Abstract
Abstract 2788
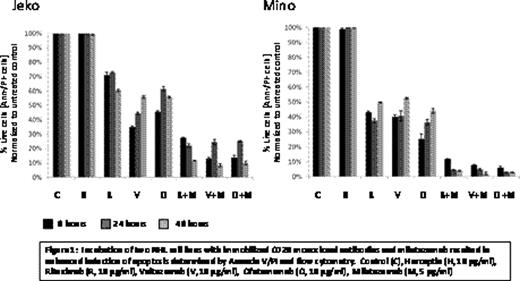
Preclinical studies conducted at our institution (Alinari et al. Blood Abstract 1694, 2009) demonstrated superior efficacy of milatuzumab (Immunomedics, Inc.), a humanized anti-CD74 antibody, in combination with rituximab in mantle cell lymphoma (MCL) cell lines, MCL patient (pt) primary tumor cells, and an in vivo preclinical model of human MCL, compared to either agent alone. Veltuzumab (Immunomedics, Inc.), a novel humanized anti-CD20 antibody, has been reported to have several advantages over rituximab including slower off-rates, shorter infusion times, higher potency, and improved therapeutic responses in animal models. Phase II clinical testing of veltuzumab demonstrated single agent activity in pts with relapsed and refractory non-Hodgkin's lymphoma (NHL). In vitro, veltuzumab combined with milatuzumab also resulted in enhanced apoptosis compared to either agent alone, similar to that observed with rituximab and milatuzumab (Fig 1). As a result of the anti-tumor activity observed in vitro with combined veltuzumab and milatuzumab, we initiated a phase I/II trial in pts with relapsed or refractory B-cell NHL after at least 1 prior therapy to determine the safety, tolerability, and overall response rate with this combination. Patients received veltuzumab 200 at mg/m2 weekly combined with escalating doses of milatuzumab at 8, 16, and 20 mg/kg twice per wk of wks 1–4, 12, 20, 28, and 36. All pts received pre-medication with acetominophen, diphenhydramine, and famotidine prior to each veltuzumab dose and acetominophen, diphenhydramine, and hydrocortisone 50 mg before and after each milatuzumab dose. Dose limiting toxicity was defined during weeks 1–4. Six pts with grade 2 (n=2) or 3 (n=4) follicular NHL, have completed at least 4 weeks of combined veltuzumab and milatuzumab. Median age was 63.5 years (range 44–81), and pts received a median of 3.5 prior therapies (range 3 – 5), including 2 pts with prior autologous stem cell transplant. Three of 6 patients were refractory to rituximab, defined as having less than a partial response to the last rituximab-containing regimen. Dose escalation has reached 16 mg/kg milatuzumab, and no dose limiting toxicities have been observed to date. However, 3 of 6 pts experienced grade 3 infusion reactions during or immediately following the milatuzumab infusion; 1 pt treated with 8 mg/kg milatuzumab during weeks 1 and 12, and 2 pts receiving 16 mg/kg during weeks 3 and 12. Grade 3 infusion reactions consisted of fever, rigors, nausea, vomiting, diarrhea, and in 1 case a diffuse macular rash. Grade 3–4 hematologic toxicity occurred in only 2 pts consisting of grade 4 lymphopenia, and no infections have been observed. The most frequently observed grade 1–2 toxicities were fatigue, transient hyperglycemia, dyspnea, hypoalbuminemia, and thrombocytopenia. Human anti-veltuzumab and anti-milatuzumab antibodies, collected in all 6 pts pretreatment and day 1 of weeks 4 and 12, have not been detected in any pt. Plasma cytokine levels of IL-10, IL-12, TNF-α and INF-γ were checked pre- and post- veltuzumab infusion on day 1 and pre- and post-milatuzumab infusion on day 2. While elevations in cytokine levels were observed, there was no correlation with infusion reactions or response. In the first cohort, one pt achieved a PR maintained at week 20, 1 pt experienced stable disease at week 10, and 1 pt developed progressive disease at week 20. In the second cohort, two pts achieved a PR at week 10, and 1 pt had stable disease at week 10. Two of 3 responding pts were rituximab-refractory. Due to the observed infusion reactions with milatuzumab, the protocol has been amended to include additional premedication with intravenous antihistamine, and dexamethasone 20 mg pre-milatuzumab and 10 mg post-milatuzumab. The schedule of treatment was also modified so that the antibodies are no longer administered on the same day. Dose escalation will continue and updated results will be presented. The first 2 cohorts will be expanded to 6 pts to determine if the modifications will limit the infusion reactions and permit prolonged dosing in responding pts. In summary, 3 of 6 pts with relapsed or refractory NHL treated to date including heavily pretreated and rituximab-refractory pts, responded to combination therapy, achieving a PR. The primary observed toxicity has been infusion reactions due to milatuzumab.
Christian:Immunomedics, Inc.: Research Funding. Off Label Use: The use of the monoclonal antibodies veltuzumab and milatuzumab is experimental in the treatment of non-Hodgkin's lymphoma. Benson:Immunomedics, Inc: Research Funding. Jones:Immunomedics, Inc.: Research Funding. Wegener:Immunomedics, Inc.: Employment, shareholders. Goldenberg:Immunomedics, Inc.: Employment, shareholders. Baiocchi:Immunomedics, Inc.: Research Funding. Blum:Immunomedics, Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal