Abstract
Abstract 1456
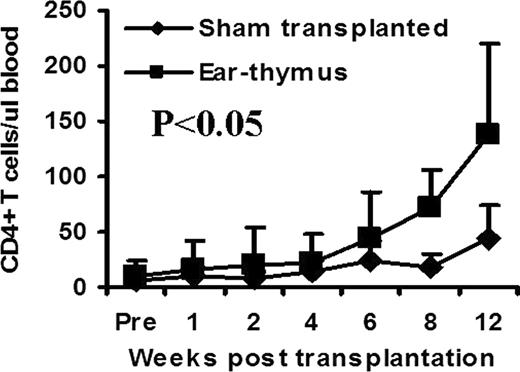
The use of microscopy in medicine has revolutionized medical research, diagnosis, and treatment. Unfortunately, current use of microscopy is mostly limited to 2 dimensional structures. The introduction of next generation microscopy, such as confocal and multiphoton, has enabled study of 3 dimensional structures deep in living tissues. Because there is a limit as to how deep these microscopic techniques can detect signal in tissue, many tissues/organs can not be studied due to inaccessibility (e.g., thymus). We have been using an ear-heart murine model to study immune tolerance. In this model, a heart from a newborn mouse is transplanted subcutaneously into the ear pinna. If the heart is not rejected (e.g., syngeneic setting), it can survive and beat indefinitely. In this study, we tested a hypothesis that other tissues can also be transplanted into the mouse ear pinna and function. Skin on the mouse ear pinna is extremely thin (<15 μm), thus allowing for visualization of cellular and subcellular changes in transplanted tissues in 4 dimensions (3D plus time) in real time using existing technologies such as multiphoton microscopy. We transplanted a variety of C57BL/6 adult (lung, trachea, aorta, kidney, bone marrow, thymus, spleen, lymph node, skeletal muscle, thyroid gland, adrenal gland) and fetal (colon, ileum, stomach, heart, lung, kidney, bone marrow, thymus, spleen, skeletal muscle) tissues subcutaneously into syngeneic mouse ear pinna. All of these tissues were able to survive at least 4–8 weeks after transplantation. Many of these tissues maintained normal or close to normal structures for at least 4–8 weeks. We chose an ear-thymus model to test whether the engrafted tissues can function. Thymic tissue from C57BL/6 newborn mice (<48 hrs) was transplanted into BALB/c nude mice (lacking thymic tissue). The numbers of CD4+ and CD8+ T cells were followed by flow cytometric analysis in peripheral blood over time. CD4+ T cell counts were significantly higher in thymic tissue recipients compared with sham transplanted control group (Figure, P<0.05). As an internal control, B220+ B cells, which are normal in nude mice and are not produced in thymus, remained similar between transplanted and sham transplanted groups at all time points. To test whether this model is useful for high-resolution imaging in live animals, we transplanted GFP+ C57BL/6 T cell depleted bone marrow into thymic tissue recipients. After bone marrow transplantation, almost all hematopoietic cells were replaced by GFP+ cells. Using two photon microscopy technology, we were able to obtain 4 dimension images of the transplanted thymic tissue at the cellular level in living animals. Because surgical exposure is not required, we were able to perform imaging of living tissues repeatedly in these animals indefinitely. We conclude that multiple tissues are able to survive and function for a long period of time when transplanted into ear pinna. Our innovative ear-tissue transplant model has the potential to allow many living tissues to be visualized at the cellular and subcelluar level in real time and in live animals.
Chao:Genzyme: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal