Abstract
Abstract 2280
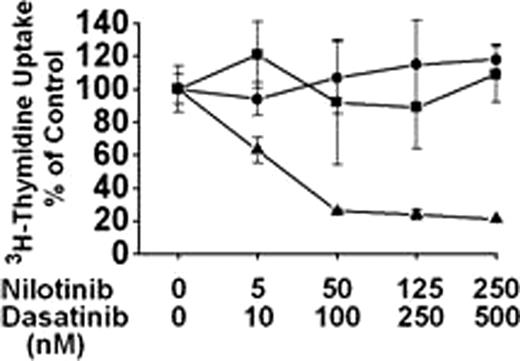
In most patients with chronic myeloid leukemia (CML), complete cytogenetic remission can be achieved with the BCR/ABL tyrosine kinase inhibitor (TKI) imatinib. However, not all patients are long-term responders. A major cause of acquired resistance against imatinib is the development of BCR/ABL mutations in subclones. In most of these patients, a second generation TKI is prescribed. However, the T315I mutant of BCR/ABL introduces resistance against most TKI, including nilotinib and dasatinib. One approach to overcome drug resistance in BCR/ABL T315I+ CML cells may be to apply drug combinations. Recent data suggest that the mechanisms through which dasatinib and nilotinib act on BCR/ABL differ from each other and that both drugs act on multiple additional targets in CML cells. Here, we show that dasatinib and nilotinib cooperate with each other in producing growth inhibition in imatinib-sensitive and imatinib-resistant CML cells, including subclones bearing BCR/ABL T315I. The drug combination was tested on leukemic cells obtained from 9 patients with chronic phase (CP) CML and 3 with blast phase (PB) of CML. Samples were assessed from 4 patients at the time of diagnosis, and against cells from 8 patients (CP, n=5; BP, n=3) who had developed resistance against one or more BCR/ABL TKI. In all 3 patients in PB, the T315I mutant was detectable. As expected, nilotinib and dasatinib failed to inhibit proliferation of cells harbouring BCR/ABL T315I when applied as single agents. However, the combination xnilotinib+dasatinibx produced synergistic effects in most samples, including primary CML cells and Ba/F3 cells harbouring BCR/ABL T315I. Interestingly, in all 3 patients with BP (BCR/ABL T315I+), strong cooperative or even synergistic growth-inhibitory effects were observed in primary CML cells, resulting in substantial anti-leukemic effects seen at reasonable (pharmacologic) drug concentrations (< 1 μ M) (figure). Based on these results, we treated one patient with TKI-resistant CML in hematologic relapse in whom 2 BCR/ABL mutant-bearing subclones, one clinically resistant against nilotinib (F359V) and one clinically resistant against dasatinib (F317L) had been detected, with a combination of nilotinb (800 mg p.o. daily) and dasatinib (50 mg/day p.o., days 1–5 every third week). A transient hematologic response was obtained in this patient, and except for mild bone pain, no side effects were recorded. Moreover, we were able to show that during treatment with xnilotinib+dasatinibx, the number of CD34+/CD38-/CD33+ CML stem cells decreased from clearly measurable levels (0.005%) to nearly undetectable levels (0.0002%). Finally, ex vivo analyses of leukemic blood cells confirmed, that the combination xnilotinib+dasatinibx produced strong cooperative growth-inhibitory effects in both disease-components, i.e. the F359V-bearing subclone and the F317L-bearing subclone. In summary, our data show that the combination of dasatinib and nilotinib can override acquired TKI resistance in CML, and can suppress growth of various imatinib-resistant subclones including cells that bear BCR/ABL T315I or other BCR/ABL mutants. Whether this combination can suppress imatinib-resistant subclones in CML for prolonged time periods or even can eradicate neoplastic stem cells remains in CML patients to be determined.
Synergistic effects of nilotinib and dasatinib on primary leukemic cells obtained from a patient with a BCR/ABL T315I+ blast phase of CML
Valent: Novartis: Research Funding; Bristol-Myers Squibb: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal