Abstract
Abstract 534
There is increasing information about reduced intensity conditioning regimen AlloHSCT (Allogeneic Hematopoietic Stem Cell Transplantation) in children. The safety of this approach is now well established but data regarding efficacy are limited and the role in pediatric cancer has yet to be defined.
We report results of a French pediatric AlloHSCT protocol with ATG-fludarabine (180 mg/m2) - Busilvex (3.2 at 4.8 mg/kg/d for 2 days) conditioning regimen. Related, unrelated bone marrow (BM) and Peripheral blood stem cell (PBSC) donors and Cord blood units (CB) were allowed. In case of CB a TBI 2 grays, Cyclophosphamide 50 mg/kg, Fludarabine 100 mg/m2 conditioning regimen was recommended. GVH prophylaxis consists in cyclosporine alone. A rapid discontinuation of systemic immunosuppression and re-injecting donor lymphocytes to initiate graft-versus-tumor effect are based on tumor assessment and blood chimerism. Inclusion criteria are children with malignancies that can be potentially cure by allograft but a conventional conditioning regimen being impossible due to toxicity and children with solid tumor or hematological malignancy remaining unresponsive to the reference strategies according to French best practices in pediatrics.
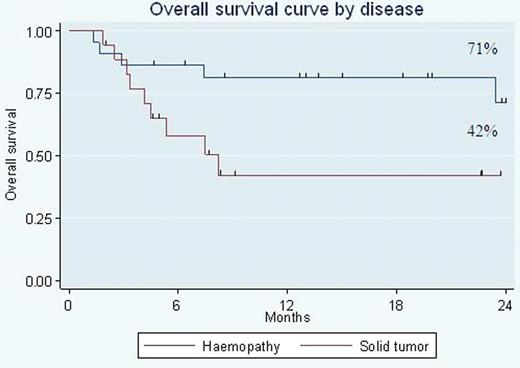
From April 2007 to April 2010, 40 RIC AlloHSCT were performed in 10 different French pediatric graft centers: 13 Hodgkin Lymphoma, 7 acute myeloblastic leukaemia, 2 acute lymphoblastic leukaemia, 6 neuroblastoma, 8 rhabdomyosarcoma, 3 desmoplastic tumor and 1 Ewing sarcoma. Median age at transplantation was 15 years and median time from diagnosis to transplant was 18 months. Before transplant, 15 patients are in complete response and 25 patients (14/18 solid tumors) have active disease (11 progressive, 14 partial response). 21 had already received a myeloablative therapy (18 autograft and 3 allograft). Graft source was PBSC in 17 cases (7 related and 10 unrelated), BM in 18 (10 related and 8 unrelated), and 5 CB. The RIC Bu-flu conditioning regimen permits rapid engraftment without major toxicity contrary to the Cy-TBI in CB. 1 patient had primary graft failure: 1 CB and 5 patients experienced secondary graft failure: 3 CB, 1 PBSC and 1 BM. Median time to reach an ANC of 0.5 109/l was 16 days. Median time to reach a platelet count of 20 × 109/l was 2 days. Platelet count did not decrease below 20 109/l in 10 allografts. At day 30 post-transplant, chimerism is mainly donor for 30 and partial for 6 children. At day 100 post transplant, 4 out of 6 with initial mixed chimerism were converted into full donor chimerism. 8 patients received DLI and 17 patients experienced acute graft versus host disease (GvH) (2 grade IV and 15 grade ≤ II). A low day 365 TRM of 5% is reported in these heavily pre-treated patients. With a median follow-up of 15 months, the estimated 2 yr overall survival (OS) was respectively 57 % (71% for hematological malignancies and 42% solid tumors) (fig 1) and event free survival (EFS) 36% (50% for hematological malignancies and 19% solid tumors). Univariate analysis of EFS and OS showed no effect of related versus unrelated stem cell sources and BM versus PBSC. Our analysis identified a group of patients, who had no measurable disease at transplant, with a 2 yr OS and EFS of 86%. In term of efficacy, we observe a graft versus lymphoma effect in patients with advanced active Hodgkin lymphoma. Concerning solid tumors, all children included had a very bad prognosis and detectable disease before transplant. Our results may suggest that an immune-mediated effect cannot be excluded in some refractory solid pediatric tumors particularly in neuroblastoma. The main cause of failure of this approach is disease progression. Immunologic approaches after transplantation may help cure more of these very-high-risk patients.
Even if further follow up is needed, this prospective study suggest that RIC regimen provides promising outcome in children previously not eligible for myeloablative AlloHSCT.
This study “RICE” was registered at www.clinicaltrials.gov as NCT 007 50 126
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal