Abstract
Abstract 919
Patients (pts) with Rai Stage III/IV CLL have a shorter median survival compared to Rai Stage I/II pts. We previously reported significant improvement in progression-free survival (PFS) with FluCam compared to single-agent fludarabine (Flu) in pts with previously treated CLL (Engert ASH 2009; EHA 2010). Here we report the efficacy and safety of FluCam and Flu in a subset of these pts with advanced stage CLL.
In a Phase 3, multi-center, controlled trial, pts with relapsed/refractory CLL were randomized to receive either IV Flu 25 mg/m2 on days 1–5 every 28 days or IV alemtuzumab (Cam) in combination with IV Flu (FluCam). Prior to cycle 1, FluCam pts were required to undergo Cam escalation (3 mg, 10 mg, 30 mg); once the 30 mg dose was tolerated, pts began cyclic administration of Flu 30 mg/m2 IV and Cam 30 mg IV on days 1–3 every 28 days. Pts could receive up to 6 cycles of either therapy depending on response and toxicity. Randomization was accomplished utilizing the minimization method to ensure balance between treatment arms with respect to study center, disease status, prior therapy, Rai Stage, age, gender, and lymph node size. All pts received prophylaxis with cotrimoxazole and famciclovir until CD4+ counts were >200 cells/mcL. The primary endpoint of the study was PFS with FluCam compared to Flu. A pre-specified subgroup analysis in Rai Stage III/IV pts compared PFS, overall response rate (ORR=CR + PR), complete response rate (CR), overall survival (OS) and safety in FluCam vs Flu treated pts. Differences in PFS between the 2 treatment arms were tested using the log-rank test.
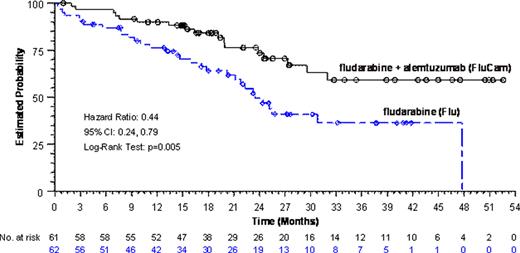
Of the 335 pts enrolled in this study, 123 (37%) were Rai Stage III or IV (61 FluCam pts, 62 Flu pts). For the overall study population, FluCam significantly prolonged PFS compared with Flu (24.1 months (mos) vs 15.4 mos, respectively; p = 0.002; HR: 0.618; 95% CI 0.46–0.83). Data presented herein focus on the subgroup of CLL pts with Rai Stage III/IV disease. Demographics for this subset of FluCam vs Flu treated pts include: median age (65 yrs [36-80] vs 64 yrs [46-80]); gender (70% male vs 61% male); and prior Flu exposure (10% vs 8%) respectively. Pts received a median of 6 cycles in the FluCam arm and a median of 5 cycles in the Flu arm. FluCam significantly prolonged PFS compared with Flu (24.5 mos vs 11.8 mos, respectively; p < 0.001; HR 0.46; 95% CI 0.29–0.73). Similarly, the ORR (77% vs 56%; p = 0.016) and CR (16% vs 3%; p = 0.014) improved significantly with FluCam compared to Flu. With a median follow-up of 21 mos, pts treated with FluCam had significantly improved median OS compared with those treated with Flu (not reached vs 23.5 mos, respectively, p = 0.005 HR 0.44; 95% CI 0.24–0.79), representing a 56% reduction in the risk of death (Figure 1). Treatment-related adverse events (AE) occurred in 97% and 88% of Rai Stage III/IV pts receiving FluCam (n=59) and Flu (n=60) arms, respectively. There were no clinically relevant differences in the AE profile in pts with Rai Stage III/IV between arms. In the Rai Stage III/IV subgroup, the AEs that occurred ≥10% and more frequently in the FluCam arm relative to the Flu arm included infusion-related events and leukopenia. Reported all-cause grade 3/4 hematologic AEs in the FluCam vs Flu arms were neutropenia (47% vs 48%), leukopenia (29% vs 8%), thrombocytopenia (15% vs 20%), and anemia (7% vs 23%), respectively. The total frequency of treatment-emergent infections was 42% in the FluCam arm vs 52% in the Flu arm. No CMV infections were reported in either arm in this subset of pts. All-cause serious AEs (SAE) occurred in 37% and 45% in the FluCam and Flu arms, respectively. The most commonly reported all-cause SAEs in ≥5% of pts in either arm were pyrexia (5% vs 2%), neutropenia (3% vs 5%), febrile neutropenia (2% vs 8%) and anemia (0% vs 8%) for FluCam vs Flu respectively. Deaths occurring on therapy or within 30 days after last dose were 3% on the FluCam arm vs 8% on the Flu arm.
Data presented in this subset analysis of Rai Stage III/IV CLL pts demonstrate a survival advantage, longer PFS and improved ORR/CR in pts treated with FluCam compared to Flu. The safety profile of FluCam in pts with advanced stage was acceptable and in most respects, comparable to the Flu alone arm. Overall, FluCam is a compelling treatment option in pts with previously treated advanced CLL.
Engert:Genzyme: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Off Label Use: Alemtuzumab (Campath, MabCampath) is indicated for the treatment of CLL. This trial examined the use of alemtuzumab in combination with fludarabine monophospate. Gercheva:Genzyme: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Pilipenko:Genzyme: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Robak:Genzyme: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Wu:Genzyme: Employment, Equity Ownership. Sirard:Genzyme: Employment, Equity Ownership. Elter:Genzyme: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal