Abstract
Abstract 2361
Reduced intensity allogeneic transplants represent a potentially curative therapy in older patients with haematological malignancies. However the upper age limit for transplantation using a sibling or unrelated donor is unclear and few studies have addressed this important issue. In the UK in vivo T cell depletion utilising alemtuzumab is commonly used as a strategy to reduce the risk of acute and chronic graft-versus-host disease (GVHD) but this manoeuvre impairs immune reconstitution and may pose a particular problem in older patients.
We analysed the outcome of patients over the age of 60 after an alemtuzumab based reduced intensity allograft from five UK Transplant Centres with the aim of identifying factors determining long term outcome and also factors affecting the duration of inpatient admission during the first 100 days post transplant. A modified Charlson's comorbidity index score (Sorror modified HCT comorbidity index) was applied to analyse the transplant outcomes of these patients according to the presence of transplant co-morbidities.
We have studied the outcome of 161 patients (89 male, 72 female) after an alemtuzumab conditioned reduced allograft allograft for a haematological malignancy. The median age of the patients was 62 years(range 60–72). 115 patients had a transplant for myeloid malignancies (87 acute myeloid leukaemia, 21 myelodysplastic syndrome, 2 chronic myeloid leukaemia, 5 myelofibrosis) and 46 for lymphoid malignancies (18 Non_Hodgkin's lymphoma, 14 chronic lymphocytic leukaemia, 4 T-prolymphocytic leukaemia, 7 multiple myeloma and 3 acute lymphoblastic leukaemia). 93 patients received an unrelated donor transplant and 68 had a sibling transplant. The great majority of patients were transplanted using a Fludarabine/Melphalan conditioning regimen(n=118) whilst 13 received a combination of fludarabine and busulphan, 21 BEAM (BCNU, cytarabine, etoposide, melphalan) and 9 a combination of BEAM and fludarabine. The median follow up was 19.1 months (range 2–94 months).
The one year overall survival for the whole group was 52% and the predicted two year OS 46%. The transplant related mortality (TRM) was 19% in the first 100 days post transplant and 23% in the first year post transplant. 40 patients (25%) relapsed. 25 patients (21%) developed Grade III-IV acute GvHD and 16 (10%) patients developed chronic extensive GvHD. There was a weakly negative correlation between Age and Bed Days(p:0.05843) but the correlation between high comorbidity index (3 or greater than 3) and increased bed days is significant(P:0.0013).
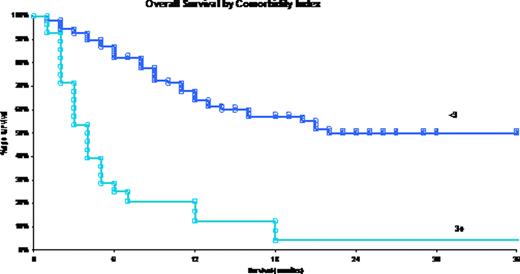
Transplant outcomes were affected by Sorror modified comorbidity index for HCT.A score of 3 and above was significantly associated with decreased overall survival (P:0.001) and interestingly with decreased disease free survival (P:0.02).
CMV status and stem cell dose did not have any impact on overall survival and disease free survival and CR1 at transplantation was showed a trend towards increased overall survival(P:0.05).
Reduced intensity alemtuzumab based stem cell transplant can be delivered safely in patients above the age of 60. It appears that a Sorror comorbidity score of 3 and above has a negative impact on overall survival and disease free survival of these patients and significantly increases inpatient days.These observations require confirmation in a larger cohort of patients but suggest that the selection of patients above the age of 60 for a reduced intensity transplants will be facilitated by incorporation of the Sorror HCI comorbidity index into a transplant algorithm.
Correlations between patient age/bed days and between comorbidity index/bed days until D+100
| . | Correlation with Bed days . | Test . |
|---|---|---|
| Bed Days | Median 56 | |
| Range 8-100 | ||
| IQR 32-80 | ||
| Age | R=-0.05843 | Spearman's Rank Correlation Coefficient |
| Comorbidity Index | P=0.0013 | Mann Whitney non-parametric test |
| . | Correlation with Bed days . | Test . |
|---|---|---|
| Bed Days | Median 56 | |
| Range 8-100 | ||
| IQR 32-80 | ||
| Age | R=-0.05843 | Spearman's Rank Correlation Coefficient |
| Comorbidity Index | P=0.0013 | Mann Whitney non-parametric test |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal