Abstract
Abstract 3751
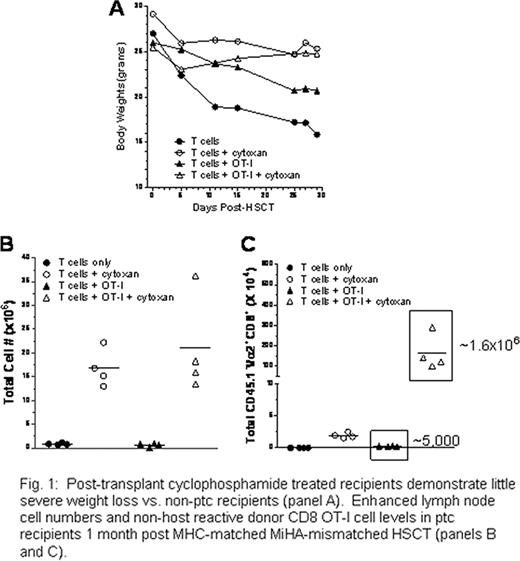
Following myeloablative as well as reduced intensity conditioning regimens, recipients of allogeneic T cell replete (TCR) HLA matched sibling)or unrelated hematopoietic stem cell transplants (HSCT) experience significant acute as well as chronic graft vs. host disease (GVHD). Recent studies have reported that post-transplant cyclophosphamide (PTC) can function as an effective single agent for prophylaxis of acute and chronic GVHD (Biol. Blood Marrow Transpl. 16:482, 2010; Blood. 116:3224, 2010). To investigate the fate of non-host alloreactive donor cells, which are critical to providing immune reconstitution post-transplant, T cell replete HSCT were performed between MHC-matched allogeneic donor–recipient pairs. C3H.SW (H2b) mice were lethally conditioned 4 hrs prior to receipt of B6-CD45.1 (H2b) T cells and bone marrow. Recipients developed severe GVHD as assessed by weight loss (Fig. 1A) and clinical analyses during the first 4–5 wks post-transplant. Recipients had inverted CD4/CD8 ratios as well as an activated phenotype (CD44hiCD62Llo), severe B and T cell lymphopenia, and virtually undetectable thymic tissue. A single dose of post-transplant cyclophosphamide at day 3 (66mg/kg/body weight) blocked onset of weight loss and clinical signs of GVHD as well as lethality in C3H.SW recipients, consistent with the reduction of alloreactive anti-recipient T cells. In contrast to non-cyclophosphamide treated animals, treated recipients expressed typical CD4/CD8 ratios, significant numbers of spleen and lymph node cells (Fig. 1B) and readily detectable thymi 7–8 weeks post-HSCT. Notably, the majority of T as well as B cells in the periphery of these recipients exhibited a non-activated phenotype and were derived from donor bone marrow. Together with the observation that approximately 50% of T cells exhibited a CD44hiCD62Llo phenotype characteristic of TN, the findings are consistent with de novo derived lymphopoiesis in the thymus and marrow of post-transplant cyclophosphamide treated recipients. To assess the impact of D.3 cyclophosphamide treatment on a non-host reactive T cell population, B6 OT-I CD8 T cells (Vα2/Vβ5 TcR) were added (1-2×106) to the donor inoculum. In non-PTC treated recipients, low numbers of OT-I T cells (below input levels) were detected by 3–4 weeks post-HSCT. In contrast, in PTC-treated recipients, significant numbers of OT-I CD8 T cells were present in the recipient spleen and lymph nodes (Fig. 1C), consistent with the finding of CFSE labeled OT-I cells exhibiting division following cyclophosphamide treatment. Notably, the overall numbers of OT-I CD8 T cells in cyclophosphamide treated recipients was greater than the input levels, indicating that these cells a) survived post-cyclophosphamide treatment and b) underwent lymphopenic expansion. Consistent with these findings, CFSE studies at 72 hrs post-HSCT illustrated OT-I T cells exhibited low proliferation vs. a population of presumed B6 anti-C3H.SW alloreactive T cells. We therefore conclude that non-host reactive donor T cells undergo sufficient repair following cyclophosphamide induced alkylation to enable greater survival in contrast to rapidly dividing anti-host (GVHD) reactive populations. Vaccination studies in cyclophosphamide treated and non-treated HSCT recipients are being performed using heat shock fusion protein-transfected tumor cells to investigate the capacity to elicit anti-tumor (i.e., GVL) responses in the presence and absence of GVHD.
Post-transplant cyclophosphamide treated recipients demonstrate little severe weight loss vs. non-ptc recipients (panel A). Enhanced lymph node cell numbers and non-host reactive donor CD8 OT-I cell levels in ptc recipients 1 month post MHC-matched MiHA-mismatched HSCT (panels B and C).
Post-transplant cyclophosphamide treated recipients demonstrate little severe weight loss vs. non-ptc recipients (panel A). Enhanced lymph node cell numbers and non-host reactive donor CD8 OT-I cell levels in ptc recipients 1 month post MHC-matched MiHA-mismatched HSCT (panels B and C).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal