Abstract
Abstract 34
We performed randomized phase III study to compare the regimen related toxicities (RRT) of two different conditioning regimens, cyclophosphamide (CyATG) vs. cyclophosphamide plus fludarabine (CyFluATG) given in addition to anti-thymocyte globulin (ATG) for allogeneic hematopoietic cell transplantation (alloHSCT) for bone marrow failure syndrome including severe aplastic anemia (AA) and hypoplastic myelodysplastic syndrome (MDS).
CyATG consisted of Cyclophosphamide (Cy) 50 mg/kg (D –5 to –2). CyFluATG arm received fludarabine (Flu) 30 mg/m2 (D –6 to –2) and Cy 50 mg/kg (D –3 to –2). Thymoglobuline 3 mg/kg, lymphoglobulin 15 mg/kg on days -4 to -2 or alemtuzumab 20mg on day -4 were infused in both arms. Patients were stratified by stem cell donor (related vs. unrelated).
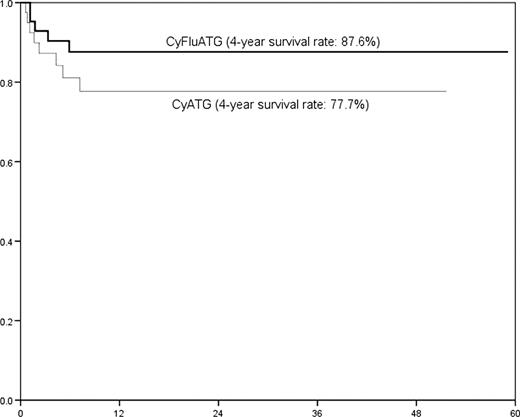
Total 83 patients (40 patients to Cy-ATG and 43 patients to Cy-Flu-ATG) were enrolled from February 2003. All patients except for one patient, who had assigned to Cy-Flu-ATG arm and died during conditioning, received full planed regimen and all planned patients were included in this analysis. Median age was 34 (15-60) years and male patients were 42/83 (50.6%). AA patients were 79 and MDS were 4. Matched sibling donors were 53 (63.9%). ATG was used in form of thymoglobulin (n=75, 90.4%), and some patients had received ALG (n=5, 6.0%) or alemtuzumab (n=3, 3.6%). Median duration from diagnosis to transplantation was 4.7 (0.2-177.7) months. Age, gender, donor type were not different in both arms (Table 1). Various TRT were similar between Cy-ATG and Cy-Flu-ATG (Table 2); granulocyte graft failure rate (p=0.959), platelet graft failure rate (p=0.625), acute GvHD (p=0.388), chronic GvHD (p=0.991), CMV antigenemia (p=0.550), hematuria (p=0.480). However, pulmonary complications (p=0.005) was significantly lower in CyFluATG arm. Infection rate (p=0.130) and sinusoidal obstruction syndrome (SOS, p=0.101) seemed lower in CyFluATG arm but were not statistically significant. Any RRTs were significantly higher in CyATG arm (80.0% vs. 39.5%; p<0.001) but any treatment-related toxicities were similar in both arms (85% vs. 79.1%; p=0.483). Figure 1 shows that 4-year survival rates (77.7% vs. 87.6%) were higher in CyFluATG arm without any statistical significance (p=0.265) and this trend was similar in MRD (81.9 vs. 92.1%; p=0.354) and AD (69.3 vs. 80.2%; p=0.442).
Characteristics of patients between Cy-ATG and Cy-Flu-ATG
| Characteristics . | Cy-ATG . | Cy-Flu-ATG . | p-value . |
|---|---|---|---|
| Gender, n (%) | 0.586 | ||
| Male | 19 (47.5) | 23 (53.5) | |
| Female | 21 (52.5) | 20 (46.5) | |
| Age, median (range) | 34.5 (15±59) | 34.0 (18±60) | 0.365 |
| Months from diagnosis to SCT, median (range) | 4.8 (0.2–147.2) | 4.6 (0.9–177.7) | 0.982 |
| Diagnosis, n (%) | 0.617 | ||
| AA | 39 (97.5) | 40 (93.0) | |
| MDS | 1 (1.9) | 3 (7.0) | |
| Infused CD34+ cell dose (?106/kg), mean±SD | 5.76±4.89 | 5.25±5.30 | 0.449 |
| ATG, n (%) | 0.360 | ||
| Thymoglobulin | 38 (95.0) | 37 (86.0) | |
| ALG | 1 (2.5) | 4 (9.3) | |
| Alemtuzumab | 1 (2.5) | 2 (4.7) | |
| HLA-A, B, C and DR molecular matching, n (%) | 0.699 | ||
| Full matched | 30 (75.0) | 30 (69.8) | |
| 1 locus mismatched | 3 (7.5) | 3 (7.0) | |
| 2 loci mismatched | 3 (7.5) | 2 (4.7) | |
| Not determined | 4 (10.0) | 8 (18.6) | |
| Donor, n (%) | 0.834 | ||
| MSD | 26 (65.0) | 27 (62.8) | |
| AD | 14 (35.0) | 16 (37.2) |
| Characteristics . | Cy-ATG . | Cy-Flu-ATG . | p-value . |
|---|---|---|---|
| Gender, n (%) | 0.586 | ||
| Male | 19 (47.5) | 23 (53.5) | |
| Female | 21 (52.5) | 20 (46.5) | |
| Age, median (range) | 34.5 (15±59) | 34.0 (18±60) | 0.365 |
| Months from diagnosis to SCT, median (range) | 4.8 (0.2–147.2) | 4.6 (0.9–177.7) | 0.982 |
| Diagnosis, n (%) | 0.617 | ||
| AA | 39 (97.5) | 40 (93.0) | |
| MDS | 1 (1.9) | 3 (7.0) | |
| Infused CD34+ cell dose (?106/kg), mean±SD | 5.76±4.89 | 5.25±5.30 | 0.449 |
| ATG, n (%) | 0.360 | ||
| Thymoglobulin | 38 (95.0) | 37 (86.0) | |
| ALG | 1 (2.5) | 4 (9.3) | |
| Alemtuzumab | 1 (2.5) | 2 (4.7) | |
| HLA-A, B, C and DR molecular matching, n (%) | 0.699 | ||
| Full matched | 30 (75.0) | 30 (69.8) | |
| 1 locus mismatched | 3 (7.5) | 3 (7.0) | |
| 2 loci mismatched | 3 (7.5) | 2 (4.7) | |
| Not determined | 4 (10.0) | 8 (18.6) | |
| Donor, n (%) | 0.834 | ||
| MSD | 26 (65.0) | 27 (62.8) | |
| AD | 14 (35.0) | 16 (37.2) |
The comparison of treatment-related toxicities between Cy-ATG and Cy-Flu-ATG
| Factors . | Cy-ATG . | Cy-Flu-ATG . | p-value . |
|---|---|---|---|
| Graft failure, n (%) | 5 (12.5) | 7 (16.3) | 0.625 |
| Granulocyte | 1 (2.5) | 1 (2.3) | 0.959 |
| Platelet | 5 (12.6) | 7 (16.3) | 0.625 |
| Acute GvHD, n (%) | |||
| Any grades | 6 (15.0) | 10 (23.3) | 0.388 |
| Grade 3/4 | 2 (5.0) | 1 (2.3) | 0.514 |
| Chronic GvHD, n (%) | |||
| Any | 5 (12.5) | 5 (11.6) | 0.991 |
| Extensive | 4 (10.0) | 3 (7.0) | 0.369 |
| CMV antigenemia, n (%) | 24 (60.0) | 23 (53.5) | 0.550 |
| Infection, n (%) | 32 (80.0) | 28 (65.1) | 0.130 |
| Interstitial pneumonitis, n (%) | 0 (0.0) | 0 (0.0) | – |
| Pulmonary complications, n (%) | 14 (35.0) | 4 (9.3) | 0.005 |
| SOS, n (%) | 5 (12.5) | 1 (2.3) | 0.101 |
| Hematuria, n (%) | 10 (8.7) | 8 (29.6) | 0.480 |
| Any regimen-related toxicities, n (%) | 32 (80.0) | 17 (39.5) | <0.001 |
| Any treatment-related toxicities, n (%) | 34 (85.0) | 34 (79.1) | 0.483 |
| Factors . | Cy-ATG . | Cy-Flu-ATG . | p-value . |
|---|---|---|---|
| Graft failure, n (%) | 5 (12.5) | 7 (16.3) | 0.625 |
| Granulocyte | 1 (2.5) | 1 (2.3) | 0.959 |
| Platelet | 5 (12.6) | 7 (16.3) | 0.625 |
| Acute GvHD, n (%) | |||
| Any grades | 6 (15.0) | 10 (23.3) | 0.388 |
| Grade 3/4 | 2 (5.0) | 1 (2.3) | 0.514 |
| Chronic GvHD, n (%) | |||
| Any | 5 (12.5) | 5 (11.6) | 0.991 |
| Extensive | 4 (10.0) | 3 (7.0) | 0.369 |
| CMV antigenemia, n (%) | 24 (60.0) | 23 (53.5) | 0.550 |
| Infection, n (%) | 32 (80.0) | 28 (65.1) | 0.130 |
| Interstitial pneumonitis, n (%) | 0 (0.0) | 0 (0.0) | – |
| Pulmonary complications, n (%) | 14 (35.0) | 4 (9.3) | 0.005 |
| SOS, n (%) | 5 (12.5) | 1 (2.3) | 0.101 |
| Hematuria, n (%) | 10 (8.7) | 8 (29.6) | 0.480 |
| Any regimen-related toxicities, n (%) | 32 (80.0) | 17 (39.5) | <0.001 |
| Any treatment-related toxicities, n (%) | 34 (85.0) | 34 (79.1) | 0.483 |
Overall survival
Off Label Use: Cyclophosphamide, Fludarabine and thymoglobulin were used in conditioning regimens of this phase III clinical trial.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal