Abstract
The major limitation for the development of curative cancer therapies has been an incomplete understanding of the molecular mechanisms driving cancer progression. Human models to study the development and progression of chronic myeloid leukemia (CML) have not been established. Here, we show that BMI1 collaborates with BCR-ABL in inducing a fatal leukemia in nonobese diabetic/severe combined immunodeficiency mice transplanted with transduced human CD34+ cells within 4-5 months. The leukemias were transplantable into secondary recipients with a shortened latency of 8-12 weeks. Clonal analysis revealed that similar clones initiated leukemia in primary and secondary mice. In vivo, transformation was biased toward a lymphoid blast crisis, and in vitro, myeloid as well as lymphoid long-term, self-renewing cultures could be established. Retroviral introduction of BMI1 in primary chronic-phase CD34+ cells from CML patients elevated their proliferative capacity and self-renewal properties. Thus, our data identify BMI1 as a potential therapeutic target in CML.
Introduction
Chronic myeloid leukemia (CML) was the first cancer to be associated with a defined chromosomal abnormality, the Philadelphia chromosome (Ph), which occurs as a consequence of reciprocal translocation of chromosomes 9 and 22 (t9;22)(q34;q11).1–3 In the pre–tyrosine kinase inhibitor (TKI) era, CML was characterized by distinct clinical stages evolving from a chronic phase (CP) through an accelerated phase (AP) into blast crisis (BC).4,5 The BCR-ABL oncoprotein is necessary and sufficient for initiating the chronic phase of the disease when preferential expansion of myeloid progenitors and differentiated progeny is observed.6–8
While overexpression of BCR-ABL in murine bone marrow (BM) was sufficient to induce transplantable leukemias in almost all recipient mice,9 attempts to develop human CML models did not result in overt hematologic disease. When cord blood CD34+ cells were retrovirally transduced with p210 BCR-ABL and transplanted into nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice, enhanced numbers of erythroid and megakaryocytic cells were observed, but after 5 months, only a few mice developed signs of a myeloproliferative disease.10 When primary CML samples were injected into NOD/SCID mice in an attempt to model the disease, recipients showed an accumulation of abnormal populations of cells that recapitulated the onset of the disease, rather than the blast crisis, and no reproducible human hematopoietic malignancies were generated, so far.11
Various pathways downstream of BCR-ABL have been implicated in the transformation process, including the activation of β-catenin,12,13 signal transducer and activator of transcription 5 (STAT5),14–16 Rac GTPases,17,18 and mitogen-activated protein kinase/extracellular signal–related kinase (MEK/ERK).19 Even though many of these pathways are activated by BCR-ABL and are required for the onset of disease, little is known about the molecular mechanisms that cooperate with BCR-ABL in the transition from CP- to BC-CML. Pathways that might cooperate with BCR-ABL in the transition from CP-CML into BC-CML include the Wnt and Hedgehog pathways.12,20,21 Recent findings report that the expression of the polycomb gene, BMI1, which is implicated in normal and leukemic stem cell proliferation,22,23 is significantly more highly expressed in patients with advanced disease than in patients in CP.24 BMI1 has been linked to leukemogenesis since its discovery as a cooperating partner of c-Myc in the induction of B-cell lymphomas.25 When HOXA9 and MEIS1 were coexpressed in bmi1−/− cells, leukemia did not occur in secondary transplanted animals, suggesting that BMI1 has an important role in the maintenance of leukemic stem cells in this model.22 We have classified BMI1 as a strong intrinsic regulator of human stem cells, as BMI1-expressing cells engrafted efficiently in the NOD/SCID mice even after in vitro culturing.26 However, recipient mice did not show signs of disease, documenting that BMI1 by itself is not sufficient to induce leukemia in human cells (unpublished observations,26 ), comparable with what has been observed in murine cells.27
Here, we report that coexpression of BMI1 and BCR-ABL in human cord blood (CB) CD34+ cells is sufficient to induce transplantable leukemia in NOD/SCID mice. In vitro, both myeloid- and lymphoid-transformed cell lines could be established, depending on extrinsic cues. Retroviral introduction of BMI1 in primary CP CD34+ cells from CML patients elevated their proliferative capacity and self-renewal properties. In conclusion, our data indicate that BMI1 collaborates with BCR-ABL in leukemic transformation, and our human-based system should provide a useful model to study the pathology of leukemias and test new drug entities.
Methods
Primary cell isolation
CB and CML patient samples were obtained after informed consent in accordance with the Declaration of Helsinki and protocol approval by the Medical Ethical Committee of the University Medical Center Groningen. All analyzed CML samples were obtained at the time of diagnosis before treatment. The percentage of CD34+ cells ranged from 6% to 45%, and all cells were Ph+, as determined by karyotyping. After Ficoll separation of mononuclear cells, CD34+ cells were enriched using the magnetically activated cell sorting (MACS) CD34 progenitor kit (Miltenyi Biotec).
Cell lines and ex vivo culture of primary cells
Propagation of 293T, PG13, and MS5 cells, and myeloid-driven MS5 coculture experiments, were performed as previously described.28–30 Lymphoid-driven MS5 cocultures contained essentially the same components as in the myeloid-driven cultures, with the exception of hydrocortisone and horse serum and the presence of 50 μg/mL ascorbic acid (Sigma-Aldrich). The CD34+ cells from CP CML patients were cultured on MS5 cells in myeloid-supportive Gartners medium, supplemented with interleukin (IL)–3 (Gist-Brocades), IL-6 (Gist-Brocades), granulocyte colony-stimulating factor (G-CSF; Rhone-Poulenc Rorer; 20 ng/mL each), and stem cell factor (SCF) and FLT3 ligand (FLT3-L; (Amgen; 100 ng/mL each). For the sequential plating of MS5 cocultures, suspension and adherent cells were harvested by trypsinization, whereas human CD45+ cells were obtained using a magnetically activated cell sorting (MACS) CD45 progenitor kit (Miltenyi Biotec), after which cells were replated on new MS5 stromal cells. The stroma-independent culture assays were performed in Iscove modified Dulbecco medium (IMDM; PAA Laboratories) supplemented with 20% fetal calf serum (FCS), 2mM glutamine, penicillin and streptomycin, SCF, and IL-3 (20 ng/mL each).
Colony-forming cell and human long-term culture-initiating cell assays
Primary and secondary colony-forming cell (CFC) and long-term culture-initiating cell (LTC-IC) assays on MS5 stromal cells were performed as previously described.30 Briefly, for the CFC assays, 5000 sorted green fluorescent protein–positive (GFP+) cells were plated directly after transduction, and 25 000 GFP+ cells were used at later time points. For the colony replating experiments, 2 weeks after the primary plating, the colonies from one plate were collected, washed 3 times with phosphate-buffered saline (PBS), and all the cells were plated in new methylcellulose for an additional 2 weeks. For the lymphoid CFC assays, 10 000 cells were plated in methylcellulose containing 4 ng/mL IL-7. Cultures were scored using a Leica DM-IL inverted microscope (Leica Microsystems) at a total magnification of ×40.
For the LTC-IC assays, newly transduced cells were sorted on MS5 stromal cells in limiting dilutions from 10 to 7290 cells per well in 96-well plates. Cultures were demidepopulated weekly and fed with new medium. After 5 weeks of culture, the wells containing cobblestone areas were scored, after which the medium from the wells was aspirated and replaced with methylcellulose-containing cytokines. After an additional 2 weeks of culture, wells were scored as positive or negative to yield the LTC-IC frequency. Stem cell frequencies were calculated using L-Calc software (StemCell Technologies)
Immunoblotting and cytospins
Antibodies against BMI1 (Upstate), Abl, and STAT5 (Santa Cruz Biotechnology) were used in a 1:1000 dilution. Anti-actin antibody (Santa Cruz Biotechnology) was used in a 1:2000 dilution, and anti–β-tubulin antibody (Roche Diagnostics) was used in a 1:1000 dilution. May-Grünwald Giemsa staining was used to stain cytospins. Cytospin preparations were evaluated and photographed using a Leica DM3000 microscope (Leica Microsystems) equipped with a Leica DFC420C digital camera at a total magnification of ×400.
RNA extraction and real-time polymerase chain reaction analysis
RNA was isolated from 1 × 105 cell using RNeasy kit from QIAGEN, reverse transcribed using M-MuLV reverse transcriptase (Fermentas), and real-time amplified using iQ SYBR Green mix (Bio-Rad) on a MyIQ thermocycler (Bio-Rad) and quantified using MyIQ Version 2.0 software (Bio-Rad). Hypoxanthine-guanine phosphoribosyltransferase (HPRT) expression was used to calculate relative expression levels. Sequences and conditions are available on request.
Retroviral and lentiviral transduction of CB CD34+ cells
The murine stem cell virus (MSCV)-BMI1-internal ribosomal entry site (IRES)-GFP (MiGR1 BMI1) vector was cloned as previously reported.26 The MSCV-BCR-ABL-IRES-GFP (MiGR1 BCR-ABL, expressing p210 BCR-ABL) vector was kindly provided by Dr M.A.S. Moore (Memorial Sloan-Kettering Cancer Center, New York, NY), and the BCR-ABL gene was removed from this vector and inserted into the MSCV-IRES-tNGFR (truncated nerve growth factor receptor; MiNR1) vector by digestion with EcoRI. MiNR1-infected cells were stained with anti–NGFR-phycoerythrin (PE) antibody (Becton Dickinson) for analysis. Transductions of CB CD34+ cells were performed as described previously.29–31 The CD34+ cells from chronic CML patients were prestimulated for 48 hours in the presence of IL-3, IL-6, G-CSF, SCF, and FLT3-L. Retroviral supernatants were concentrated in Centriprep YK tubes (Millipore) at 3000g for 30 minutes at 4°C. Four consecutive rounds of 8-12 hours were performed.
Lentiviral vector–expressing short hairpins against human BMI1 (CS-H1–short-hairpin RNA [shRNA]-elongation factor 1-α [EF-1α]–enhanced GFP [EGFP]) and scrambled lentiviral vectors were a kind gift from Dr Iwama (Chiba University, Chiba, Japan). Lentiviral particles were produced by cotransfection of 293T cells with 0.7 μg of pcDNA3-vesicular stomatitis virus G (VSVg)-reticuloendotheliosis virus REV), 3 μg of pMDLg-RRE, and 3 μg CS-H1–scrambled RNAi or CS-H1-BMI1 RNAi. The lentiviral supernatants were collected 24 hours later and were either used directly or stored at −80°C until further use.
In vivo transplantations into NOD/SCID mice
Eight- to 10-week-old female NOD/SCID mice (NOD.CB17-Prkdcscid/J) were purchased from Charles River Laboratory. Before transplantations, mice were sublethally irradiated (3 Gy). Two independent transductions and transplantation experiments were performed. In the first experiment, the mice were injected with 4.6 × 105 and, in the second experiment, with 3.8 × 105 nonsorted CD34+ cells into the retro-orbital vein immediately after transduction. Human cell engraftment was analyzed in the BM, peripheral blood (PB), spleen, and liver by flow cytometry after 6-25 weeks of transplantation. The BM (2 femurs) from 2 leukemic mice in experiment 2 (m5 and m7) was used for secondary transplantations into sublethally irradiated NOD/SCID mice in duplicate, whereby one-third of leukemic bone marrow cells of the primary mice was transplanted into each secondary recipient.
Flow cytometric analysis and sorting procedures
Antibodies were obtained from Becton Dickinson. Sorting of the CB into stem/progenitor fractions was performed as previously reported.32,33 To deplete CLPs from the HSC fraction anti-CD2, CD3, CD4, CD7, CD8, CD10, CD19, CD20, and CD56 were included in our lineage cocktail. Analyses were performed on a FACSCalibur (Becton Dickinson), and sorting was performed on a MoFlo (Dako Cytomation). Data were analyzed using FlowJo Version 7.6.1 software (TreeStar).
LM-PCR
Ligation-mediated polymerase chain reaction (LM-PCR) was performed as previously described.34 PCR products were analyzed on a 2% agarose gel, gel-purified using the QIAquick Gel Extraction kit (QIAGEN), and sequence analyses was performed using the rvLTRIII primer.
Results
BMI1 collaborates with BCR-ABL in inducing leukemia in NOD/SCID mice
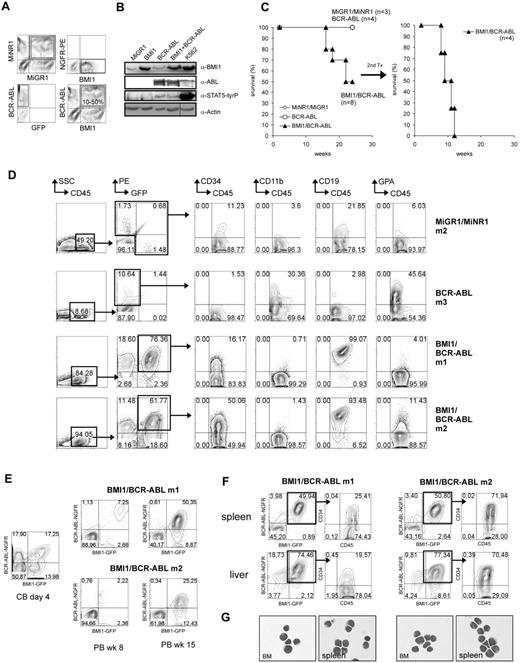
CB CD34+ cells were transduced with retroviral BMI1-IRES2-EGFP and BCR-ABL-IRES2-tNGFR vectors, and transplantations into sublethally irradiated NOD/SCID mice were performed. A control group of cells was cotransduced with MiGR1 and MiNR1 empty vectors. Efficiencies of double-transduced cells typically ranged from 10% to 50% (Figure 1A), and Western blot analysis of transduced cells confirmed the expression of the introduced proteins (Figure 1B). Sublethally irradiated NOD/SCID mice received transplants of unsorted transduced cells in 2 independent experiments (n = 8 for BMI1/BCR-ABL, n = 4 for BCR-ABL mice, and n = 3 for control MiNR1/MiGR1 mice; Table 1). The recipients of cells transduced with control MiNR1/MiGR1 retroviruses displayed no overt pathology (Figure 1C-D and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The recipients of BCR-ABL–transduced CB cells remained healthy throughout the observation time of 25 weeks. The engraftment efficiencies ranged between 1.5% and 10.6%, and differentiation of donor-derived cells was skewed toward the erythroid lineage (Figure 1D and supplemental Figure 2), in line with previously published observations.10 Our previous studies indicated that BMI1 expression by itself was also not sufficient to induce leukemia in mice, in line with data published by others.26,27 In contrast, 4 of 8 mice that received cotransduced BMI1/BCR-ABL cells died 16-22 weeks after transplantation (Figure 1C-D and supplemental Figure 3). Mice appeared sick and lethargic, with strongly elevated peripheral nucleated white blood cell counts, moderate splenomegaly, lymphadenopathy, and more than 20% poorly differentiated lymphoblasts in the bone marrow, as revealed by May-Grünwald Giemsa staining and phenotyping (Figure 1D-G). A strong proliferative advantage of BMI1/BCR-ABL cells was observed, compared with single-transduced populations, as the percentages of double-transduced cells in the PB and BM increased from 10%-20% at the beginning of the experiment to 60%-80% at week 18 (Figure 1E). Fluorescence-activated cell sorting (FACS) analysis of BM, spleen, and liver confirmed the lymphoblastic phenotype, as the leukemic cells were highly positive for CD34 (16%-91%) as well as CD19 (93%-99%) antigens (Figure 1D,F,G and supplemental Figure 3). Within the time frame of our experiments, no signs of leukemic development were observed in 4 mice, possibly due to relatively low levels of engraftment of double-transduced cells.
Introduction of BCR-ABL and BMI1 in human cord blood CD34+ cells induces lymphoid leukemia upon transplantation in NOD/SCID mice. (A) Cord blood CD34+ cells were transduced with MiGR1 and MiNR1 empty vector controls, with MiGR1 BMI1 or MiNR1 BCR-ABL vectors, or cotransduced with both vectors (BMI1/BCR-ABL). Efficiencies after 3 transduction rounds are shown. (B) Expression of BMI1, BCR-ABL, and phosphorylated STAT5 was analyzed by Western blotting of transduced CB CD34+ cells. A vertical line has been inserted to indicate a repositioned gel lane. (C) Transduced CB CD34+ cells were transplanted into sublethally irradiated NOD/SCID mice, and Kaplan-Meier survival curves are shown. BM of 2 mice was used for secondary transplantation into sublethally irradiated NOD/SCID mice in duplicate, and Kaplan-Meier survival curves of these mice are shown as well. (D) FACS analysis of BM of engrafted NOD/SCID mice at week 16. (E) Transduced CB CD34+ cells were transplanted into sublethally irradiated NOD/SCID mice, and the percentage of double-positive cells was evaluated directly after transduction and at later time points in the PB of the recipient mice. (F) FACS analysis of the spleen and the liver of the diseased mice showed infiltration of the organs with primitive CD34+ human cells. (G) May- Grünwald Giemsa staining of cytospins from BM and spleen of diseased mice.
Introduction of BCR-ABL and BMI1 in human cord blood CD34+ cells induces lymphoid leukemia upon transplantation in NOD/SCID mice. (A) Cord blood CD34+ cells were transduced with MiGR1 and MiNR1 empty vector controls, with MiGR1 BMI1 or MiNR1 BCR-ABL vectors, or cotransduced with both vectors (BMI1/BCR-ABL). Efficiencies after 3 transduction rounds are shown. (B) Expression of BMI1, BCR-ABL, and phosphorylated STAT5 was analyzed by Western blotting of transduced CB CD34+ cells. A vertical line has been inserted to indicate a repositioned gel lane. (C) Transduced CB CD34+ cells were transplanted into sublethally irradiated NOD/SCID mice, and Kaplan-Meier survival curves are shown. BM of 2 mice was used for secondary transplantation into sublethally irradiated NOD/SCID mice in duplicate, and Kaplan-Meier survival curves of these mice are shown as well. (D) FACS analysis of BM of engrafted NOD/SCID mice at week 16. (E) Transduced CB CD34+ cells were transplanted into sublethally irradiated NOD/SCID mice, and the percentage of double-positive cells was evaluated directly after transduction and at later time points in the PB of the recipient mice. (F) FACS analysis of the spleen and the liver of the diseased mice showed infiltration of the organs with primitive CD34+ human cells. (G) May- Grünwald Giemsa staining of cytospins from BM and spleen of diseased mice.
Summary of NOD/SCID engraftment studies
| Sample . | Chimerism (%) . | Phenotype . | ||
|---|---|---|---|---|
| Bone marrow . | Spleen . | Liver . | ||
| MiNR1/MiGR1 m1 | 0.7 | n.d. | n.d. | No leukemia |
| MiNR1/MiGR1 m2 | 1.1 | n.d. | n.d. | No leukemia |
| MiNR1/MiGR1 m3 | 2.1 | n.d. | n.d. | No leukemia |
| BCR-ABL m1 | 1.5 | n.d. | n.d. | No leukemia, lymphoid/erythroid engraftment |
| BCR-ABL m2 | 2.2 | n.d. | n.d. | No leukemia, erythroid engraftment |
| BCR-ABL m3 | 10.6 | n.d. | n.d. | No leukemia, myeloid/erythroid engraftment |
| BCR-ABL m4 | 1.8 | n.d. | n.d. | No leukemia, lymphoid/erythroid engraftment |
| BMI1/BCR-ABL m1 | 76.4 | 49.9 | 74.5 | Leukemia, lymphoid, and erythroid engraftment |
| BMI1/BCR-ABL m2 | 61.8 | 50.8 | 77.3 | Leukemia, lymphoid, and erythroid engraftment |
| BMI1/BCR-ABL m3 | 1.2 | n.d. | n.d. | No leukemia |
| BMI1/BCR-ABL m4 | 0.5 | n.d. | n.d. | No leukemia |
| BMI1/BCR-ABL m5* | 53.8 | 59.2 | 63.8 | Leukemia, lymphoid, and erythroid engraftment |
| BMI1/BCR-ABL m6 | 5.6 | n.d. | n.d. | No leukemia |
| BMI1/BCR-ABL m7* | 89.4 | 59.1 | 59.3 | Leukemia, lymphoid, and erythroid engraftment |
| BMI1/BCR-ABL m8 | 4.3 | n.d. | n.d. | No leukemia |
| Sample . | Chimerism (%) . | Phenotype . | ||
|---|---|---|---|---|
| Bone marrow . | Spleen . | Liver . | ||
| MiNR1/MiGR1 m1 | 0.7 | n.d. | n.d. | No leukemia |
| MiNR1/MiGR1 m2 | 1.1 | n.d. | n.d. | No leukemia |
| MiNR1/MiGR1 m3 | 2.1 | n.d. | n.d. | No leukemia |
| BCR-ABL m1 | 1.5 | n.d. | n.d. | No leukemia, lymphoid/erythroid engraftment |
| BCR-ABL m2 | 2.2 | n.d. | n.d. | No leukemia, erythroid engraftment |
| BCR-ABL m3 | 10.6 | n.d. | n.d. | No leukemia, myeloid/erythroid engraftment |
| BCR-ABL m4 | 1.8 | n.d. | n.d. | No leukemia, lymphoid/erythroid engraftment |
| BMI1/BCR-ABL m1 | 76.4 | 49.9 | 74.5 | Leukemia, lymphoid, and erythroid engraftment |
| BMI1/BCR-ABL m2 | 61.8 | 50.8 | 77.3 | Leukemia, lymphoid, and erythroid engraftment |
| BMI1/BCR-ABL m3 | 1.2 | n.d. | n.d. | No leukemia |
| BMI1/BCR-ABL m4 | 0.5 | n.d. | n.d. | No leukemia |
| BMI1/BCR-ABL m5* | 53.8 | 59.2 | 63.8 | Leukemia, lymphoid, and erythroid engraftment |
| BMI1/BCR-ABL m6 | 5.6 | n.d. | n.d. | No leukemia |
| BMI1/BCR-ABL m7* | 89.4 | 59.1 | 59.3 | Leukemia, lymphoid, and erythroid engraftment |
| BMI1/BCR-ABL m8 | 4.3 | n.d. | n.d. | No leukemia |
n.d. indicates not determined.
Used for secondary transplantation.
To determine whether self-renewing leukemic stem cells were still present within the leukemic graft in the primary recipients, secondary transplantations were performed. BM of BMI1/BCR-ABL mice 5 and 7 was transplanted into sublethally irradiated NOD/SCID recipients. Robust engraftment was observed in all transplanted animals, resulting in a fatal leukemia within 8-12 weeks (Figure 1C), with high infiltration of lymphoid CD34+/CD19+ blast cells in BM, spleen, and liver (supplemental Figure 3). Retroviral integration-site analyses were performed by LM-PCR in primary and secondary recipients, and these studies indicated that different clones from 1 transduction experiment gave rise to a similar leukemia in primary mice (nrs 5 and 7; supplemental Figure 4). Comparison of integration sites between primary and secondary recipients revealed that similar clones could give rise to leukemia upon serial transplantation (5 vs 5.1 and 5.2 and 7 vs 7.1 and 7.2; supplemental Figure 4). Sequence analysis of several integration sites showed no integrations in close proximity to known oncogenes (data not shown). Deletions of the Ikaros gene are frequently observed in BCR-ABL–induced lymphoblastic leukemia.35 However, we did not detect Ikaros deletions in the leukemic cells isolated from secondary recipient animals (supplemental Figure 5). Expression levels of BMI1 and BCR-ABL in the BM of primary and secondary recipients is provided in supplemental Figure 6A-B. Taken together, these data show that constitutive expression of BMI1 and BCR-ABL can functionally collaborate to induce acute lymphoid leukemia.
Coexpression of BMI1 and BCR-ABL in human CD34+ cells promotes long-term myeloid or lymphoid expansion and stem cell self-renewal
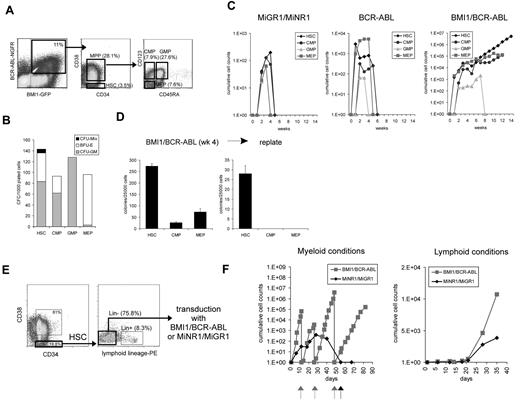
To better understand the mechanism underlying the leukemic transformation, we performed ex vivo long-term experiments. BMI1, BCR-ABL, or double-transduced CB CD34+ cells were cultured on MS5 stromal cells under myeloid growth conditions. After 5 weeks, both suspension and adherent human CD45+ cells were harvested from the cultures, and the cells were seeded onto new stroma to evaluate their replating capacity (Figure 2A-B). Cells transduced with MiGR1/MiNR1 only transiently expanded more than a 3- to 5-week period, but could not be replated (Figure 2B). BMI1-transduced cells could be replated once after the first 5 weeks of culture on MS5, in agreement with our previous observations,26 but a third replating did not result in the establishment of new, expanding, self-renewing cultures. While the BCR-ABL–transduced cells initially displayed a very strong proliferative potential, secondary cultures could not be established. In strong contrast, the double-transduced BMI1/BCR-ABL cells displayed a strong proliferative advantage (Figure 2A), and expanding cultures could be maintained for more than 20 weeks by sequential replating (Figure 2B). Four independent BMI1/BCR-ABL lines were generated, displaying a doubling time of approximately 1.5 days (Figure 2A). Phase-dark cobblestone area forming cells (CAFCs) appeared within 3 days after plating onto MS5 and reappeared upon each replating of BMI1/BCR-ABL cells (Figure 2C). These CAFCs represent an immature population of hematopoietic stem/progenitor cells and have been shown to contain self-renewal potential, based on their serial replating potential, particularly in cases when oncogenes are overexpressed.30 Progenitors were maintained in BMI1/BCR-ABL cultures, as determined by CFC assays using suspension cells from MS5 cocultures at week 9 (Figure 2D). While in previous studies we have observed that CB CD34+ cells transduced with BMI1 were able to maintain progenitors for up to 16 weeks in liquid culture, this was not observed in MS5 cocultures.26 This is in line with a more moderate long-term proliferative advantage of BMI1-transduced cells in MS5 stromal cocultures, compared with liquid cultures, we have observed in our previous studies and described here.26 FACS analysis of long-term BMI1/BCR-ABL cultures revealed the expression of CD45 and CD117 as well as myeloid and erythroid markers (CD33, CD71, and GPA) (supplemental Table 1). Cytospin preparations showed the presence of blast-like, as well as erythroid and myeloid precursor, cells (Figure 2E). Despite the presence of cells with an immature morphology, the expression of CD34 was lost upon long-term culturing under myeloid conditions. This indicates that a population of cells that lack CD34 expression, but most likely express CD117, is able to maintain long-term self-renewal properties under these conditions. Long-term culture-initiating cell (LTC-IC) assays using freshly transduced CB CD34+ cells indicated that stem cell frequencies in the BMI1/BCR-ABL double-transduced cells were ∼ 240-fold higher compared with controls (Figure 2F).
Coexpression of BCR-ABL and BMI1 in human CD34+ cells promotes long-term stem cell self-renewal and myeloid and lymphoid expansion in vitro. (A) BMI1/BCR-ABL coexpression results in the immortalization of human CB CD34+ cells on MS5 stroma under myeloid conditions. The difference in proliferative capacity between the groups is shown. (B) A detailed representation of the experiment in panel A is shown. After 5 weeks, both suspension and adherent human CD45+ cells were harvested from the cultures, and the cells were seeded onto new stroma to evaluate their replating capacity. Arrows indicate time points when replating was performed. (C) An example of week 18 phase-dark CAFCs that could be harvested and replated to initiate new expanding cocultures. (D) The number of hematopoietic progenitors was evaluated in CFC assays in methylcellulose. A representative experiment is shown, where transduced CB CD34+ cells were analyzed after 9 weeks of expansion on MS5 BM stroma. (E) Cytospin preparations of suspension cells from cocultures described in (A) at week 16, indicating the presence of blast-like, as well as erythroid and myeloid precursor, cells. (F) Stem cell frequencies were determined in LTC-IC assays using freshly transduced CB CD34+ cells. (G) Long-term expansion of the transduced cells under lymphoid culture conditions. (H) Representative cytospin at week 8 from double-transduced cultures. (I) Clonogenic assays in methylcellulose were performed to evaluate the capacity to form progenitor colonies in the presence of IL-7. Microscopic images of the colonies are shown as the insets in the graph. (J) Immediately after transduction, the cells were plated in stroma-free liquid cultures in the presence of IL-3 and SCF. Long-term expanding BMI1/BCR-ABL cultures could be maintained for more than 23 weeks.
Coexpression of BCR-ABL and BMI1 in human CD34+ cells promotes long-term stem cell self-renewal and myeloid and lymphoid expansion in vitro. (A) BMI1/BCR-ABL coexpression results in the immortalization of human CB CD34+ cells on MS5 stroma under myeloid conditions. The difference in proliferative capacity between the groups is shown. (B) A detailed representation of the experiment in panel A is shown. After 5 weeks, both suspension and adherent human CD45+ cells were harvested from the cultures, and the cells were seeded onto new stroma to evaluate their replating capacity. Arrows indicate time points when replating was performed. (C) An example of week 18 phase-dark CAFCs that could be harvested and replated to initiate new expanding cocultures. (D) The number of hematopoietic progenitors was evaluated in CFC assays in methylcellulose. A representative experiment is shown, where transduced CB CD34+ cells were analyzed after 9 weeks of expansion on MS5 BM stroma. (E) Cytospin preparations of suspension cells from cocultures described in (A) at week 16, indicating the presence of blast-like, as well as erythroid and myeloid precursor, cells. (F) Stem cell frequencies were determined in LTC-IC assays using freshly transduced CB CD34+ cells. (G) Long-term expansion of the transduced cells under lymphoid culture conditions. (H) Representative cytospin at week 8 from double-transduced cultures. (I) Clonogenic assays in methylcellulose were performed to evaluate the capacity to form progenitor colonies in the presence of IL-7. Microscopic images of the colonies are shown as the insets in the graph. (J) Immediately after transduction, the cells were plated in stroma-free liquid cultures in the presence of IL-3 and SCF. Long-term expanding BMI1/BCR-ABL cultures could be maintained for more than 23 weeks.
Because the in vivo leukemic transformation in NOD/SCID mice was biased toward lymphoid leukemia, we questioned whether ex vivo long-term cultures could also be established under lymphoid-permissive conditions. Single- or double-transduced CB CD34+ populations were expanded on MS5 under lymphoid growth conditions. A proliferative advantage was particularly observed in the BMI1/BCR-ABL double-transduced cells, and long-term self-renewing cultures could only be established by the sequential replating of BMI1/BCR-ABL cultures (Figure 2G). Morphologic analysis revealed the generation of immature lymphoblastic cells (Figure 2H), which was confirmed by FACS analysis showing the presence of CD34+ and CD19+ cells (supplemental Table 2). Clonogenic growth in methylcellulose in the presence of IL-7 was observed with BMI1/BCR-ABL, but not with BCR-ABL, cells and only to a limited extent in the control groups (Figure 2I).
To determine the necessity of a BM microenvironment, we performed stroma-free liquid cultures driven by IL-3 and SCF. While control cells were only able to expand for a limiting period of 5-9 weeks, the BMI1/BCR-ABL cells could initiate cultures that maintained proliferative activity for more than 25 weeks, resulting in strong expansion of > 1 × 1010-fold (Figure 2J). BMI1 cells were able to expand for ∼ 13-20 weeks, as previously reported.26 Importantly, although BCR-ABL–transduced cells displayed a strong enhanced initial expansion at week 1, compared with control cultures, no long-term propagating cultures could be established, suggesting that BCR-ABL+ cells still depend on additional cues from the BM microenvironment. Phenotypical FACS analysis of BMI1/BCR-ABL cultures revealed biphenotypic expression of erythroid and myeloid differentiation markers for more than a period of 15 weeks (supplemental Table 3).
Together, these data indicate that coexpression of BMI1 and BCR-ABL in human CD34+ cells results in a strong proliferative advantage and elevated long-term self-renewal potential under lymphoid- as well as myeloid-permissive conditions.
HSCs are the most effective target cells for long-term expansion and self-renewal induced by coexpression of BMI1 and BCR-ABL
To further distinguish which population of cells may be responsible for the initiation of long-term cultures, transduced hematopoietic stem cells (HSCs), common myeloid progenitors (CMPs), megakaryocyte-erythroid progenitors (MEPs), and granulocyte-monocyte progenitors (GMPs) were sorted, as illustrated in Figure 3A. The purity of progenitor sorts was determined by CFC assays (Figure 3B). All stem and progenitor compartments transduced with the control MiNR1/MiGR1 vectors generated progeny in MS5 cocultures, whereby maximal expansion was observed in cultures initiated with HSCs. However, none of the cultures could be maintained beyond 5 weeks (Figure 3C). Interestingly, MEPs transduced with BCR-ABL showed a strong, but transient, proliferative capacity. Coexpression of BMI1 and BCR-ABL conferred the long-term self-renewal and expansion to the HSC population, but much less efficiently to the progenitor subpopulations (Figure 3C). After 4 weeks of MS5 coculture, only the cultures initiated with BMI1/BCR-ABL–transduced HSCs contained self-renewing progenitors (Figure 3D).
Coexpression of BCR-ABL and BMI1 results in long-term expansion and self-renewal of HSCs, but not CMPs, GMPs, or MEPs. (A) Sorting strategies of the transduced cells on the basis of combinatorial expression of cell-surface markers. Four different populations of cells were sorted from each group, as indicated. (B) CFC assay from the MiGR1/MiNR1 group revealed high purity of the sorted populations. (C) HSCs, CMPs, GMPs, and MEPs from all the groups were grown on MS5 stroma to evaluate their proliferative capacity. Maximal expansion was observed in cultures initiated with HSCs, and moreover, only the BMI1/BCR-ABL HSCs could be maintained over the long term. (D) Cells were plated weekly in methylcellulose, and colonies were scored on the basis of their morphology; a representative example at week 4 is shown. The HSC fraction yielded the highest progenitor frequencies, and only this fraction contained self-renewal properties, as determined by secondary replating assays. (E) Sorting scheme for Lin− HSCs that were used in transduction experiments. (F) Transduced Lin− HSCs were cultured on MS5 BM stromal cells under myeloid or lymphoid conditions. Arrows indicate time of replating.
Coexpression of BCR-ABL and BMI1 results in long-term expansion and self-renewal of HSCs, but not CMPs, GMPs, or MEPs. (A) Sorting strategies of the transduced cells on the basis of combinatorial expression of cell-surface markers. Four different populations of cells were sorted from each group, as indicated. (B) CFC assay from the MiGR1/MiNR1 group revealed high purity of the sorted populations. (C) HSCs, CMPs, GMPs, and MEPs from all the groups were grown on MS5 stroma to evaluate their proliferative capacity. Maximal expansion was observed in cultures initiated with HSCs, and moreover, only the BMI1/BCR-ABL HSCs could be maintained over the long term. (D) Cells were plated weekly in methylcellulose, and colonies were scored on the basis of their morphology; a representative example at week 4 is shown. The HSC fraction yielded the highest progenitor frequencies, and only this fraction contained self-renewal properties, as determined by secondary replating assays. (E) Sorting scheme for Lin− HSCs that were used in transduction experiments. (F) Transduced Lin− HSCs were cultured on MS5 BM stromal cells under myeloid or lymphoid conditions. Arrows indicate time of replating.
To further deplete common lymphoid progenitors (CLPs) from the HSC fraction, we sorted CD34+/CD38−/Lin− cells using an extended lineage cocktail containing antibodies against CD2, CD3, CD4, CD7, CD8, CD10, CD19, CD20, and CD56 (Figure 3E). After MoFlo sorting, cells were transduced with MiNR1/MiGR1 or BMI1/BCR-ABL and plated onto MS5 under myeloid and lymphoid coculture conditions, and similar results were obtained as in previous experiments (Figure 3F and supplemental Table 4).
Enhanced proliferation and self-renewal of BCR-ABL+ CP CML CD34+ cells by retroviral introduction of BMI1
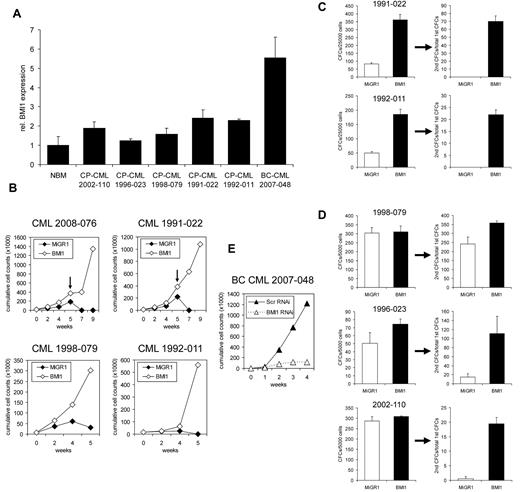
Finally, we isolated CD34+ cells from CML patients in CP that expressed relatively low amounts of endogenous BMI1, compared with BC CML (Figure 4A), and asked whether retroviral introduction of BMI1 in BCR-ABL+ cells would affect long-term growth and self-renewal. Upon overexpression of BMI1, we observed increased proliferation capacity of the transduced CML cells on MS5 stromal cells under myeloid conditions, and cultures could be replated for an additional 4 weeks in 2 of 4 CML patients (Figure 4B). As depicted in Figure 4C, when nonadherent cells from week 5 cocultures were analyzed for the presence of progenitors, overexpression of BMI1 resulted in the production of more colony-forming units (CFUs), which had retained self-renewing ability, as determined by sequential replating of the colonies in methylcellulose assays. Progenitor-replating activity was also studied in 3 additional CP-CML samples directly after transduction with BMI1 or MiGR1 control vectors, and these studies further confirmed that overexpression of BMI1 enhances the self-renewal of CP-CML progenitor cells (Figure 4D). Although increased expansion and self-renewal was observed in MS5 cocultures, maturation of the progeny was not impaired, and no signs of blast crisis were observed within this time frame of the experiment (data not shown). In line with these observations, the CD34 percentage was not maintained, but declined, throughout the culture period under myeloid conditions.
Enhanced proliferation of BCR-ABL+ CP CML CD34+ cells by retroviral introduction of BMI1. (A) RNA was isolated from CD34+ cells of CP-CML patients; one BC-CML patient and normal bone marrow (NBM) and BMI1 expression was determined by quantitative real-time polymerase chain reaction. (B) CD34+ cells from CP-CML patients were transduced with control or MiGR1 BMI1 vectors and cells were cultured on MS5 stromal cells, and cumulative cell counts in suspension are shown. Where indicated by the arrows, cultures were harvested and replated onto new stroma. (C) Suspension cells from week 5 cocultures of transduced CP-CML samples were used to perform CFC assays. Progenitor self-renewal was analyzed by replating the cells in secondary CFC assays, and only the BMI1-overexpressing cells had replating ability. (D) CD34+ cells from CP-CML patients were transduced with control or MiGR1 BMI1 vectors, transduced cells were sorted by MoFlo, and CFC frequencies as well as CFC replating capacity were determined in methylcellulose assays. (E) CD34+ cells from a BC CML sample were transduced with lentivirus encoding shRNA sequences against BMI1 or scrambled and then cultured on MS5 stromal cells.
Enhanced proliferation of BCR-ABL+ CP CML CD34+ cells by retroviral introduction of BMI1. (A) RNA was isolated from CD34+ cells of CP-CML patients; one BC-CML patient and normal bone marrow (NBM) and BMI1 expression was determined by quantitative real-time polymerase chain reaction. (B) CD34+ cells from CP-CML patients were transduced with control or MiGR1 BMI1 vectors and cells were cultured on MS5 stromal cells, and cumulative cell counts in suspension are shown. Where indicated by the arrows, cultures were harvested and replated onto new stroma. (C) Suspension cells from week 5 cocultures of transduced CP-CML samples were used to perform CFC assays. Progenitor self-renewal was analyzed by replating the cells in secondary CFC assays, and only the BMI1-overexpressing cells had replating ability. (D) CD34+ cells from CP-CML patients were transduced with control or MiGR1 BMI1 vectors, transduced cells were sorted by MoFlo, and CFC frequencies as well as CFC replating capacity were determined in methylcellulose assays. (E) CD34+ cells from a BC CML sample were transduced with lentivirus encoding shRNA sequences against BMI1 or scrambled and then cultured on MS5 stromal cells.
To study the necessity of BMI1 expression for long-term expansion of BC CML samples, we transduced CD34+ cells isolated from a BC CML patient with lentiviral vectors encoding shRNAi sequences against BMI1 or scrambled, and performed long-term cocultures on MS5 stroma. As shown in Figure 4E, long-term expansion of BC CML cells was severely impaired by the down-modulation of BMI1.
Taken together, these data suggest that BMI1 might collaborate with BCR-ABL in the progression of the disease of the CML patients.
Discussion
While it is clear that in more than 95% of the CML cases, leukemia is induced by the p210 BCR-ABL oncogene, it is highly likely that additional mutations or alterations are required to induce the transition from CP into BC. Our current data indicate that elevated levels of the polycomb-group protein, BMI1 can act as a collaborating event with BCR-ABL to induce leukemic transformation of human cells. Interestingly, a recent report indicated that BMI1 expression levels were significantly higher in patients with advanced-phase than in patients with CP CML.24 In addition, the level of BMI1 at diagnosis correlated with the time to transformation into BC, and low BMI1 expression levels were associated with an improved overall survival.24 Some previous reports have also indicated that BMI1 can act as a collaborating factor in the process of cellular transformation. In a provirus integration screen, BMI1 was originally identified as a co-operating factor with Myc in the induction of B-cell lymphomagenesis.25 BMI1 appears not to be sufficient to induce leukemia by itself, as we and others observed previously.26,27 Even though we cannot exclude the possibility that BMI1 might enhance the lifespan of lymphoid cells, in our current studies, in which nonsorted BMI1, BCR-ABL, and double-transduced BMI1/BCR-ABL cells were injected into NOD/SCID mice, only double-transduced populations were capable of initiating a transplantable leukemia. Several reports have highlighted the role that BMI1 fulfills in both normal as well as leukemic stem cell self-renewal.22,23,26,27 Thus, it appears likely that BMI1 contributes to the process of leukemic transformation by acting as a stem cell self-renewal factor in CML as well.
Although the mechanisms by which BMI1 contributes to BCR-ABL–induced leukemia remain to be elucidated, it is well documented that the p16INK4a/p19ARF locus, which is required to bypass the senescence of embryonic fibroblasts, is repressed by BMI1.36 In addition, in hematopoietic cells, the targeted deletion of BMI1 resulted in the increased expression of p16 and p19.37 Deletion of p16/p19 in bmi1−/− HSCs only partly restored self-renewal, and overexpression of BMI1 could still increase progenitor levels in the absence of p16/p19, indicating that other BMI1 targets must exist as well.37 Indeed, we also observed a strong down-regulation of p16 and p14 (human homolog of p19) in BMI1/BCR-ABL–transduced cells (data not shown). Although direct downstream targets have not been described yet, the forced expression of BMI1 promotes symmetric cell divisions of HSCs and expansion of immature multipotent progenitors.27 Thus, BMI1 might contribute to tumorigenesis by bypassing senescence and maintaining the lifespan of stem cells, as well as increasing self-renewal by allowing symmetric cell divisions. This would also imply that the BMI1-expressing stem cells might be more prone to acquire yet additional mutations that ultimately aid in the development of leukemia. Recently, we observed that down-modulation of BMI1 in human CB CD34+ cells impairs long-term expansion and self-renewal.38 This was associated with enhanced levels of apoptosis and coincided with elevated levels of reactive oxygen species accumulation. In line with these observations, it was recently shown that bmi1−/− mice are characterized by elevated levels of reactive oxygen species, impaired mitochondrial function, and impaired DNA damage responses.39 These data suggest that protection against oxidative stress might be one of the functions of BMI1 in HSCs and leukemic stem cells.
Deletion of both the p16INK4a/p19ARF locus, as well as deletion of IKAROS and PAX5, have been implicated in the acute lymphoid, but not myeloid, leukemias initiated by BCR-ABL.35,40,41 We have currently no indications that IKAROS or PAX5 expression levels are changed in BMI1/BCR-ABL–transduced cells, but since we observed a strong bias toward a lymphoid leukemia in our NOD/SCID mouse model, it is possible that BMI1-mediated repression of the p16INK4a/p14ARF locus drives transformation along the lymphoid lineage. A recent murine model demonstrated that in the absence of p19ARF, a lymphoid progenitor served as the target for transformation by BCR-ABL.42 Another study showed that the growth and survival of aged lymphoid progenitors was increased by BMI1-mediated repression of p16 and p19.43 In light of these findings, we have questioned whether a lymphoid progenitor, rather than an HSC, was the target of transformation in our model. However, our data pinpoint to a multipotent progenitor or HSC as the most efficient target cell, although we cannot exclude the possibility that, in vivo, the lymphoid progenitor might also be a target cell of transformation. In vitro, both myeloid as well as lymphoid long-term self-renewing cultures could be established, most efficiently from HSC-transduced cell populations. Although, in vivo, we observed a strong lymphoid bias in transplanted NOD/SCID animals, it would be interesting to study whether mouse models that allow more myeloid engraftment, such as was recently elegantly used to study mixed lineage leukemia (MLL-AF9)–induced transformation,44 also allow in vivo transformation along the myeloid lineage of BMI1/BCR-ABL–transduced cells.
Treatment of CML patients with the inhibitor imatinib leads to response rates of more than 95%.45 Yet, the leukemia-initiating cells are not targeted efficiently,46,47 and patients might need to stay on therapy lifelong. A significant proportion of patients develop resistance to therapy, often due to mutations in the kinase domain of BCR-ABL.48,49 Thus, identification of additional targets that facilitate the eradication of BCR-ABL+ leukemia-initiating cells is needed. Our data indicate that self-renewal of Ph+ cells is enhanced by overexpression of BMI1, both in CB model systems as well as in primary CP-CML patient samples. Because, in CML patients, BMI1 levels also increase upon progression toward BC and correlate with a poor prognosis,24 BMI1 appears to be an attractive candidate for targeting. Indeed, we observed that lentiviral down-modulation of BMI1 in a BC CML sample was sufficient to impair long-term expansion on MS5 stroma. Our model system allows the identification of BMI1-downstream pathways that collaborate with BCR-ABL–induced leukemic transformation. Inhibitors against BMI1 (or downstream targets) are currently being evaluated, and these should also be tested for their efficacy in the eradication of the BCR-ABL+ LSC in CML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We greatly appreciate the help of Drs J. J. Erwich, A. van Loon, and H. H. de Haan and colleagues (Obstetrics Departments of University Medical Center in Groningen, Martini Hospital Groningen, and Sophia Hospital in Zwolle) for collecting cord blood. We thank Dr P. Valk (Erasmus University Rotterdam) for sequencing BCR-ABL kinase domains and Dr E. van den berg (Department of Genetics, University Medical Center Groningen) for the karyotyping of CML samples.
This work was supported by grants from the European Union (Eurythron Marie Curie Training Network EU FP6 to G.d.H., NWO-VENI (2004) to J.J.S., NWO-VIDI (2008) to J.J.S., NWO-VICI (2007) to G.d.H., KWF (RUG 2009-4275) to J.J.S., E.V., and G.d.H., and Landsteiner stichting voor bloed transfusie research to R.v.O.
Authorship
Contribution: A.R. and S.J.H. performed experiments and analyzed data; S.O. provided technical assistance; B.D., A.A., and R.v.O. assisted in NOD/SCID engraftment studies; V.v.d.B. performed LM-PCR studies; E.V. and G.d.H. analyzed and discussed data; and A.R. and J.J.S. designed the experiments, analyzed and discussed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jan Jacob Schuringa, Department of Hematology, or Gerald de Haan, Department of Stem Cell Biology, University of Groningen, University Medical Center Groningen, Hanzeplein 1, 9700 RB Groningen, The Netherlands; e-mail: j.schuringa@int.umcg.nl or g.de.haan@med.umcg.nl.
References
Author notes
A.R. and S.J.H. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal