Abstract
Abstract 4336
Recent reports have suggested that aside from the tyrosine kinase inhibitory effect, dasatinib also promotes a clonal expansion of cytotoxic T-cells in patients with Philadelphia-chromosome positive (Ph+) leukemias. We sought to determine whether there is an absolute lymphocytosis in patients with Ph+ Acute Lymphocytic Leukemia (ALL) treated at our institution on 3 consecutive protocols.
We collected data on 122 patients with Ph+ ALL treated with 3 different regimens (hyperCVAD, hyperCVAD + imatinib, and hyperCVAD + dasatinib) from 1994 to 2010, who achieved a complete hematological response (CHR). We examined potential differences between the absolute numbers lymphocytes in peripheral blood (PB) and percentage lymphocytes in bone marrow (BM) at 3 stages: pretreatment, at CHR, and 3–6 months after CHR.
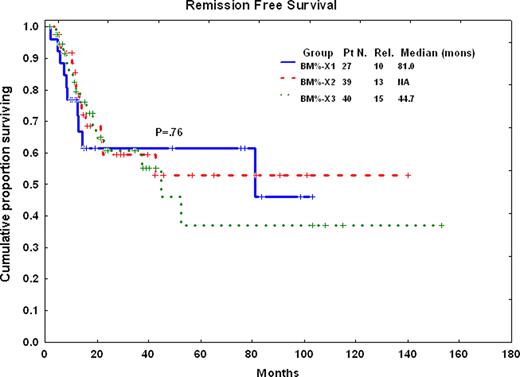
Thirty four (28%) patients received hyperCVAD, 43 (35%) hyperCVAD + imatinib, and 45 (37%) hyperCVAD + dasatinib. Twenty five (74%) of patients treated with hyperCVAD, 14 (33%) of patients treated with hyperCVAD + imatinib, and 5 (11%) of patients treated with hyperCVAD + dasatinib have relapsed on these sequential studies. The median PB absolute lymphocyte count for the 3 groups are as follow: Pretreatment: hyperCVAD: 3570.5/uL, hyperCVAD + imatinib: 2262/uL, hyperCVAD + dasatinib: 2376/uL (p = 0.1); at CR: hyperCVAD: 291.5/uL, hyperCVAD + imatinib: 168/uL, hyperCVAD + dasatinib: 239/uL (p = 0.3); 3 to 6 months after CR: hyperCVAD: 485/uL, hyperCVAD + imatinib: 450/uL, hyperCVAD + dasatinib: 632.5/uL (p = 0.26). The median BM lymphocyte percentages are as follow: Pretreatment: hyperCVAD: 3%, hyperCVAD + imatinib: 6%, hyperCVAD + dasatinib: 5% (p = 0.5). CHR: hyperCVAD: 6%, hyperCVAD + imatinib: 4%, hyperCVAD + dasatinib: 4% (p = 0.4). Three to 6 months after CHR: hyperCVAD: 5%, hyperCVAD + imatinib: 3%, hyperCVAD + dasatinib: 7% (p = 0.005). We then divided all patients by their 3 to 6 month BM lymphocyte percentages to 3 groups (< 3%, 3–6%, >6%). There was no difference in remission-free survival for these 3 groups (p=0.76)(Figure 1)
Patients who received hyperCVAD + dasatinib had a statistically significant higher BM lymphocyte percentages, 3 to 6 month after achieving CHR than patients treated with the imatinib-containing regimen or chemotherapy alone. Whether this is a clonal population of effector T-cells and whether it plays an important role on the activity of dasatinib compared to imatinib or chemotherapy alone will need to be further investigated.
Ravandi:Novartis: Honoraria, Speakers Bureau; Bristol- Myere- Squibb: Honoraria, Research Funding. Kantarjian:BMS, Pfizer and Novartis: Research Funding; Novartis: Consultancy. Cortes:Bristol Myers Squibb: Research Funding; Novartis: Research Funding; Pfizer: Consultancy, Research Funding. O'Brien:Novartis: Research Funding; BMS: Research Funding. Thomas:Novartis: Honoraria; Bristol-Meyer-Squibb: Honoraria; Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal