Abstract
Abstract 520
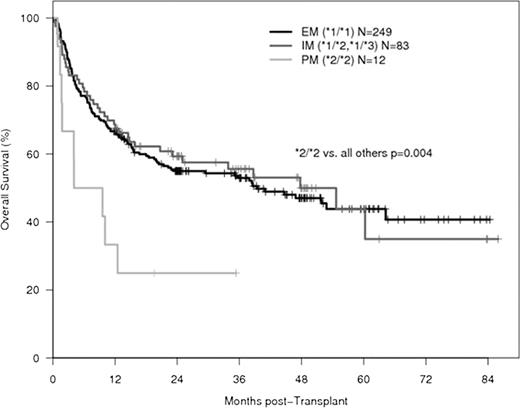
Patients with allelic variations of cytochrome P450 enzymes that affect drug metabolism have been shown to have worse outcomes after hematopoietic stem cell transplantation (HSCT). One study demonstrated higher treatment-related mortality in carriers of the cytochrome P450 2C19 (CYP2C19) loss-of-function allele, *2, however the underlying cause remains unclear. Extensive metabolizers (EM) have two wild-type alleles of CYP2C19 (i.e., *1/*1), while intermediate metabolizers (IM) and poor metabolizers (PM) are heterozygous or homozygous for common allelic variants of CYP2C19 that produce null mutations (i.e., *1/*2, *1/*3 for IM and *2/*2, *2/*3 and *3/*3 for PM). We hypothesized that allelic variations in CYP2C19 may lead to worse outcomes after HSCT due to altered metabolism of CYP2C19 substrates or other drugs commonly given to patients receiving HSCT. We analyzed DNA samples from 344 patients who received high-dose CPA conditioning and total body irradiation prior to allogeneic HSCT between 2002 and 2008. Genotyping was performed to detect the *1,*2 and *3 allelic variants of CYP2C19. Non-relapse mortality (NRM), relapse, progression-free survival (PFS) and overall survival (OS) were determined for EM, IM and PM. We collected kidney and liver function test results and recorded all medications in the PM group. The EM, IM and PM phenotype was found in 249 (72.4%), 83 (24.1%) and 12 (3.5%) patients, respectively. CYP2C19 PM had significantly worse overall survival (3-yr: 25%) than EM and IM (55%) (Figure, adjusted HR=2.78, p=0.004). PFS was also significantly worse in PM (3-yr: 25%) versus EM and IM (53%) (adjusted HR=2.38, p=0.008). No significant difference in NRM and relapse was seen (p=0.10 and p=0.42, respectively). Compared to the three surviving patients, the nine PM who died had higher maximum post-transplant concentrations of creatinine (2.59 ± 2.33 mg/dl vs. 1.37 ± 0.06 mg/dl), total bilirubin (16.7 ± 18.4 mg/dl vs. 1.5 ± 0.8 mg/dl), AST (310 ± 144 U/l vs. 156 ± 107 U/l), and alkaline phosphatase (787 ± 811 U/l vs. 207 ± 84 U/l). A total of 192 different medications were administered to the twelve PM. Of the drugs metabolized by CYP2C19, seven of the nine patients who died received voriconazole and four received omeprazole. None of the three surviving patients received either drug. The significantly worse overall survival in CYP2C19 *2 homozygotes who carry two non-functional alleles may have multiple causes, one of them being administration of triazole drugs, in particular voriconazole. Voriconazole is an effective antifungal commonly given to patients after HSCT. It undergoes extensive hepatic metabolism, primarily by CYP2C19. PM have significantly higher concentrations of voriconazole than EM and IM, which may not only lead to direct voriconazole toxicity but also to exacerbated drug-drug interactions and altered concentrations of immunosuppressants and antibiotics. Since more than a thousand patients who receive HSCT each year are homozygous for CYP2C19 *2, further investigation into the causes of poor outcomes in this patient subset is warranted. In the interim, we propose testing for the CYP2C19 *2 allele in patients undergoing HSCT and careful monitoring of drug levels in the homozygotes, in particular voriconazole and immunosuppressants.
No relevant conflicts of interest to declare.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal