Abstract
Abstract 2734
CXCR4 is a transmembrane G-protein-coupled receptor expressed by hematopoietic stem cells (HSC) and leukemia cells. CXCR4's ligand is SDF-1 (CXCL12), a chemokine constitutively secreted in the bone marrow stromal microenvironment. CXCR4+ HSCs and leukemia cells migrate to marrow niches in response to SDF-1. High level CXCR4 expression may be a prognostic indicator in acute leukemias. We hypothesized that surface CXCR4 expression (s-CXCR4) by leukemias may change dynamically in response to chemotherapy (chemo), and that chemo-mediated upregulation of s-CXCR4 may represent a mechanism of acquired chemoresistance due to enhanced protective SDF-1/CXCR4 signaling.
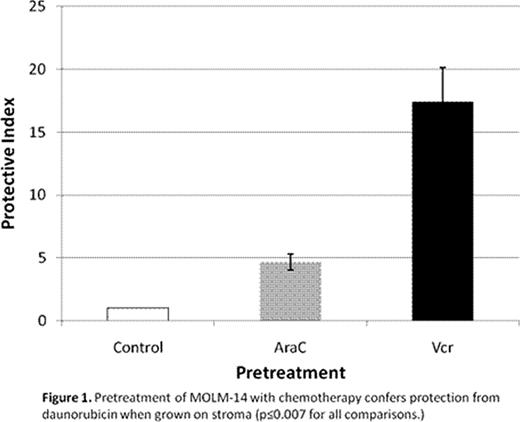
ALL (697, HB1119, NALM-6, SEMK2) and AML (MOLM-14, MV4-11) cell lines were treated for 48 hrs with dose ranges of 6 chemo agents (dauno, araC, etop, vcr, dex, and mtx). Levels of s-CXCR4 (by FACS) varied at baseline, with NALM-6 [mean fluorescence index (MFI) 4444] and MOLM-14 (MFI 108) having the highest and lowest values. These differences were functional, as NALM-6 demonstrated striking SDF-1-induced chemotaxis, while MOLM-14 did not. Chemo exposure resulted in s-CXCR4 upregulation in 697, MOLM-14, and MV4-11 (“upregulators”), and downregulation in HB1119, NALM-6, and SEMK2 (“downregulators”). For example, MOLM-14 had an average 7-fold increase in MFI from baseline, while HB1119 had a 4-fold decrease. Each cell line responded to all chemo agents in a consistent manner (i.e., either up- or downregulation). To measure stromal protection from chemo-induced apoptosis, cells were treated for 48 hrs in the presence or absence of normal human bone marrow stroma feeder layers and analyzed by FACS, after staining with Annexin V and 7-AAD. IC10 through IC90 values were calculated using Calcusyn. Protective index (PI) was defined as the IC value on stroma divided by the IC value off stroma: therefore, PI >1 indicated stromal protection, PI <=1 indicated no stromal protection. In general, upregulators were protected from chemo-induced apoptosis when plated on stroma, while downregulators showed no or minimal protection (average PI for all chemo agents 2.2 vs. 1.3, p=0.003). To determine the kinetics and mechanism of s-CXCR4 modulation, cells were treated with chemo (IC50 doses) and harvested at 10 time points over 96 hrs. The changes in s-CXCR4 were time-, drug-, and cell line-dependent, with MOLM-14 being the most consistent upregulator of s-CXCR4. Simultaneous qRT-PCR measurement of CXCR4 and NRF1 (positively-regulating transcription factor for CXCR4) suggested specific molecular mechanisms of s-CXCR4 modulation. For example, dauno-induced upregulation of s-CXCR4 in MOLM-14 started at 3 hrs, but NRF1-driven CXCR4 transcription did not increase until 48 hrs, suggesting that changes in intracellular CXCR4 localization were responsible for early increases in s-CXCR4. AraC-induced upregulation of s-CXCR4 in NALM-6, on the other hand, correlated temporally with increases in NRF1-driven CXCR4 transcription. Vcr produced increases in NRF1-driven CXCR4 transcription in SEMK2 and simultaneous decreases in s-CXCR4, suggesting CXCR4 degradation prior to cell surface localization. Finally, to test the functionality of chemo-induced changes in s-CXCR4, MOLM-14 cells were split into 3 aliquots and pretreated for 72 hours with chemo (araC and vcr at IC50 doses) or vehicle control. Viable cells were then isolated using Ficoll. The chemo-pretreated cells showed s-CXCR4 upregulation and enhanced SDF-1-mediated chemotaxis compared to control-treated cells. Cells from each aliquot were plated on and off stroma and treated for an additional 72 hours with a full dose range of dauno. AraC and vcr (but not control) pretreatment resulted in striking stromal protection from dauno-induced apoptosis (fig 1).
Surface CXCR4 expression is dynamically altered by chemotherapy exposure in ALL and AML cells, and may be driven by transcription, changes in localization of CXCR4, CXCR4 degradation, or a combination of mechanisms. Chemotherapy-induced upregulation of surface CXCR4 results in increased SDF-1-mediated chemotaxis and a stromal-mediated survival advantage. Upregulation of surface CXCR4 may therefore represent a mechanism of chemoresistance in acute leukemias that is potentially reversible with CXCR4-targeted therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal