Abstract
Abstract 2127
The addition of tyrosine kinase inhibitors imatinib and dasatinib to combination chemotherapy regimens has improved the outcome of patients (pts) with Philadelphia-chromosme-positive (Ph+) acute lymphoblastic leukemia (ALL). We examined whether the dynamics of minimal residual disease (MRD) measured by multiparameter flow cytometry (MFC) or reverse transcription quantitative polymerase chain reaction (RQ-PCR) was different among pts treated with the combination of hyperCVAD with imatinib or dasatinib.
From April 2001 to September 2006, 54 pts with newly diagnosed Ph+ ALL were treated with the combination of hyperCVAD and imatinib; from October 2006 to July 2009, 42 pts were treated with the hyperCVAD and dasatinib regimen. The median ages for the two groups were 50 and 51 years [ranges, (27 - 84) and (21 - 78)]. Fifty one (94%) and 40 (95%) achieved complete remission (CR) on the two regimen and were followed by serial bone marrow assessments for MRD. MFC was performed using 4 or 6 color combinations of antibodies to lymphoblast and myeloid antigens (e.g., CD10, CD13, CD15, CD19, CD20, CD22, CD25, CD33, CD34, CD38, CD58, CD66c, and CD81), with a sensitivity of 0.01%. RQ-PCR for BCR-ABL was performed using TaqMan primer/probes for the e1a2, e13a2 (b2a2), and e14a2 (b3a2) BCR-ABL transcripts in a single tube with normalization to total ABL transcripts.
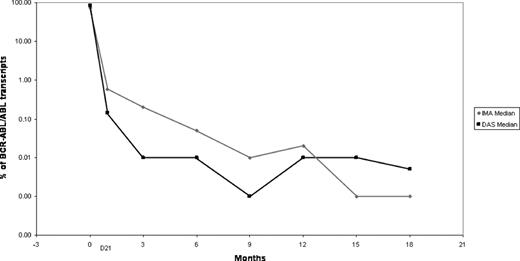
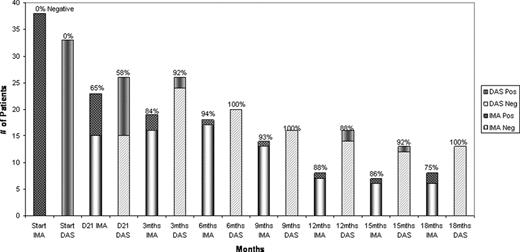
The dynamics of the MRD by RQ-PCR and MFC over the initial 18 month period after achieving CR are shown in the attached figures 1 and 2, respectively. The decline in the median value of BCR-ABL/ABL was more rapid with dasatinib, with more patients reaching the level of <0.01 at 3 and 6 months (p <0.05 and p=0.05, respectively). Similarly, a higher proportion of patients receiving dasatinib were negative by MFC at 3 and 6 months after therapy; 82% and 92% were negative at 3 months, and 94% and 100% negative at 6 months for imatinib and dasatinib, respectively.
Dasatinib containing regimen produces a deeper and more rapid decline in the BCR-ABL and MRD by MFC in the first 6 months of treatment. Whether this would translate to an improved long-term disease-free and overall survival remains to be investigated.
Ravandi: Bristol Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria, Speakers Bureau. Off Label Use: frontline use of dasatinib in acute lymphoblastic leukemia. Thomas: Novartis: Honoraria; Bristol-Meyer-Squibb: Honoraria; Pfizer:; Amgen: Research Funding. Cortes: Bristol Myers Squibb: Research Funding; Novartis: Research Funding. Kantarjian: Novartis Pharmaceuticals: Research Funding; Bristol Myers Squibb: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal