Abstract 236
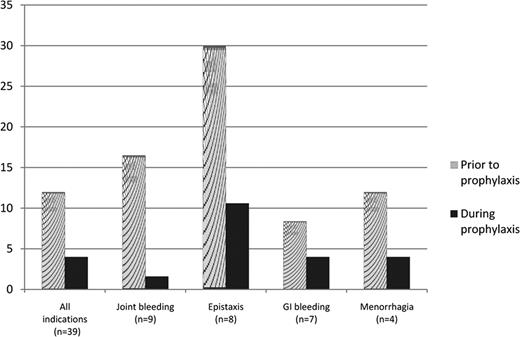
The bleeding patterns of severe von Willebrand Disease (VWD), mainly type 3, adversely affect short- and long-term quality of life, and may be life threatening. The index case of VWD, described by Erik von Willebrand in 1926, was a girl who had a history of serious bleeds from mucous membranes and ankles, and died during her fourth menstrual period. There is no doubt that there is a role for long-term prophylaxis with VWF-containing concentrates, but the experience is scarce and restricted to a retrospective follow-up of 37 patients in Sweden, and a few other small cohorts in Europe. All studies have shown favorable results; however, unresolved issues remain, such as to whom prophylaxis should be given, optimal dose and dose interval, and when to start. The von Willebrand Disease Prophylaxis Network (VWD PN) was formed to investigate the role of prophylaxis in clinically severe VWD that is non-responsive to other treatment(s). The VWD International Prophylaxis (VIP) Study, a combination of prospective and retrospective studies, is an initiative of the VWD PN. Using a retrospective study design, the effect of prophylaxis on bleeding frequency was studied among 39 individuals enrolled from 11 centers in Europe and North America. Data were collected from center records, diaries, and bleeding logs. The period of study was one year prior to start of prophylaxis plus at least 6 months following onset of treatment. Subject demographics, VWD type, and primary bleeding indication for prophylaxis were collected. Annualized bleeding rates were calculated for the period prior to onset of prophylaxis, during prophylaxis for the total group, and by primary bleeding indication defined as the most frequent bleeding symptom. In 4 cases, data were not available to identify the primary indication. Differences (after – before) were calculated. The Wilcoxan Signed Rank test of the differences in the medians was used. The median age (range) at onset of prophylaxis was 29 years (2 to 76). Fifty-one percent were female and 49% male. The vast majority were of European descent, 84.6%, with 12.8% and 2.6% reported as Hispanic and of African descent, respectively. Type 3 VWD accounted for the largest number: 24 (61.5%). Two subjects (5.1%) were type 1, 7 (18.0%) type 2A, 5 (12.8%) type 2B, and 1 (2.6%) type 2M. The usual number of infusions of VWF during prophylaxis was 2 to 3 times per week, with a median usual dose of 45 U VWF:RCo per kg per infusion. The median (range) number of bleeding episodes per year prior to the onset of prophylaxis was 12 (2 – 54), compared to a median (range) of 4 (0 – 24) during prophylaxis, p<0.0001. Changes in bleeding for the total group, as well as the most common primary indications for prophylaxis, are shown in the figure. In 7 cases (not included in the figure) the bleeding pattern was mixed with no primary indication (N=3), or the primary indications occurred with low frequency (N=4). Annualized bleeding rates were lower during prophylaxis for all primary indications, and significantly so (p<0.05) for joint bleeding, epistaxis and GI bleeding. When we examined the effect of prophylaxis by age for subjects <18, and those ≥18, we found that it was similar in both groups. The median number of bleeds per year during prophylaxis was significantly lower, p=0.001 and p<0.0001 respectively, compared to the number prior to prophylaxis. While the primary indications of epistaxis and joint bleeding occurred with similar frequency in both age groups, GI bleeding and menorrhagia were not reported as the primary bleeding indication for prophylaxis for anyone <18. We conclude from this international, multi-center cohort that prophylactic treatment of VWD is efficacious. A network-initiated prospective study is underway, the objectives of which are to provide guidelines for dosing, and address issues of cost-effectiveness and quality of life.
Disclosures:
Berntorp:CSL Behring: Honoraria, Research Funding. Abshire:CSL Behring: Honoraria, Research Funding. Federici:CSL Behring: Honoraria, Research Funding.
*
Asterisk with author names denotes non-ASH members.
© 2010 by The American Society of Hematology
2010

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal