Cd40l−/−Apoe−/− platelets were injected (Figure 2E). Double immunohistochemistry revealed that CCL2 was abundantly present in Mac3+ macrophages and in FVIII+ endothelial cells in plaques of all treatment groups (Figure 2F-G). Interestingly, treatment with activated Cd40l+/+Apoe−/−platelets, but not Cd40/−/−Apoe−/− platelets, increased the endothelial deposition of CCL2 (Figure 2H). Plasma levels of TNF-α, IL-6, IL-10, interferon-γ, or IL-12 were not affected (data not shown).
Platelet CD40L promotes leukocyte adhesion to the atherosclerotic arterial wall. (A-D) Intravital microscopy of adhering, rhodamine-labeled leukocytes in carotid arteries of Apoe−/− mice treated with activated Cd40l+/+Apoe−/−, Cd40l−/−Apoe−/− platelets, or vehicle every 5 days for 10 weeks (n = 4-6). *P < .05. (E) Plasma CCL2 levels after repeated injection of Cd40l−/−Apoe−/− platelets into Apoe−/− mice compared with injection of Cd40l+/+Apoe−/− platelets and vehicle-treated mice (n = 9). *P < .05. (F) Double immunohistochemistry for FVIII (blue) and CCL2 (red) of a CD40l+/+Apoe−/− atherosclerotic lesion. (G) Double immunohistochemistry for Mac3 (blue) and CCL2 (red) of a CD40l+/+Apoe−/− atherosclerotic lesion. (H) Endothelial (luminal) deposition of CCL2, quantified by immunohistochemical staining, is decreased in the Cd40l−/−Apoe−/− treatment group compared with the Cd40l+/+Apoe−/− group (n = 6). *P < .05.
Platelet CD40L promotes leukocyte adhesion to the atherosclerotic arterial wall. (A-D) Intravital microscopy of adhering, rhodamine-labeled leukocytes in carotid arteries of Apoe−/− mice treated with activated Cd40l+/+Apoe−/−, Cd40l−/−Apoe−/− platelets, or vehicle every 5 days for 10 weeks (n = 4-6). *P < .05. (E) Plasma CCL2 levels after repeated injection of Cd40l−/−Apoe−/− platelets into Apoe−/− mice compared with injection of Cd40l+/+Apoe−/− platelets and vehicle-treated mice (n = 9). *P < .05. (F) Double immunohistochemistry for FVIII (blue) and CCL2 (red) of a CD40l+/+Apoe−/− atherosclerotic lesion. (G) Double immunohistochemistry for Mac3 (blue) and CCL2 (red) of a CD40l+/+Apoe−/− atherosclerotic lesion. (H) Endothelial (luminal) deposition of CCL2, quantified by immunohistochemical staining, is decreased in the Cd40l−/−Apoe−/− treatment group compared with the Cd40l+/+Apoe−/− group (n = 6). *P < .05.
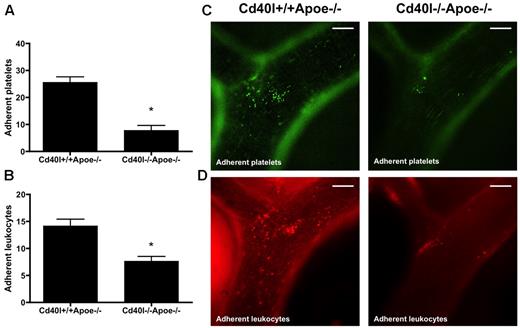
Adhesion of both platelets and leukocytes is impaired after injection of activated Cd40l−/−Apoe−/− platelets. Intravital microscopy of the endothelial wall of carotid arteries in 17-week-old Apoe−/− mice shows an impaired adhesion of intravenously administered calcein-labeled Cd40l−/−Apoe−/− platelets (green) (A). Subsequent rhodamine 6G injection shows a decreased adhesion of leukocytes (red) in Apoe−/− mice injected with Cd40l−/−Apoe−/− platelets compared with mice injected with Cd40l+/+Apoe−/− platelets (B) (n = 6). *P < .05. (C-D) Representative images of the external carotid artery. Scale bars represent 100 μm.
Adhesion of both platelets and leukocytes is impaired after injection of activated Cd40l−/−Apoe−/− platelets. Intravital microscopy of the endothelial wall of carotid arteries in 17-week-old Apoe−/− mice shows an impaired adhesion of intravenously administered calcein-labeled Cd40l−/−Apoe−/− platelets (green) (A). Subsequent rhodamine 6G injection shows a decreased adhesion of leukocytes (red) in Apoe−/− mice injected with Cd40l−/−Apoe−/− platelets compared with mice injected with Cd40l+/+Apoe−/− platelets (B) (n = 6). *P < .05. (C-D) Representative images of the external carotid artery. Scale bars represent 100 μm.
Surprisingly, circulating levels of the platelet chemokine CCL5 did not change (CCL5: Cd40l+/+Apoe−/− platelets 215 ± 18 pg/mL vs Cd40l−/−Apoe−/− platelets 210 ± 22 pg/mL vs vehicle injection 242 ± 26 pg/mL). Notably, immunofluorescent staining for CCL5 revealed its deposition at carotid lesion sites immediately after injection of the activated platelets. However, no differences were observed between the Cd40l+/+Apoe−/− and Cd40l−/−Apoe−/− groups compared with vehicle controls (CCL5 deposition: Cd40l+/+Apoe−/− platelets 34.8% ± 5.3% vs Cd40l−/−Apoe−/− platelets 38.6% ± 3.9% vs vehicle 20.2% ± 5.0% of luminal lining). This indicates that deposition of CCL5 did not require platelet CD40L. Plasma levels of IL-1β, a cytokine excreted by platelets, and of soluble VCAM, were not affected by platelet CD40L deficiency (data not shown).
In vitro, coculture of activated platelets and macrophages did not influence the release of cytokines (data not shown). Furthermore, the phagocytic activity of macrophages, cocultured with activated Cd40l+/+Apoe−/− or Cd40l−/−Apoe−/− platelets, was not affected (supplemental Figure 1).
CD40L facilitates platelet-endothelium interactions
To investigate the contribution of CD40L to platelet adhesion to the vascular endothelium, we injected calcein-labeled, thrombin-activated platelets from Cd40l−/−Apoe−/− or Cd40l+/+Apoe−/− mice into Apoe−/− mice and recorded their adhesion to the carotid artery by intravital microscopy. A significantly reduced number of platelets adhering to the endothelium of the carotid artery was seen in Apoe−/− mice injected with calcein AM-labeled Cd40l−/−Apoe−/− platelets compared with Cd40l+/+Apoe−/− platelets (Figure 3A,C). On subsequent rhodamine injection, we observed a corresponding decrease in leukocyte adhesion (Figure 3B,D), suggesting that CD40L is critical to the platelet function of facilitating leukocyte adhesion to endothelium in inflamed and/or atherosclerotic vessels.
Platelet CD40L accelerates early stages of atherosclerosis
The results so far indicate that platelet CD40L plays a key role in platelet-platelet interactions during thrombus formation, PLA formation, platelet-endothelium interactions, and leukocyte recruitment to the arterial wall. We therefore investigated the contribution of platelet CD40L to atherosclerosis in vivo using an established model of platelet-mediated atherosclerotic plaque formation in Apoe−/− mice, driven by repeated injections of thrombin-activated platelets.23
After these injections, no differences in body weight (vehicle 28.3 ± 0.4 g; Cd40l+/+Apoe−/− 27.0 ± 0.4 g; Cd40l−/−Apoe−/− 28.1 ± 0.6 g) or in cholesterol levels (vehicle 328 ± 28 mg/dL; Cd40l+/+Apoe−/− 336 ± 29 mg/dL; Cd40l−/−Apoe−/− 309 ± 21 mg/dL) were observed. Moreover, no macroscopic or microscopic complications of the injections of activated platelets could be observed in more than 20 organs analyzed, especially no signs of hemorrhage or thrombosis.
Platelet CD40L accelerates plaque development
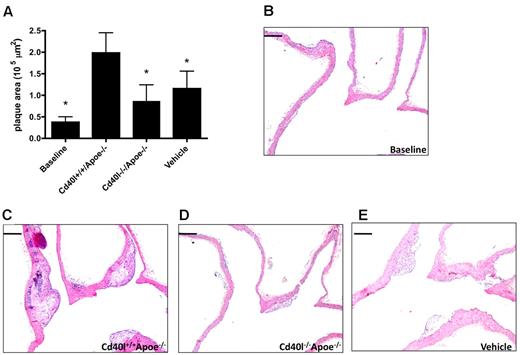
To analyze the effects of platelet CD40L in the initiation of atherosclerosis, Apoe−/− mice were injected with thrombin-activated platelets every 5 days from the age of 5 weeks to the age of 17 weeks. At the time of death, predominantly early atherosclerotic lesions (intimal thickening and intimal xanthoma) were present in the aortic arch and its main branch points. As expected, injection of activated Cd40l+/+Apoe−/− platelets significantly increased atherosclerotic plaque area compared with the vehicle-treated group. Interestingly, injection of activated Cd40l−/−Apoe−/− platelets prevented this increase in atherosclerosis, and average plaque area was comparable with that in vehicle-treated animals (Figure 4A-D). The decrease in atherosclerosis in the aortic arch was the result of a reduced number of plaques (Cd40l+/+Apoe−/− 2.6 ± 0.3 vs Cd40l−/−Apoe−/− 1.7 ± 0.2 plaques), whereas the distribution of initial and advanced plaques was not affected (Cd40l+/+Apoe−/−: 71.2% ± 13.0% initial; 28.8% ± 13.0% advanced; Cd40l−/−Apoe−/−: 92.4% ± 5.2% initial; 7.6% ± 5.2% advanced). Similar results were detected in the aortic root (Figure 4E).
Platelet CD40L promotes atherosclerosis initiation. (A) Plaque area of the aortic arch, including the main branch points (brachiocephalic trunk [BCT], left common carotid artery [LCC], left subclavian artery) of Apoe−/− mice, injected every 5 days for 12 weeks with activated Cd40l+/+Apoe−/− or Cd40l−/−Apoe−/− platelets, or vehicle (n = 11/group). (B-D) Representative longitudinal sections (hematoxylin and eosin staining) of the aortic arch and main branch points. Note the early, foam cell-rich atherosclerotic lesions formed in the brachiocephalic trunk. Scale bar represents 200 μm. (E) Plaque area in the aortic root of the same mice (n = 9/group). *P < .05.
Platelet CD40L promotes atherosclerosis initiation. (A) Plaque area of the aortic arch, including the main branch points (brachiocephalic trunk [BCT], left common carotid artery [LCC], left subclavian artery) of Apoe−/− mice, injected every 5 days for 12 weeks with activated Cd40l+/+Apoe−/− or Cd40l−/−Apoe−/− platelets, or vehicle (n = 11/group). (B-D) Representative longitudinal sections (hematoxylin and eosin staining) of the aortic arch and main branch points. Note the early, foam cell-rich atherosclerotic lesions formed in the brachiocephalic trunk. Scale bar represents 200 μm. (E) Plaque area in the aortic root of the same mice (n = 9/group). *P < .05.
In this early phase of the disease, when lesions are small and rich in foam cell, no difference in plaque composition was observed between the treatment groups (Table 1). However, plaques of animals injected with Cd40l−/−Apoe−/− platelets contained a reduced number of macrophages, reflecting the inhibition of leukocyte recruitment in absence of platelet CD40L (Table 1). These data suggest that CD40L from activated platelets accelerates the initial stages of atherosclerosis.
Plaque phenotypic characteristics of initial atherosclerosis
| . | Cd40l+/+Apoe−/− . | Cd40l−/−Apoe−/− . | Vehicle . |
|---|---|---|---|
| % CD45 | 3.8 ± 0.4 | 3.6 ± 0.6 | 3.7 ± 0.4 |
| % Mac3 | 68.6 ± 4.4 | 79.16 ± 2.9 | 68.9 ± 6.4 |
| MAC-3 Abs | 126.6 ± 17.4 | 103.0 ± 15.8* | 139.3 ± 21.3 |
| % α-SMA | 4.7 ± 2.2 | 5.2 ± 3.1 | 3.1 ± 1.8 |
| % SR | 3.7 ± 2.0 | 2.4 ± 1.3 | 3.9 ± 2.6 |
| . | Cd40l+/+Apoe−/− . | Cd40l−/−Apoe−/− . | Vehicle . |
|---|---|---|---|
| % CD45 | 3.8 ± 0.4 | 3.6 ± 0.6 | 3.7 ± 0.4 |
| % Mac3 | 68.6 ± 4.4 | 79.16 ± 2.9 | 68.9 ± 6.4 |
| MAC-3 Abs | 126.6 ± 17.4 | 103.0 ± 15.8* | 139.3 ± 21.3 |
| % α-SMA | 4.7 ± 2.2 | 5.2 ± 3.1 | 3.1 ± 1.8 |
| % SR | 3.7 ± 2.0 | 2.4 ± 1.3 | 3.9 ± 2.6 |
P < .05 vs Cd40l+/+Apoe−/−.
Platelet CD40L promotes progression toward advanced atherosclerosis
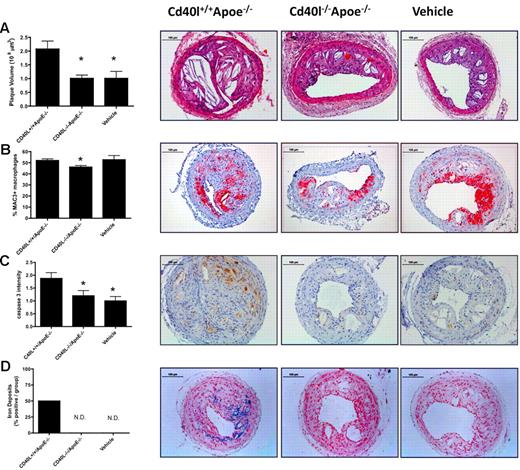
To investigate whether platelet CD40L also has a role in more advanced stages of atherosclerosis, we used a collar-induced atherosclerosis model.39 Six weeks after collar placement, advanced plaques were present in the right carotid artery of all treatment groups. Again, injection of activated Cd40l+/+Apoe−/− platelets significantly increased plaque volume, whereas injection of activated Cd40l−/−Apoe−/− platelets prevented this increase and plaque volumes were comparable with those of the vehicle-treated group (Figure 5A). From these data, we can conclude that platelet CD40L, besides its role in the initiation of atherosclerosis, also contributes to plaque progression to advanced lesions.
Platelet CD40L promotes advanced plaque development. Silastic collars were placed in 14-week-old Apoe−/− mice fed a 0.21% cholesterol diet. Activated Cd40l+/+Apoe−/− or Cd40l−/−Apoe−/− platelets, or vehicle were injected once every 5 days for 6 weeks. (A) Plaque volume was calculated from the plaque areas measured in 6 slices. (B) Macrophage infiltration: representative staining and percentage of Mac3+ cells of all plaque cells. (C) Cleaved caspase-3 was determined by immunohistochemistry and graded from 0 (not present) to 3 (highly present). (D) Iron deposits (percentage of mice containing lesions with iron depositions) were determined by Perl iron staining (n = 9/group); quantification (left) and representative figures (right) are shown. *P < .05 vs Cd40l+/+Apoe−/−. Scale bars represent 100 μm. N.D. indicates not detected.
Platelet CD40L promotes advanced plaque development. Silastic collars were placed in 14-week-old Apoe−/− mice fed a 0.21% cholesterol diet. Activated Cd40l+/+Apoe−/− or Cd40l−/−Apoe−/− platelets, or vehicle were injected once every 5 days for 6 weeks. (A) Plaque volume was calculated from the plaque areas measured in 6 slices. (B) Macrophage infiltration: representative staining and percentage of Mac3+ cells of all plaque cells. (C) Cleaved caspase-3 was determined by immunohistochemistry and graded from 0 (not present) to 3 (highly present). (D) Iron deposits (percentage of mice containing lesions with iron depositions) were determined by Perl iron staining (n = 9/group); quantification (left) and representative figures (right) are shown. *P < .05 vs Cd40l+/+Apoe−/−. Scale bars represent 100 μm. N.D. indicates not detected.
Platelet CD40L induces plaque macrophage infiltration and iron deposition
Extensive phenotypic analysis of plaques in the collar-induced atherosclerosis model revealed that the plaques from Apoe−/− mice treated with activated Cd40l−/−Apoe−/− platelets contained less macrophages compared with treatment with Cd40l+/+Apoe−/− platelets, as indicated by a decrease in Mac3+ cells (Figure 5B). In addition, the number of apoptotic, cleaved caspase-3+ cells was significantly smaller in mice injected with Cd40l−/−Apoe−/− platelets (Figure 5C). The number of CD3+ T cells and CD45+ leukocytes did not differ between the experimental groups (Table 2) Interestingly, no iron depositions and only limited amounts of erythrocyte deposits were found in plaques from Apoe−/− mice treated with activated Cd40l−/−Apoe−/− platelets or vehicle (Figure 5D), whereas these were abundantly present in plaques from mice treated with activated Cd40l+/+Apoe−/− platelets. Collagen content in plaques did not differ between mice injected with platelets but was significantly lower than in vehicle-treated mice (vehicle 52.4% ± 2.1%; Cd40l+/+Apoe−/− 43.9% ± 1.3%; Cd40l−/−Apoe−/− 44.0% ± 2.9%; Table 2). As reported previously,43 injection of activated platelets caused an increase in the amount of intimal smooth muscle cells, which was not observed in the Cd40l−/−Apoe−/− group (Table 2). Collectively, these data indicate that platelet CD40L deficiency prevents plaque macrophage infiltration, subsequent macrophage death, and iron deposition probably resulting from intraplaque hemorrhages, in platelet-mediated atherosclerosis.
Plaque phenotypic characteristics of collar-induced atherosclerosis
| . | Cd40l+/+Apoe−/− . | Cd40l−/−Apoe−/− . | Vehicle . |
|---|---|---|---|
| % CD3 | 5.9 ± 0.7 | 5.1 ± 1.0 | 4.0 ± 0.4 |
| % CD45 | 14.4 ± 2.3 | 15.6 ± 2.3 | 10.0 ± 1.8 |
| % Mac3 | 52.0 ± 1.4 | 46.1 ± 1.4* | 52.7 ± 3.7 |
| MAC-3 Abs | 121.0 ± 15.3 | 126.2 ± 11.7 | 118.6 ± 16.4 |
| Caspase-3+ | 1.9 ± 0.2 | 1.2 ± 0.2* | 1.0 ± 0.17 |
| % α-SMA | 19.7 ± 6.0 | 15.5 ± 3.0* | 12.1 ± 2 |
| % SR | 43.9 ± 1.3 | 44.0 ± 2.9 | 52.4 ± 2.1 |
| % LC | 14.3 ± 3.6 | 16.9 ± 3.2 | 17.4 ± 2.4 |
| . | Cd40l+/+Apoe−/− . | Cd40l−/−Apoe−/− . | Vehicle . |
|---|---|---|---|
| % CD3 | 5.9 ± 0.7 | 5.1 ± 1.0 | 4.0 ± 0.4 |
| % CD45 | 14.4 ± 2.3 | 15.6 ± 2.3 | 10.0 ± 1.8 |
| % Mac3 | 52.0 ± 1.4 | 46.1 ± 1.4* | 52.7 ± 3.7 |
| MAC-3 Abs | 121.0 ± 15.3 | 126.2 ± 11.7 | 118.6 ± 16.4 |
| Caspase-3+ | 1.9 ± 0.2 | 1.2 ± 0.2* | 1.0 ± 0.17 |
| % α-SMA | 19.7 ± 6.0 | 15.5 ± 3.0* | 12.1 ± 2 |
| % SR | 43.9 ± 1.3 | 44.0 ± 2.9 | 52.4 ± 2.1 |
| % LC | 14.3 ± 3.6 | 16.9 ± 3.2 | 17.4 ± 2.4 |
P < .05 vs Cd40l+/+Apoe−/−.
Absence of CD40L on platelets impedes the progression of established atherosclerotic plaques
In a third approach, we examined the potential effect of platelet CD40L on established atherosclerotic plaques. In this group, platelet injections started in Apoe−/− mice that already contained established plaques (baseline, 17 weeks old, Figure 6A), and treatment was continued for 12 more weeks. Lesion size in 29-week-old Apoe−/− animals injected with activated Cd40l+/+Apoe−/−platelets was 5-fold increased compared with baseline, whereas injection of activated Cd40l−/−Apoe−/− platelets significantly reduced lesion progression with plaques similar in size to those in animals of the vehicle-treated group (Figure 6). Plasma cholesterol levels in all 29-week-old Apoe−/− animals increased compared with baseline (baseline: 318.5 ± 53.2 mg/dL; Cd40l+/+Apoe−/−: 523.7 ± 27.6 mg/dL; Cd40l−/−Apoe−/−: 502.0 ± 39.8 mg/dL; vehicle: 469.0 ± 41.0 mg/dL). Although animals injected with Cd40l+/+Apoe−/− platelets displayed a 2-fold increase in lesion area compared with animals injected with Cd40l−/−Apoe−/− platelets, no differences in plaque phenotype could be observed (Table 3).
Platelet CD40L contributes to the progression of established plaques. Seventeen-week-old Apoe−/− mice were injected with activated Cd40l+/+Apoe−/− or Cd40l−/−Apoe−/− platelets, or vehicle once every 5 days for 12 weeks. (A) Plaque area was quantified in the aortic arch and main branch points at 29 weeks and compared with the baseline lesion size of 17-week-old Apoe−/− mice. (B-E) Representative longitudinal sections (hematoxylin and eosin staining; n = 8/group). *P < .05 vs Cd40l+/+Apoe−/−. Scale bars represent 200 μm.
Platelet CD40L contributes to the progression of established plaques. Seventeen-week-old Apoe−/− mice were injected with activated Cd40l+/+Apoe−/− or Cd40l−/−Apoe−/− platelets, or vehicle once every 5 days for 12 weeks. (A) Plaque area was quantified in the aortic arch and main branch points at 29 weeks and compared with the baseline lesion size of 17-week-old Apoe−/− mice. (B-E) Representative longitudinal sections (hematoxylin and eosin staining; n = 8/group). *P < .05 vs Cd40l+/+Apoe−/−. Scale bars represent 200 μm.
Plaque phenotypic characteristics of established atherosclerosis
| . | Baseline . | Cd40l+/+Apoe−/− . | Cd40l−/−Apoe−/− . | Vehicle . |
|---|---|---|---|---|
| % CD3 | 0.2 ± 0.1 | 3.4 ± 0.6 | 3.4 ± 0.4 | 2.0 ± 0.4 |
| % CD45 | 2.9 ± 0.9 | 4.6 ± 0.8 | 5.2 ± 0.5 | 2.6 ± 0.2 |
| % Mac3 | 42.1 ± 6.6 | 44.5 ± 2.8 | 58.8 ± 5.1 | 62.5 ± 5.2 |
| MAC-3 Abs | 81.8 ± 38.6 | 385.6 ± 52.2 | 310.9 ± 63.8 | 363.5 ± 84.2 |
| Caspase-3+ | 0.1 ± 0.1 | 1.3 ± 0.3 | 1.4 ± 0.4 | 0.9 ± 0.3 |
| % α-SMA | 0.0 ± 0.0 | 11.3 ± 1.3 | 11.4 ± 1.0 | 11.0 ± 0.5 |
| % SR | 1.1 ± 0.9 | 34.2 ± 5.9 | 19.0 ± 5.6* | 23.2 ± 5.9 |
| % LC | 0.0 ± 0.0 | 7.9 ± 2.5 | 5.1 ± 1.5 | 3.7 ± 1.6 |
| . | Baseline . | Cd40l+/+Apoe−/− . | Cd40l−/−Apoe−/− . | Vehicle . |
|---|---|---|---|---|
| % CD3 | 0.2 ± 0.1 | 3.4 ± 0.6 | 3.4 ± 0.4 | 2.0 ± 0.4 |
| % CD45 | 2.9 ± 0.9 | 4.6 ± 0.8 | 5.2 ± 0.5 | 2.6 ± 0.2 |
| % Mac3 | 42.1 ± 6.6 | 44.5 ± 2.8 | 58.8 ± 5.1 | 62.5 ± 5.2 |
| MAC-3 Abs | 81.8 ± 38.6 | 385.6 ± 52.2 | 310.9 ± 63.8 | 363.5 ± 84.2 |
| Caspase-3+ | 0.1 ± 0.1 | 1.3 ± 0.3 | 1.4 ± 0.4 | 0.9 ± 0.3 |
| % α-SMA | 0.0 ± 0.0 | 11.3 ± 1.3 | 11.4 ± 1.0 | 11.0 ± 0.5 |
| % SR | 1.1 ± 0.9 | 34.2 ± 5.9 | 19.0 ± 5.6* | 23.2 ± 5.9 |
| % LC | 0.0 ± 0.0 | 7.9 ± 2.5 | 5.1 ± 1.5 | 3.7 ± 1.6 |
P < .05 vs Cd40l+/+Apoe−/−.
Platelet-CD40L disturbs T-cell homeostasis
In our in vivo experiments, blood leukocyte populations were analyzed by flow cytometry 12 to 48 hours after the last injection of activated platelets.
Numbers of CD11b+Ly6C+ cells were significantly decreased in the circulation of animals injected with activated Cd40l+/+Apoe−/− platelets, but not in animals injected with activated Cd40l−/−Apoe−/− platelets or vehicle (supplemental Figure 2). No differences were observed in frequency of B220+ B cells or Ly6G+ granulocytes (data not shown).
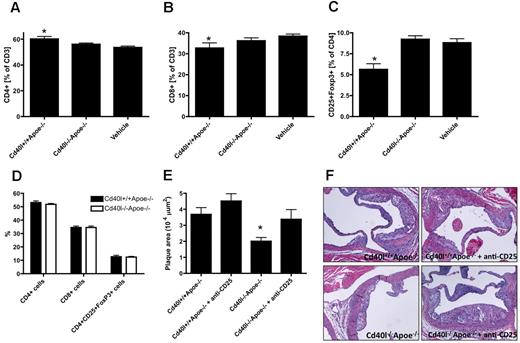
In contrast, profound effects on T-cell homeostasis were noted. Compared with vehicle-treated mice, injection of activated Cd40l+/+Apoe−/− platelets resulted in an increase of CD4+ T cells, whereas the CD8+ T-cell fraction decreased (Figure 7A-B). Remarkably, injection of activated platelets caused a decrease in CD25+Foxp3+ Tregs (Figure 7C). Interestingly, these changes in T-cell homeostasis measured 12 hours after platelet injection were only transient, as neither a difference in CD4+/CD8+ T-cell balance nor a decrease in Treg numbers was observed 48 hours after platelet infusion (Figure 7D). This indicates that injection of activated platelets transiently induces activation of T-effector cells and omits immune regulation by Tregs. These effects of activated platelets on T-cell homeostasis were prevented when animals were injected with Cd40l−/−Apoe−/− platelets, indicating that platelet CD40L is involved in regulation of T-cell homeostasis. Furthermore, these transient effects of activated platelets on T-cell homeostasis were prevented when mice were injected with Cd40l−/−Apoe−/− platelets, indicating that platelet CD40L is required for platelet-mediated changes in the T-cell compartment (Figure 7A-C). Of note, in vitro assays indicated that the suppressive function of Tregs isolated from Cd40l−/−Apoe−/− platelet-treated mice was not altered compared with control mice.
Platelet CD40L transiently disturbs T-cell homeostasis. Flow cytometric analysis of T-cell distribution in blood from adult Apoe−/− mice treated with activated Cd40l+/+Apoe−/− or Cd40l−/−Apoe−/− platelets, or vehicle 12 hours after injection. Percentage of CD4+ T cells (A), CD8+ T cells (B), and CD4+CD25+Foxp3+ regulatory T cells (C). (D) Forty-eight hours after injection of activated platelets, no differences in T-cell phenotype were observed. Anti-CD25 treatment increases atherosclerotic plaque area (E) on injection of Cd40l+/+Apoe−/− platelets and reverses the protective effect of platelet-CD40L deficiency on atherosclerosis. (E) Quantification and (F) representative figures of atherosclerotic plaques in the aortic root of Apoe−/− mice treated with activated Cd40l+/+Apoe−/− or Cd40l−/−Apoe−/− platelets in the absence or presence of an anti-CD25 antibody (n = 30). *P < .05 vs Cd40l+/+Apoe−/−.
Platelet CD40L transiently disturbs T-cell homeostasis. Flow cytometric analysis of T-cell distribution in blood from adult Apoe−/− mice treated with activated Cd40l+/+Apoe−/− or Cd40l−/−Apoe−/− platelets, or vehicle 12 hours after injection. Percentage of CD4+ T cells (A), CD8+ T cells (B), and CD4+CD25+Foxp3+ regulatory T cells (C). (D) Forty-eight hours after injection of activated platelets, no differences in T-cell phenotype were observed. Anti-CD25 treatment increases atherosclerotic plaque area (E) on injection of Cd40l+/+Apoe−/− platelets and reverses the protective effect of platelet-CD40L deficiency on atherosclerosis. (E) Quantification and (F) representative figures of atherosclerotic plaques in the aortic root of Apoe−/− mice treated with activated Cd40l+/+Apoe−/− or Cd40l−/−Apoe−/− platelets in the absence or presence of an anti-CD25 antibody (n = 30). *P < .05 vs Cd40l+/+Apoe−/−.
Although the platelet CD40L-mediated effect on T-cell homeostasis is transient and does not affect the suppressive function of Tregs, the effects on atherosclerosis are profound. Depletion of Tregs by anti-CD25 treatment in Apoe−/− mice completely prevented the protective effects of platelet CD40L deficiency on atherosclerosis (Figure 7E-F), indicating that platelet-CD40L accelerates atherosclerosis, at least in part, by transiently disturbing the T-effector/Treg balance. No differences in plaque phenotype could be observed (Table 4).
Plaque phenotypic characteristics after anti-CD25 treatment
| . | Cd40l+/+ Apoe−/− . | Cd40l+/+Apoe−/− + anti-CD25 . | Cd40l−/− Apoe−/− . | Cd40l+/+Apoe−/− + anti-CD25 . |
|---|---|---|---|---|
| % CD3 | 4.5 ± 0.8 | 4.9 ± 0.4 | 2.7 ± 0.7* | 2.9 ± 0.4 |
| % CD45 | 9.7 ± 1.7 | 8.3 ± 1.7 | 6.8 ± 1.2 | 7.4 ± 1.6 |
| % Mac3 | 56.4 ± 8.2 | 60.6 ± 4.9 | 64.2 ± 4.8 | 58.7 ± 3.2 |
| MAC-3 Abs | 144.2 ± 23.2 | 161.4 ± 19.6 | 151.6 ± 13.6 | 163.7 ± 27.2 |
| Caspase-3+ | 2.0 ± 0.4 | 1.7 ± 0.3 | 2.0 ± 0.2 | 2.0 ± 0.2 |
| % α-SMA | 24.7 ± 5.7 | 14.5 ± 2.2 | 24.6 ± 4.6 | 16.1 ± 2.7 |
| % SR | 64.2 ± 5.7 | 57.1 ± 2.2 | 55.1 ± 2.2 | 66.6 ± 3.6 |
| % LC | 17.4 ± 3.5 | 18.7 ± 2.4 | 12.9 ± 3.1 | 18.1 ± 3.7 |
| . | Cd40l+/+ Apoe−/− . | Cd40l+/+Apoe−/− + anti-CD25 . | Cd40l−/− Apoe−/− . | Cd40l+/+Apoe−/− + anti-CD25 . |
|---|---|---|---|---|
| % CD3 | 4.5 ± 0.8 | 4.9 ± 0.4 | 2.7 ± 0.7* | 2.9 ± 0.4 |
| % CD45 | 9.7 ± 1.7 | 8.3 ± 1.7 | 6.8 ± 1.2 | 7.4 ± 1.6 |
| % Mac3 | 56.4 ± 8.2 | 60.6 ± 4.9 | 64.2 ± 4.8 | 58.7 ± 3.2 |
| MAC-3 Abs | 144.2 ± 23.2 | 161.4 ± 19.6 | 151.6 ± 13.6 | 163.7 ± 27.2 |
| Caspase-3+ | 2.0 ± 0.4 | 1.7 ± 0.3 | 2.0 ± 0.2 | 2.0 ± 0.2 |
| % α-SMA | 24.7 ± 5.7 | 14.5 ± 2.2 | 24.6 ± 4.6 | 16.1 ± 2.7 |
| % SR | 64.2 ± 5.7 | 57.1 ± 2.2 | 55.1 ± 2.2 | 66.6 ± 3.6 |
| % LC | 17.4 ± 3.5 | 18.7 ± 2.4 | 12.9 ± 3.1 | 18.1 ± 3.7 |
P < .05 vs Cd40l+/+Apoe−/−.
Discussion
Our data disclose an important role for platelet CD40L in both the initiation and progression of atherosclerosis in Apoe−/− mice. Although we noted a key role for platelet CD40L in platelet-platelet interactions in thrombus formation on collagen, our results particularly point toward a crucial function in atherogenesis, putatively by mediating inflammatory processes and transiently activating the immune system. Our results add to previous reports that platelets are potent modulators of vascular inflammation. Injection of activated platelets resulted in increased platelet adhesion, increased formation of platelet-leukocyte aggregates, leukocyte recruitment into the intima, and aggravated atherosclerosis. Here we also showed that injection of activated platelets was able to alter the immune system by affecting T-cell subset distribution. Platelet activation caused an increase in effector T cells in blood and spleen, a T-cell subset generally considered to be proatherogenic,44 and a decrease in the number of Tregs, a T-cell subset known to be athero-protective.45 Surprisingly, deficiency of platelet CD40L completely prevented the deleterious proinflammatory effects of activated platelets, both systemically and in the arterial wall. In addition, we provide evidence that the activated platelet-induced decrease in Tregs was associated with CD40L. The protective effect of platelet CD40L deficiency on the immune system and on atherosclerosis could be omitted by administration of the Treg-depleting anti-CD25 antibody.
Deficiency of platelet CD40L especially affects leukocyte recruitment into the intima, thereby preventing the initiation of atherosclerosis. Platelet-induced leukocyte recruitment was reported to depend on several adhesive proteins,14 including P-selectin,23,46 VWF,47 platelet GPIb complex, and integrin αIIbβ3.22 However, in the present study, expression of these adhesive proteins was not altered in CD40L-deficient platelets, suggesting that platelet CD40L mediates leukocyte recruitment via alternative pathways.
Platelets are an essential source of chemokines and express numerous chemokine receptors.48 Several of these (primarily) platelet chemokines, such as CXCL4, CCL5, and CXCL7, are well known to affect leukocyte recruitment and atherosclerosis.48-50 Both CXCL4 and CCL5 are delivered by activated platelets and induce monocyte arrest in the carotid artery, thereby inducing atherosclerosis.50,51 In addition, CXCL4 and CCL5 have been shown to heterodimerize, which enhances monocyte recruitment.49 Disruption of this interaction inhibits atherosclerosis in mice.50 In the present study, we were able to confirm the increased presence of CCL5 on plaques of Apoe−/− mice that were repeatedly injected with activated platelets. However, repeated injections of Cd40l+/+Apoe−/− and Cd40l−/−Apoe−/− platelets both resulted in the accumulation of CCL5 on the plaque, suggesting that this phenomenon is independent of CD40L. Although the paper of Huo et al23 considered CCL5 to be the key mechanism of platelet induced atherosclerosis, we found that there are more (CD40L-dependent) factors, such as endothelial deposition of CCL2, a chemokine crucial in monocyte recruitment throughout atherogenesis,52,53 and systemic disruption of T-cell homeostasis.
Besides leukocyte recruitment, we found that CD40-CD40L interactions are involved in the formation of proinflammatory PLAs. PLAs can form when platelet P-selectin interacts with leukocyte P-selectin glycoprotein ligand.23,27 Until now, only few molecules are known that affect PLA formation and consequently prevent their proinflammatory actions. One of these molecules is growth arrest specific gene 6 (Gas6), which prevents PLA formation by down-regulating P-selectin expression on platelets.54 Interestingly, it has also been reported that administration of sCD40L increased platelet P-selectin expression and, consequently, PLA formation in human and murine platelets.55,56 We noted a diminished P-selectin exposure in CD40L-deficient thrombi on collagen, which may suggest a P-selection-dependent role of CD40-CD40L interactions in the formation of PLAs.
Compared with complete deficiency in CD40L (Cd40l−/−Apoe−/− mice),6 the impact of deficiency of platelet CD40L alone on atherosclerosis is slightly less marked. However, deficiency of platelet CD40L still attenuates atherosclerosis progression, induces a plaque phenotype that is low in inflammation, and contains no iron deposition, indicative of absence of intraplaque hemorrhage, but fails to induce true fibrotic plaques. This may be because activated platelets impede the synthesis of collagen in the plaque and induce the proliferation and migration of smooth muscle cells,43 effects that are apparently not regulated by CD40L. However, deficiency of platelet CD40L does not result in immune suppression and does not cause hemorrhage or bleeding in our study, thereby suggesting a potential use of platelet CD40L inhibition as target in the treatment of human atherosclerosis.
The extrapolation of the animal model we used to characterize platelet CD40L to human vascular disease should be applied with caution. Although patients with cardiovascular disease have an increased number of activated platelets in their circulation,57 repeated injections of thrombin-activated platelets are not physiologic. However, until platelet-specific CD40L-deficient mice are available, the currently used model is one of the best to study the role of CD40L in platelet biology in vivo in a chronic inflammatory disease, such as atherosclerosis.
In conclusion, platelet CD40L is a potent inducer of proatherogenic inflammatory processes by enhancing the interaction between platelets, leukocytes, and the endothelium. In addition, platelet CD40L disrupts T-cell homeostasis, thereby further promoting atherogenesis.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Humboldt Foundation (Sofja Kovalevskaja grant, E.L.), The Netherlands Organization for Scientific Research (VIDI grant, E.L.), The Netherlands Heart Foundation (D.L. and Dr E. Dekker postdoc and established investigator grant, E.L.), the German Research Foundation DFG (FOR809, WE1913/7-2, and 10-1, C.W.; and ZE827/1-1, A.Z.), and the Interdisciplinary Center for Clinical Research BIOMAT within the Faculty of Medicine at the Rheinisch-Westfälische Technische Hochschule Aachen University (VV-B113, C.W., A.Z.).
National Institutes of Health
Authorship
Contribution: D.L. designed and performed research, analyzed data, and wrote the paper; A.Z. and T.S. performed research and analyzed data; O.S. designed experiments, performed research, and analyzed data; L.B., I.C.A.M., E.W., P.G., R.v.K., and L.T. performed research; L.B., R.A.F., and R.J.N. contributed vital new reagents or analytical tools; N.G. designed experiments, performed research, and analyzed data; E.A.B., M.J.A.P.D., J.W.M.H., and C.W. designed research; and E.L. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Esther Lutgens, Department of Pathology, Cardiovascular Research Institute Maastricht, University of Maastricht, P. Debeyelaan 25, 6229 HX, Maastricht, The Netherlands; e-mail: E.Lutgens@path.unimaas.nl or elutgens@ukaachen.de.
References
Author notes
D.L. and A.Z. contributed equally to this study.

![Figure 4. Platelet CD40L promotes atherosclerosis initiation. (A) Plaque area of the aortic arch, including the main branch points (brachiocephalic trunk [BCT], left common carotid artery [LCC], left subclavian artery) of Apoe−/− mice, injected every 5 days for 12 weeks with activated Cd40l+/+Apoe−/− or Cd40l−/−Apoe−/− platelets, or vehicle (n = 11/group). (B-D) Representative longitudinal sections (hematoxylin and eosin staining) of the aortic arch and main branch points. Note the early, foam cell-rich atherosclerotic lesions formed in the brachiocephalic trunk. Scale bar represents 200 μm. (E) Plaque area in the aortic root of the same mice (n = 9/group). *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/20/10.1182_blood-2010-01-261206/7/m_zh89991060620004.jpeg?Expires=1770086939&Signature=liRWLMbkdn0fJnfeFBNWSKk3gca3IiCMw5LVyJkgI7DzZ8L1RnFisYldfphxd3FCNXqJHPUZSBc3PlxHbrV9PdmOmwtl0wIzMxYlOohO~MmCPIyBaB-CKeBeLMhzEA4Q1ZTv~FSDlxCsdjKLAO4jUMVZ1qfRnnubvWL6JDyNHm8nF3M3ezIzOT~85PUFkJSWLgBsPBTBnWH9Q9v0xjHbL0O9wuGyeGq043Hxo24wZgE7bG2SLuFEWxW5CeX9G5kFHK4vddXfF7ZUlMbFIPdTLj5N56vQ51xuKlIpbfKLxszxu7yFftRqlqE8djRiL30s0-s8QjZ6u3f8y-MgmBkh-A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal