Abstract
Abstract 682
The treatment outcome of adult patients with Philadelphia positive Acute Lymphoblastic Leukemia (Ph+ ALL) remains remarkably unfavorable and this is why patients under the age of 60 are usually considered good candidates for allogeneic hematopoietic stem cell transplantation (alloHSCT). The availability of Imatinib and other tyrosine kinase inhibitors (TKI) like Dasatinib and Nilotinib, seems to be changing rapidly the clinical outcome of Ph+ ALL since these drugs may reduce early relapse, increase the clinical response and the proportion of patients to whom the transplant can be offered. In addition, the strict and accurate evaluation of minimal residual disease by quantitative polymerase chain reaction (RQ-PCR) is crucial to guide the post transplant treatment including the use of TKI and donor lymphocytes infusions. Nonetheless, the complications of the transplant (engraftment, immune reconstitution, graft versus host disease, GVHD) may still offset the benefit of the procedure in many patients, the elderly and those with comorbidity, in particular.
To evaluate the role of Imatinib and molecular monitoring of minimal residual disease on the clinical outcome of adult Ph+ ALL patients undergoing or not alloHSCT.
One-hundred consecutive, untreated adult patients (median age 46, range 19–66) with Ph+ ALL (as determined by cytogenetic or molecular analysis) enrolled into Northern Italy Leukemia Group (NILG) protocol 09/00 (ClinicalTrial.gov Identifier: NCT00358072) are the object of this study. The protocol was approved by the institutional review board of all participating institutions and amended in February 2003, when imatinib became available and added to each chemotherapy course at 600 mg/d. Sixty-five patients received Imatinib during induction/consolidation (IM+ group, Bassan et al.: Journal of Clinical Oncology, 2010). Fifty-eight patients received alloHSCT from a sibling (n= 33), unrelated (n= 24) or haplo (n= 1) donor, while 42 received a conventional treatment (chemotherapy, n= 33 or an autologous transplant, n= 9) (with or without Imatinib). The median age of patients receiving or not an alloHSCT was 40 and 54 years, respectively. The conditioning regimen to alloHSCT was myeloablative in 49 and reduced intensity in 9. The stem cell source was the bone marrow (BM) in 20 and the peripheral blood (PB) in 38.
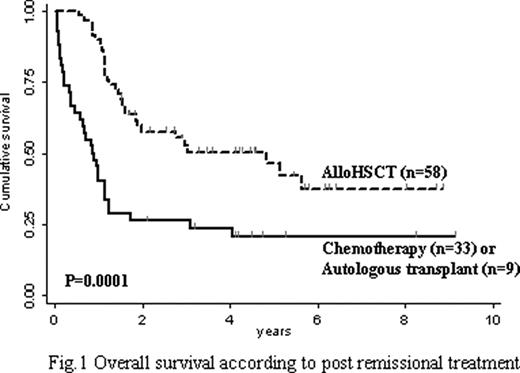
The addition of Imatinib to chemotherapy during induction/consolidation increased the proportion of patients who achieved CR (93% vs 80%, p= 0.05) and who had the opportunity to undergo alloHSCT (66% vs 43%, p= 0.02). With a median follow-up of 1.5 years, the overall survival (OS) at 5 years was 35% for the whole patient cohort (n= 100), 39% in the IM+ group and 23% in the IM-, respectively (p=0.007). At 5 years, the OS of patients receiving alloHSCT was 46% vs 21% of the others (p= 0.0001) (Figure 1). The transplant related mortality at 4 years was 23%, no matter whether Imatinib was given or not any time during treatment. The cumulative incidence of relapse (CIR) of patients undergoing alloHSCT was 32% in the IM+ vs 57% in the IM- group. Interestingly, the CIR of alloHSCT patients was 19% for patients who proved MRD negative at time of conditioning vs 51% for those bearing any positive MRD level in the bone marrow or peripheral blood (p= 0.04). Accordingly, the disease free survival probability was 67% of MRD negative vs 42% of MRD positive patients (p=0.06).
This study shows that the combined use of Imatinib and chemotherapy during induction/consolidation (1) improves the long term clinical outcome of all patients with Ph+ ALL, (2) reduces the relapse probability of patients undergoing alloHSCT and (3) is associated with a better disease free and overall survival after transplantation. Finally, alloHSCT either from a sibling or unrelated donor remains the best post remissional treatment of Ph+ adult ALL patients despite a still remarkable transplant toxicity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal